Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties
Abstract
1. Introduction
2. Results
2.1. Identification and Screening of Promising Biosurfactant-Producing Lactic Acid Bacteria
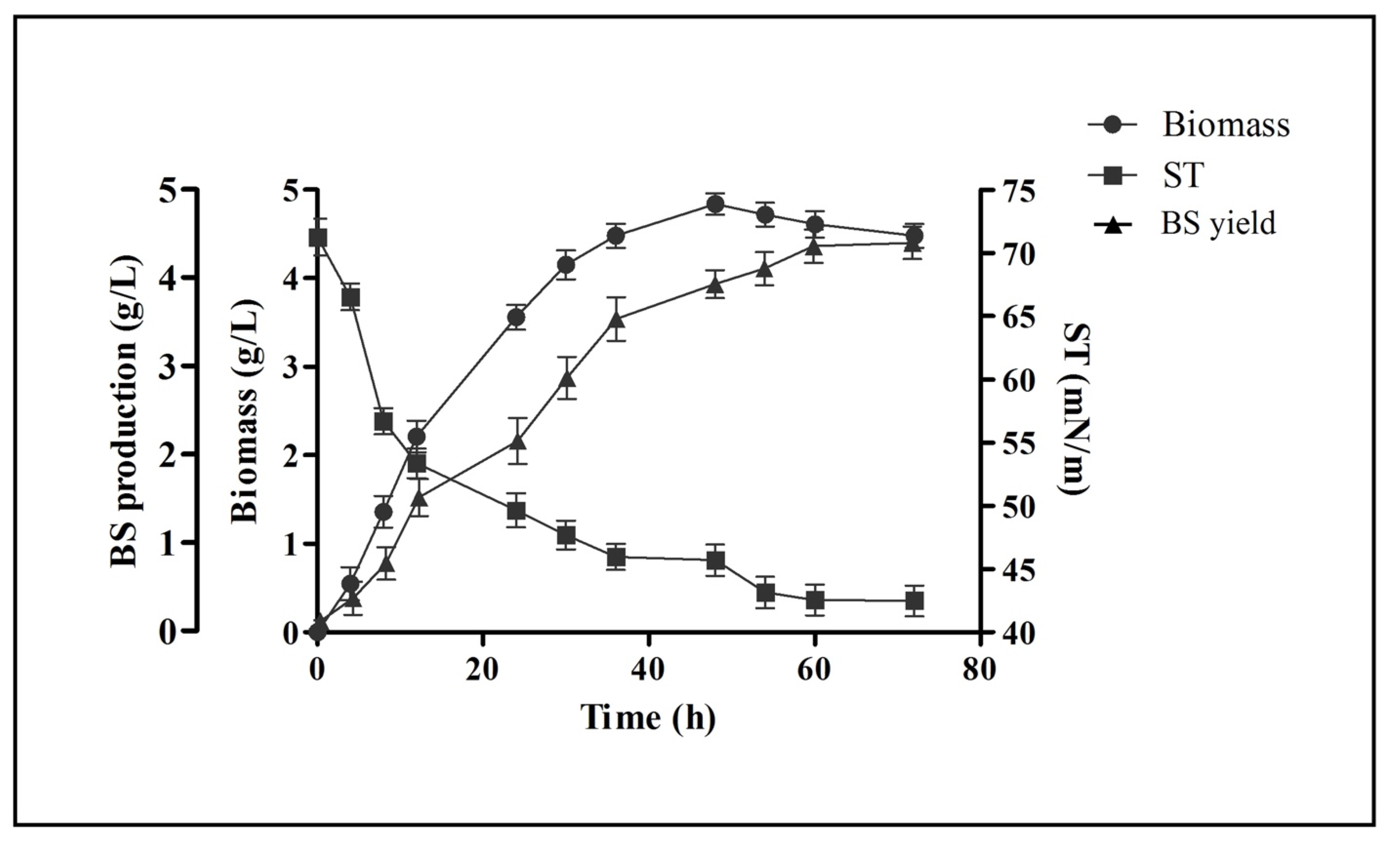
2.2. Growth Kinetics and Biosurfactant Production
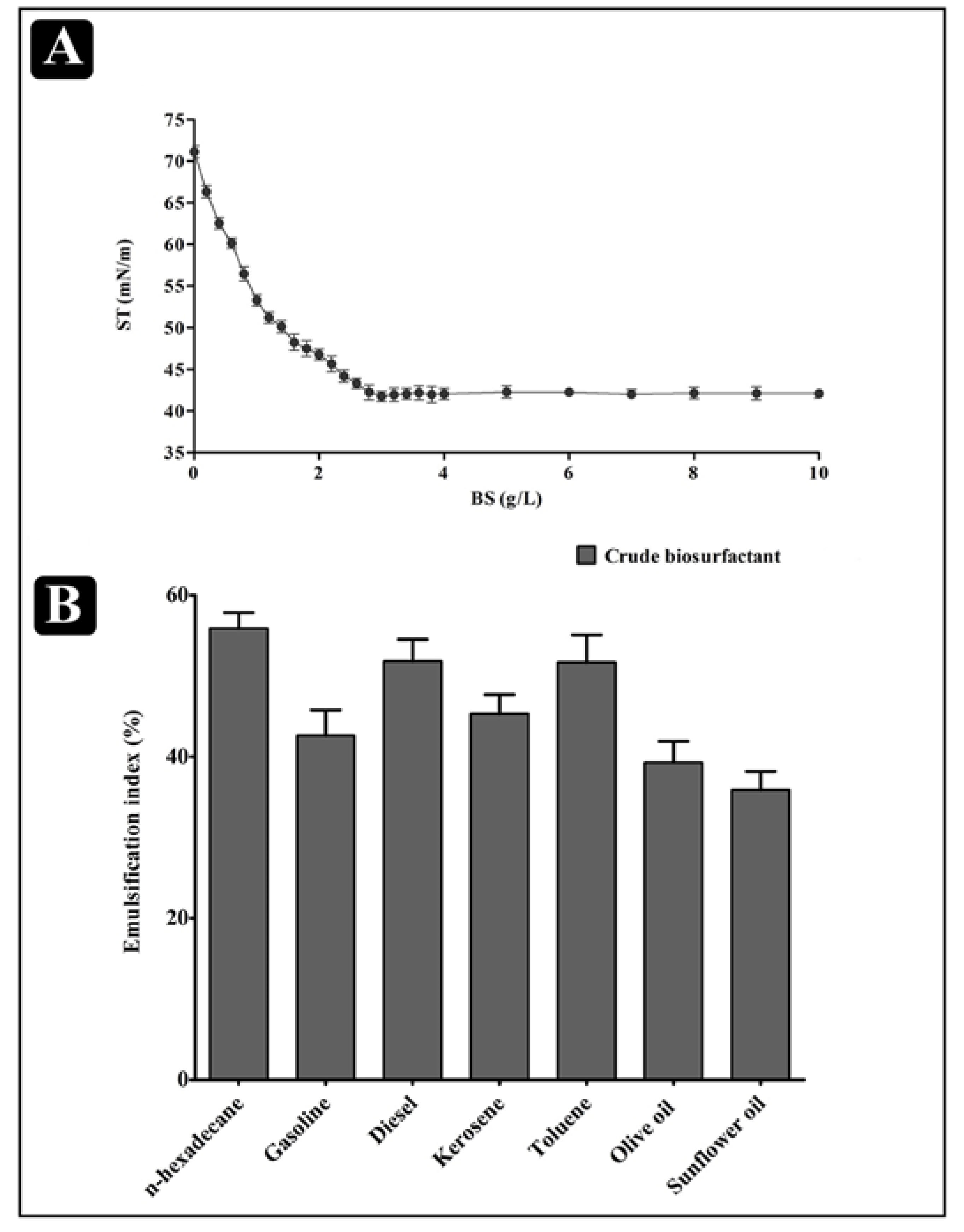
2.3. Physical Properties of the Biosurfactant
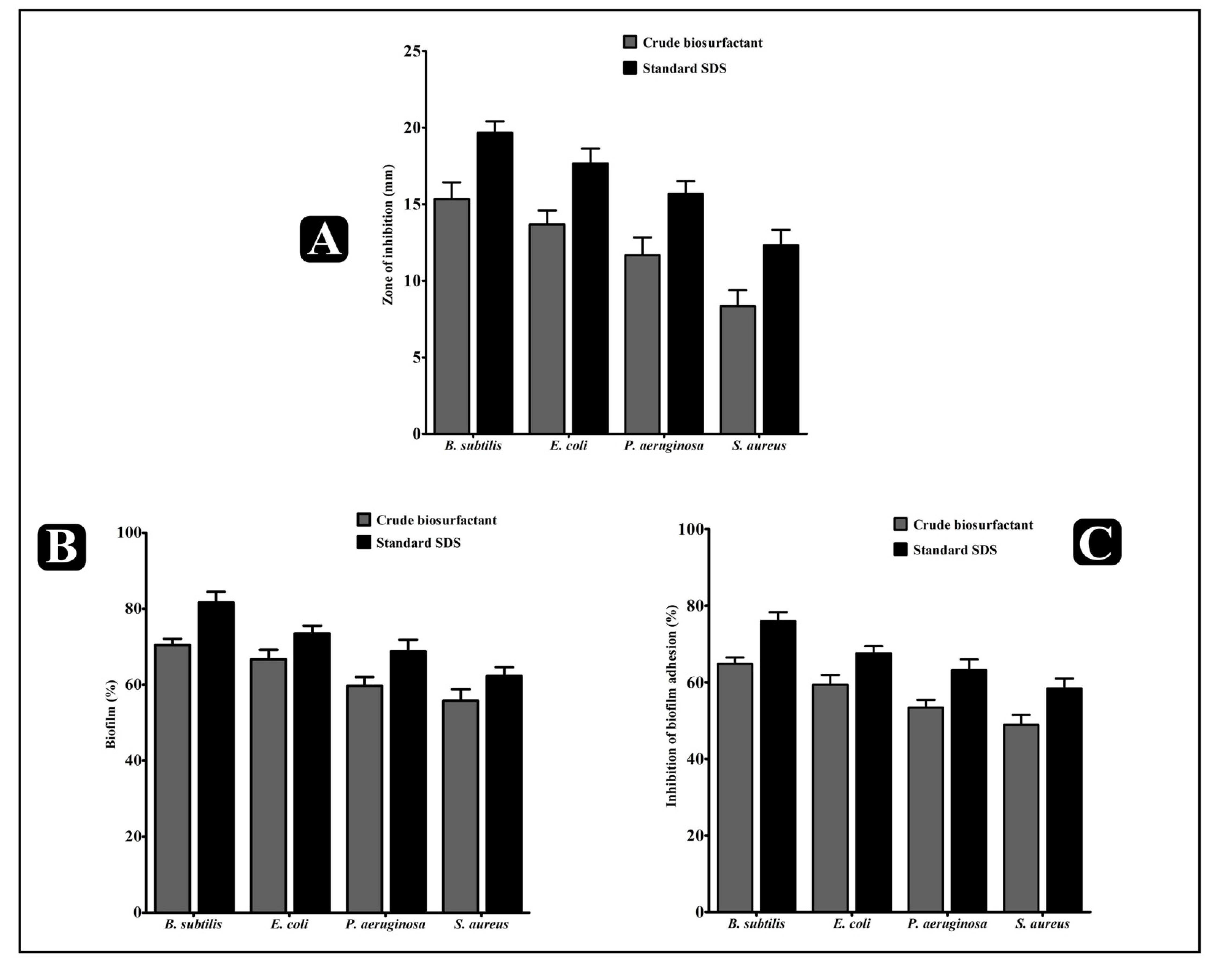
2.4. Antibacterial Activity of the L. rhamnosus Crude Biosurfactant
2.5. Antibiofilm Potential of the L. rhamnosus Crude Biosurfactant
2.6. Effect of L. rhamnosus Crude Biosurfactant on Bacterial Cells Entrapped in Biofilms
2.7. Effect of the L. rhamnosus Crude Biosurfactant on Exopolysaccharide (EPS) Production
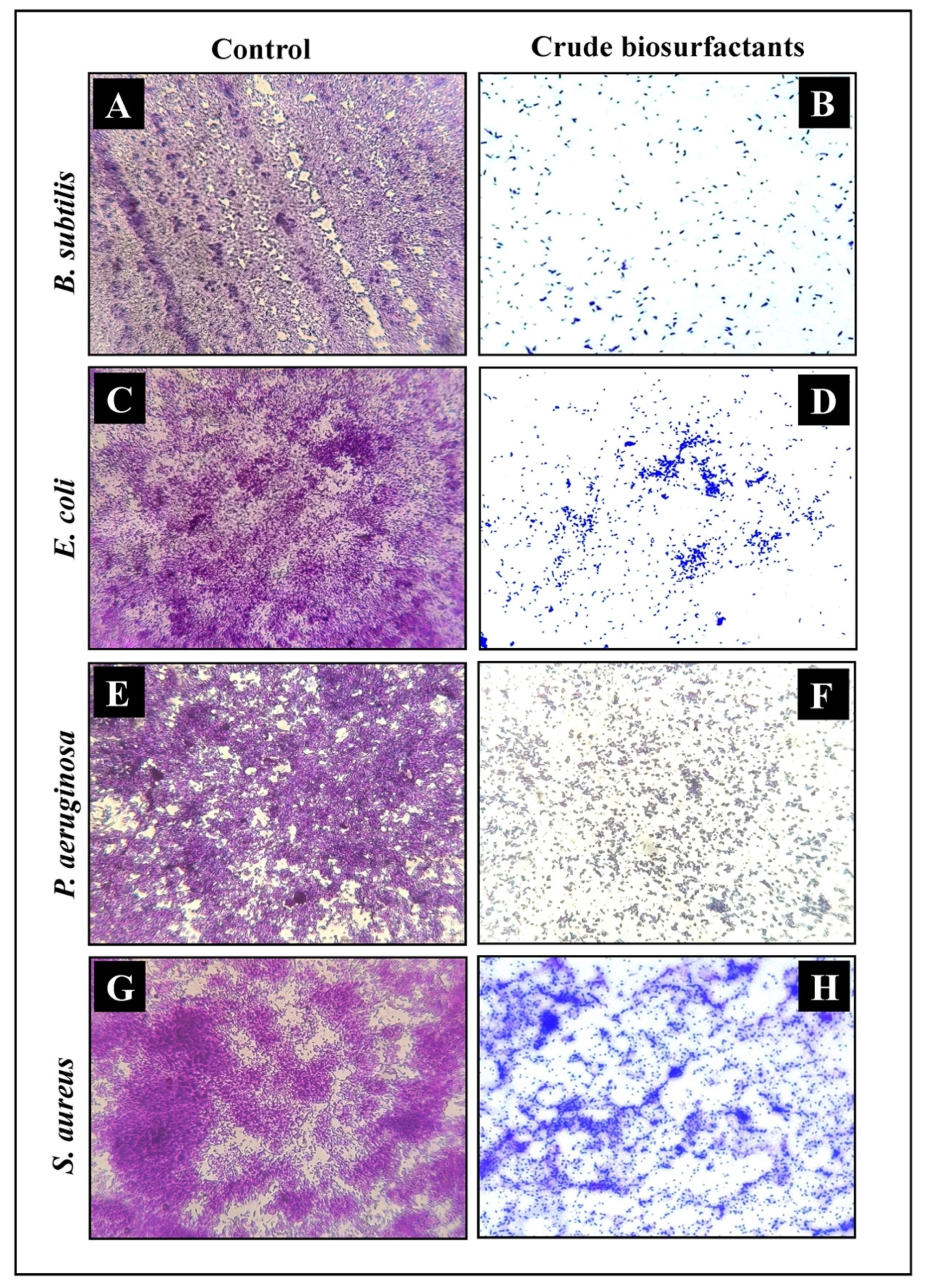
2.8. Microscopic Analysis for the Visualization of the Disrupted Biofilms by Light (LM) and Scanning Electron (SEM) Microscopy
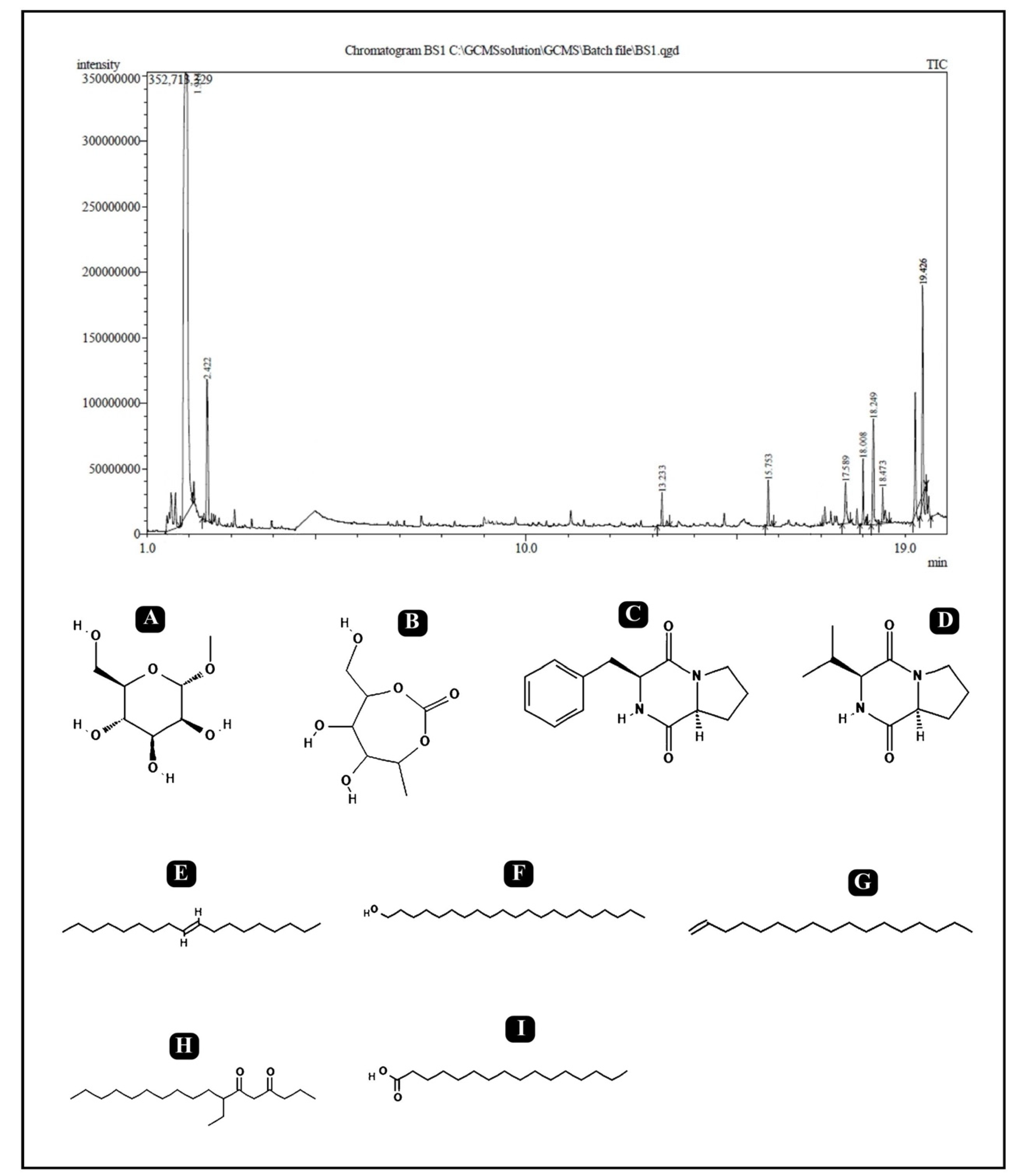
2.9. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3. Discussion
4. Materials and Methods
4.1. Isolation and Screening of Lactic Acid Bacteria
4.2. Identification of Lactic Acid Bacteria
4.3. Biosurfactant Assays
4.3.1. Emulsification Assay
4.3.2. Drop-Collapse Assay
4.3.3. Oil-Spreading Assay
4.3.4. Blue Agar Plate (BAP) Assay
4.4. Surface Tension Measurements
4.5. Control Solutions
4.6. Production of Biosurfactant and Extraction
4.7. Assessment of Biomass and Biosurfactant Concentration
4.8. Assessment of Physical Properties of Biosurfactant
4.9. Assessment of Antibacterial Activity
4.10. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
4.11. Preparation of Biofilm
4.12. Antibiofilm Assays
4.12.1. Effect of the L. rhamnosus Crude Biosurfactant on the Established Biofilms
4.12.2. Effect of the L. rhamnosus Crude Biosurfactant on Adherence of Biofilms
4.13. Microscopic Assessment
4.13.1. Determining and Visualization of Antibiofilm Activity by Light Microscopy
4.13.2. Determination and Visualization of Antibiofilm Activity by Scanning Electron Microscopy
4.14. Bacterial Metabolic Activity in the Biofilm Assays
4.15. Bacterial Cell Damage Assay
4.16. Determining the Production of Exopolysaccharide by Ruthenium Red Staining
4.17. Gas Chromatography–Mass Spectophotometry (GC–MS) Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.L.; Patel, R. The Challenge of Treating Biofilm-associated Bacterial Infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef]
- Bui, L.M.; Turnidge, J.D.; Kidd, S.P. The induction of Staphylococcus aureus biofilm formation or Small Colony Variants is a strain-specific response to host-generated chemical stresses. Microbes Infect. 2015, 17, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Begun, J.; Gaiani, J.M.; Rohde, H.; Mack, D.; Calderwood, S.B.; Ausubel, F.M.; Sifri, C.D. Staphylococcal Biofilm Exopolysaccharide Protects against Caenorhabditis elegans Immune Defenses. PLOS Pathog. 2007, 3, e57. [Google Scholar] [CrossRef]
- Prabhakara, R.; Harro, J.M.; Leid, J.G.; Keegan, A.D.; Prior, M.L.; Shirtliff, M.E. Suppression of the Inflammatory Immune Response Prevents the Development of Chronic Biofilm Infection Due to Methicillin-Resistant Staphylococcus aureus. Infect. Immun. 2011, 79, 5010–5018. [Google Scholar] [CrossRef]
- Hausner, M.; Wuertz, S. High Rates of Conjugation in Bacterial Biofilms as Determined by Quantitative In Situ Analysis. Appl. Environ. Microbiol. 1999, 65, 3710–3713. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial Biofilms: An Emerging Link to Disease Pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Adnan, M.; Alshammari, E.; Patel, M.; Ashraf, S.A.; Khan, S.; Hadi, S. Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: Necessity for green chemistry. PeerJ 2018, 6, e5049. [Google Scholar] [CrossRef]
- Van Delden, C.; Iglewski, B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998, 4, 551–560. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Minireview. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Sen, R. Surfactin: Biosynthesis, Genetics and Potential Applications. Biosurfactants 2010, 672, 316–323. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the bio-surfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.; El-Malek, F.A. Valorization of Lipopeptides Biosurfactants as Anticancer Agents. Int. J. Pept. Res. Ther. 2021, 27, 447–455. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Banat, I.M.; Van Der Mei, H.C.; Teixeira, J.A.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Sharma, S.K.; Mudhoo, A. Biosurfactants: Research Trends and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Satputea, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Caola, I.; Nollo, G.; Cavallo, M.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus -derived biosurfactant. J. Appl. Microbiol. 2015, 118, 1116–1125. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S. Simultaneous Production of Biosurfactants and Bacteriocins by ProbioticLactobacillus caseiMRTL3. Int. J. Microbiol. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, R.; Jayalakshmi, S.; Banat, I.M. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour. Technol. 2011, 102, 772–778. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-Producing Lactobacilli: Screening, Production Profiles, and Effect of Medium Composition. Appl. Environ. Soil Sci. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Saravanakumari, P.; Mani, K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 2010, 101, 8851–8854. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.; Makris, G. Probiotic bacteria and biosurfactants for nosocomial infection control: A hypothesis. J. Hosp. Infect. 2009, 71, 301–306. [Google Scholar] [CrossRef]
- Rivera, O.M.P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process. Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; van der Mei, H.C.; Teixeira, J.; Oliveira, R. Influence of Biosurfactants from Probiotic Bacteria on Formation of Biofilms on Voice Prostheses. Appl. Environ. Microbiol. 2004, 70, 4408–4410. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef]
- Heinemann, C.; van Hylckama Vlieg, J.E.; Janssen, D.B.; Busscher, H.J.; van der Mei, H.C.; Reid, G. Purification and charac-terization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol. Lett. 2000, 190, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Wittouck, S.; De Boeck, I.; Allonsius, C.N.; Pasolli, E.; Segata, N.; Lebeer, S. Large-scale phylogenomics of the Lac-tobacillus casei group highlights taxonomic inconsistencies and reveals novel clade-associated features. MSystems 2017, 2, e00061-17. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Liu, D.D.; Gu, C.T. Proposal to reclassify Lactobacillus zhaodongensis, Lactobacillus zeae, Lactobacillus argentoratensis and Lactobacillus buchneri subsp. silagei as Lacticaseibacillus zhaodongensis comb. nov., Lacticaseibacillus zeae comb. nov., Lactiplantibacillus argentoratensis comb. nov. and Lentilactobacillus buchneri subsp. silagei comb. nov., respectively and Apilactobacillus kosoi as a later heterotypic synonym of Apilactobacillus micheneri. Int. J. Syst. Evol. Microbiol. 2020, 70, 6414–6417. [Google Scholar]
- Skerman, V.B.D.; McGowan, V.; Sneath, P.H.A. Approved lists of bacterial names. Int. J. Syst. Evol. Microbiol. 1980, 30, 225–420. [Google Scholar] [CrossRef]
- Collins, M.D.; Phillips, B.A.; Zanoni, P. Deoxyribonucleic Acid Homology Studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 1989, 39, 105–108. [Google Scholar] [CrossRef]
- Jara, S.; Sánchez, M.; Vera, R.; Cofré, J.; Castro, E. The inhibitory activity of Lactobacillus spp. isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin. Anaerobe 2011, 17, 474–477. [Google Scholar] [CrossRef]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Olivares, M.; Boza, J.; Jiménez, J.; Fernández, L.; Xaus, J.; et al. The commensal microflora of human milk: New perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 2004, 15, 121–127. [Google Scholar] [CrossRef]
- Heikkila, M.P.; Saris, P.E.J. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003, 95, 471–478. [Google Scholar] [CrossRef]
- Patel, K.; Patel, M. Improving bioremediation process of petroleum wastewater using biosurfactants producing Stenotrophomonas sp. S1VKR-26 and assessment of phytotoxicity. Bioresour. Technol. 2020, 315, 123861. [Google Scholar] [CrossRef]
- Adnan, M.; Alshammari, E.; Ashraf, S.A.; Patel, K.; Lad, K.; Patel, M. Physiological and molecular characterization of bio-surfactant producing endophytic fungi Xylaria regalis from the cones of Thuja plicata as a potent plant growth promoter with its potential application. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef]
- Kracht, M.; Rokos, H.; Özel, M.; Kowall, M.; Pauli, G.; Vater, J. Antiviral and Hemolytic Activities of Surfactin Isoforms and Their Methyl Ester Derivatives. J. Antibiot. 1999, 52, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Cavallo, M.; Martinotti, M.G.; Banat, I.M. Biosurfactants and bioemulsifiers biomedical and related applica-tions–present status and future potentials. Biomed. Sci. Eng. Technol. 2012, 14, 326–335. [Google Scholar]
- Quadriya, H.; Ali, S.A.M.; Parameshwar, J.; Manasa, M.; Khan, M.Y.; Hameeda, B. Microbes Living Together: Exploiting the Art for Making Biosurfactants and Biofilms. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: Berlin/Heidelberg, Germany, 2018; pp. 161–177. [Google Scholar]
- Abisado, R.; Benomar, S.; Klaus, J.; Dandekar, A.; Chandler, J. Bacterial Quorum Sensing and Microbial Community Interac-tions. mBio 2018, 9, e02331-17. [Google Scholar]
- Mangwani, N.; Kumari, S.; Das, S. Bacterial biofilms and quorum sensing: Fidelity in bioremediation technology. Biotechnol. Genet. Eng. Rev. 2016, 32, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Velraeds, M.M.; van der Mei, H.C.; Reid, G.; Busscher, H.J. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl. Environ. Microbiol. 1996, 62, 1958–1963. [Google Scholar] [CrossRef]
- Dusane, D.; Rajput, J.; Kumar, A.; Nancharaiah, Y.; Venugopalan, V.; Zinjarde, S. Disruption of fungal and bacterial biofilms by lauroyl glucose. Lett. Appl. Microbiol. 2008, 47, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.; Nancharaiah, Y.V.; Zinjarde, S.S.; Venugopalan, V.P. Rhamnolipid mediated disruption of marine Bacillus pumilus biofilms. Colloids Surfaces B Biointerfaces 2010, 81, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.; Da Silva, J.; Lehocky, b.M.; Barros-Timmons, A.; Coelho, M.; Marrucho, I.; Coutinho, J. Production and charac-terization of a bioemulsifier from Yarrowia lipolytica. Process. Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- De JesusCortés-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Gibbs, B.F. Types, production and applications of biosurfactants. Proc. Indian Natl. Sci. Acad. Part B 2004, 70, 31–56. [Google Scholar]
- Pinto, S.; Alves, P.; Santos, A.; Matos, C.; Oliveiros, B.; Gonçalves, S.; Gudiña, E.; Rodrigues, L.; Teixeira, J.; Gil, M. Poly (di-methyl siloxane) surface modification with biosurfactants isolated from probiotic strains. J. Biomed. Mater. Re-Search Part A 2011, 98, 535–543. [Google Scholar] [CrossRef]
- Hua, Z.; Chen, J.; Lun, S.; Wang, X. Influence of biosurfactants produced by Candida antarctica on surface properties of mi-croorganism and biodegradation of n-alkanes. Water Res. 2003, 37, 4143–4150. [Google Scholar] [CrossRef]
- Satpute, S.; Bhawsar, B.; Dhakephalkar, P.; Chopade, B. Assessment of different screening methods for selecting biosurfactant producing marine bacteria. Indian J. Geo-Mar. Sci. 2008, 37, 243–250. [Google Scholar]
- Neu, T.R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 1996, 60, 151–166. [Google Scholar] [CrossRef]
- Tahmourespour, A.; Salehi, R.; Kermanshahi, R.K.; Eslami, G. The anti-biofouling effect ofLactobacillus fermentum-derived biosurfactant againstStreptococcus mutans. Biofouling 2011, 27, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.S.O.; Campos, L.M.; Melo, L.; Guedes, M.C.M.R.; Oliveira, L.G.; Silva, T.P.; Melo, R.; Rocha, V.N.; Aguiar, J.; Apolonio, A.C.; et al. Antibacterial and Antibiofilm Activities of Psychorubrin, a Pyranonaphthoquinone Isolated from Mitracarpus frigidus (Rubiaceae). Front. Microbiol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Leme, A.P.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The Role of Sucrose in Cariogenic Dental Biofilm Formation—New Insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, G.; Liu, Y.; Wang, Y.; Singh, A.P.; Vorsa, N.; Koo, H. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS ONE 2015, 10, e0145844. [Google Scholar] [CrossRef]
- Lentino, J.R. Prosthetic Joint Infections: Bane of Orthopedists, Challenge for Infectious Disease Specialists. Clin. Infect. Dis. 2003, 36, 1157–1161. [Google Scholar] [CrossRef]
- Parkinson, M. Bio-surfactants. Biotechnol. Adv. 1985, 3, 65–83. [Google Scholar] [CrossRef]
- van Hoogmoed, C.G.; van der Kuijl-Booij, M.; van der Mei, H.C.; Busscher, H.J. Inhibition of Streptococcus mutans NS Adhesion to Glass with and without a Salivary Conditioning Film by Biosurfactant- Releasing Streptococcus mitis Strains. Appl. Environ. Microbiol. 2000, 66, 659–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramage, G.; Walle, K.V.; Wickes, B.; López-Ribot, J.L. Standardized Method for In Vitro Antifungal Susceptibility Testing of Candida albicans Biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef]
- Walencka, E.; Różalska, S.; Sadowska, B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66. [Google Scholar] [CrossRef]
- Augustine, N.; Kumar, P.; Thomas, S. Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch. Microbiol. 2010, 192, 1019–1022. [Google Scholar] [CrossRef]
- Sambanthamoorthy, K.; Feng, X.; Patel, R.; Patel, S.; Paranavitana, C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014, 14, 197. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracaseiA20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef]
- Fracchia, L.; Cavallo, M.; Allegrone, G.; Martinotti, M.G. Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers. Appl. Microbiol. Microb. Biotechnol. 2010, 210, 827–837. [Google Scholar]
- Van Der Mei, H.; Free, R.; Van Weissenbruch, R.; Busscher, H.; Elving, G.; Albers, F.J. Effect of probiotic bacteria on prevalence of yeasts in oropharyngeal biofilms on silicone rubber voice prostheses in vitro. J. Med Microbiol. 2000, 49, 713–718. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An antibiotic agent pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef]
- Hassan, F.; Nasibullah, M.; Ahmad, N.; Kamal, A.; Rizvi, S.M.D.; Khan, M.S.; Khan, A.R. Synthesis, Characterization and Physicochemical Analysis of some Mannofuranoside Derivatives with Potent Antimicrobial Activity. Orient. J. Chem. 2017, 33, 2731–2741. [Google Scholar] [CrossRef]
- Arancibia, L.; Naspi, C.; Pucci, G.; Arce, M.; Colloca, C. Biological activity of 1-heneicosanol isolated from Senecio coluhua-piensis, an endemic species from Patagonia, Argentina. Pharm. Chem. J. 2016, 3, 73–77. [Google Scholar]
- Poongulali, S.; Sundararaman, M. Antimycobacterial, anticandidal and antioxidant properties of Terminalia catappa and analysis of their bioactive chemicals. Int. J. Pharm. Biol. Sci. 2016, 6, 69–83. [Google Scholar]
- Okwu, D.E.; Ighodaro, B.U. GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild. Der Pharma Chem. 2010, 2, 261–272. [Google Scholar]
- Rouis-Soussi, L.S.; Ayeb-Zakhama, E.A.; Mahjoub, A.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and antibacterial activity of essential oils from the Tunisian Allium nigrum L. EXCLI J. 2014, 13, 526. [Google Scholar] [CrossRef]
- Abubakar, M.N.; Majinda, R.R.T. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. [Google Scholar] [CrossRef]
- de Carvalho, M.P.; Abraham, W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbour Laboratory: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef]
- Płaza, G.A.; Zjawiony, I.; Banat, I.M. Use of different methods for detection of thermophilic biosurfactant-producing bacteria from hydrocarbon-contaminated and bioremediated soils. J. Pet. Sci. Eng. 2006, 50, 71–77. [Google Scholar] [CrossRef]
- Joe, M.M.; Gomathi, R.; Benson, A.; Shalini, D.; Rengasamy, P.; Henry, A.J.; Truu, J.; Truu, M.; Sa, T. Simultaneous Application of Biosurfactant and Bioaugmentation with Rhamnolipid-Producing Shewanella for Enhanced Bioremediation of Oil-Polluted Soil. Appl. Sci. 2019, 9, 3773. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Silveira, C.P.; Torres-Rodríguez, J.M.; Alvarado-Ramírez, E.; Murciano-Gonzalo, F.; Dolande, M.; Panizo, M.; Reviakina, V. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J. Med. Microbiol. 2009, 58, 1607–1610. [Google Scholar] [CrossRef][Green Version]
- Lee, K.W.K.; Periasamy, S.; Mukherjee, M.; Xie, C.; Kjelleberg, S.; Rice, S.A. Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME J. 2013, 8, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation byPseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, K.S.; Ravi, A.V.; Annapoorani, A.; Packiavathy, S.V.; Pandian, S.K. Evaluation of Anti-Quorum-Sensing Activity of Edible Plants and Fruits through Inhibition of the N-Acyl-Homoserine Lactone System in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 2010, 56, 333–339. [Google Scholar] [CrossRef]
- Nett, J.E.; Cain, M.; Crawford, K.; Andes, D. Optimizing a Candida Biofilm Microtiter Plate Model for Measurement of Antifungal Susceptibility by Tetrazolium Salt Assay. J. Clin. Microbiol. 2011, 49, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Adnan, M.; Alreshidi, M.M.; Patel, M.; Soni, A.; Redman, W. Immune responses in liver and spleen against Plasmodium yoelii pre-erythrocytic stages in Swiss mice model. J. Adv. Res. 2020, 24, 29–41. [Google Scholar] [CrossRef]
- Borucki, M.K.; Krug, M.J.; Muraoka, W.T.; Call, D.R. Discrimination among Listeria monocytogenes isolates using a mixed genome DNA microarray. Veter- Microbiol. 2003, 92, 351–362. [Google Scholar] [CrossRef]



| Strain | Colony Characteristics | Gram’s Reaction | Oil-Spreading Test | Drop-Collapse Test | BAP Test | %EI24 (n-Hexadecane) | ST (mN/m) |
|---|---|---|---|---|---|---|---|
| L. rhamnosus -MBP002 | White, circular, shiny appearance | Gram-positive, rod shaped | Positive | Positive | Positive | 32.37 ± 1.26 | 47.62 ± 1.47 |
| Bacterial Strain | L. rhamnosus Crude Biosurfactant (mg/mL) | SDS (%) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| B. subtilis | 12.5 | 25 | 0.2 | 0.4 |
| E. coli | 12.5 | 25 | 0.4 | 0.6 |
| P. aeruginosa | 25 | 50 | 0.6 | 0.8 |
| S. aureus | 50 | 100 | 0.8 | 1 |
| No. | RT | % Area | Compound Name | Class |
|---|---|---|---|---|
| 1 | 1.914 | 47.87 | Isopropyl alpha-D-mannopyranoside | Glycoside |
| 2 | 2.422 | 4.98 | 2,5-Monomethylene-l-rhamnitol | Sugar Alcohol |
| 3 | 13.233 | 0.92 | 9-Octadecene, (E)- | Fatty Acyl |
| 4 | 15.753 | 1.13 | 1-Heptadecene | Fatty Acyl |
| 5 | 17.589 | 1.51 | Pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro-3-(phenylmethyl)- Cyclo (D-phenylalanyl-L-prolyl) (Cyclo(Phe-Pro)) | Dipeptide |
| 6 | 18.008 | 1.71 | 1-Heneicosanol | Fatty alcohol |
| 7 | 18.249 | 4.86 | Cyclo(L-prolyl-L-valine) | Diketopiperazine (Dipeptide) |
| 8 | 18.473 | 1.10 | 7-Ethyl-4,6-heptadecandione | Fatty Acyl |
| 9 | 19.426 | 4.15 | n-Hexadecanoic acid | Surfactant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics 2021, 10, 1546. https://doi.org/10.3390/antibiotics10121546
Patel M, Siddiqui AJ, Hamadou WS, Surti M, Awadelkareem AM, Ashraf SA, Alreshidi M, Snoussi M, Rizvi SMD, Bardakci F, et al. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics. 2021; 10(12):1546. https://doi.org/10.3390/antibiotics10121546
Chicago/Turabian StylePatel, Mitesh, Arif Jamal Siddiqui, Walid Sabri Hamadou, Malvi Surti, Amir Mahgoub Awadelkareem, Syed Amir Ashraf, Mousa Alreshidi, Mejdi Snoussi, Syed Mohd Danish Rizvi, Fevzi Bardakci, and et al. 2021. "Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties" Antibiotics 10, no. 12: 1546. https://doi.org/10.3390/antibiotics10121546
APA StylePatel, M., Siddiqui, A. J., Hamadou, W. S., Surti, M., Awadelkareem, A. M., Ashraf, S. A., Alreshidi, M., Snoussi, M., Rizvi, S. M. D., Bardakci, F., Jamal, A., Sachidanandan, M., & Adnan, M. (2021). Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics, 10(12), 1546. https://doi.org/10.3390/antibiotics10121546













