Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions
Abstract
:1. Introduction
2. G. mellonella Larvae as a Vehicle for In Vivo Antibacterial Drug Assessment
3. Application of G. mellonella Larvae in Antifungal Drug Evaluation
4. G. mellonella Larvae as a Novel In Vivo System for Toxicity Studies
5. Application of G. mellonella Larvae to Characterise Microbial Disease Development Processes In Vivo
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 2017, 7, 12429. [Google Scholar] [CrossRef] [Green Version]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, C.; Yung, L.-Y.L.; Cai, Y.; Bay, B.-H.; Baeg, G.-H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015, 9, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.; Softley, I.; Balfour, A.; Williamson, C.; O’Brien, H.E.; Shetty, A.C.; Bruno, V.M.; Diezmann, S. Tobacco hornworm (Manduca sexta) caterpillars as a novel host model for the study of fungal virulence and drug efficacy. Virulence 2020, 11, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Montali, A.; Berini, F.; Brivio, M.F.; Mastore, M.; Saviane, A.; Cappellozza, S.; Marinelli, F.; Tettamanti, G. A silkworm infection model for in vivo study of glycopeptide antibiotics. Antibiotics 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, S.; Casey, M.; Frank, D.W.; Ausubel, F.M.; Drenkard, E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 2003, 71, 2404–2413. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.L.; Gregson, L.; Denning, D.W.; Warn, P.A. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011, 49, S107–S113. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, K.; Sheehan, G. The use of Galleria mellonella larvae to identify novel antimicrobial agents against fungal species of medical interest. J. Fungi 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.R.; Clokie, M.R.J. Clostridium difficile phages: Still difficult? Front. Microbiol. 2014, 5, 184. [Google Scholar] [CrossRef] [Green Version]
- Nale, J.Y.; Chutia, M.; Carr, P.; Hickenbotham, P.T.; Clokie, M.R.J. “Get in early”; Biofilm and wax moth (Galleria mellonella) models reveal new insights into the therapeutic potential of Clostridium difficile bacteriophages. Front. Microbiol. 2016, 7, 1383. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, J.D.; de Alvarenga, J.A.; Rossoni, R.D.; García, M.T.; Moraes, R.M.; Anbinder, A.L.; Cardoso Jorge, A.O.; Junqueira, J.C. Immunomodulatory effect of photodynamic therapy in Galleria mellonella infected with Porphyromonas gingivalis. Microb. Pathog. 2017, 110, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
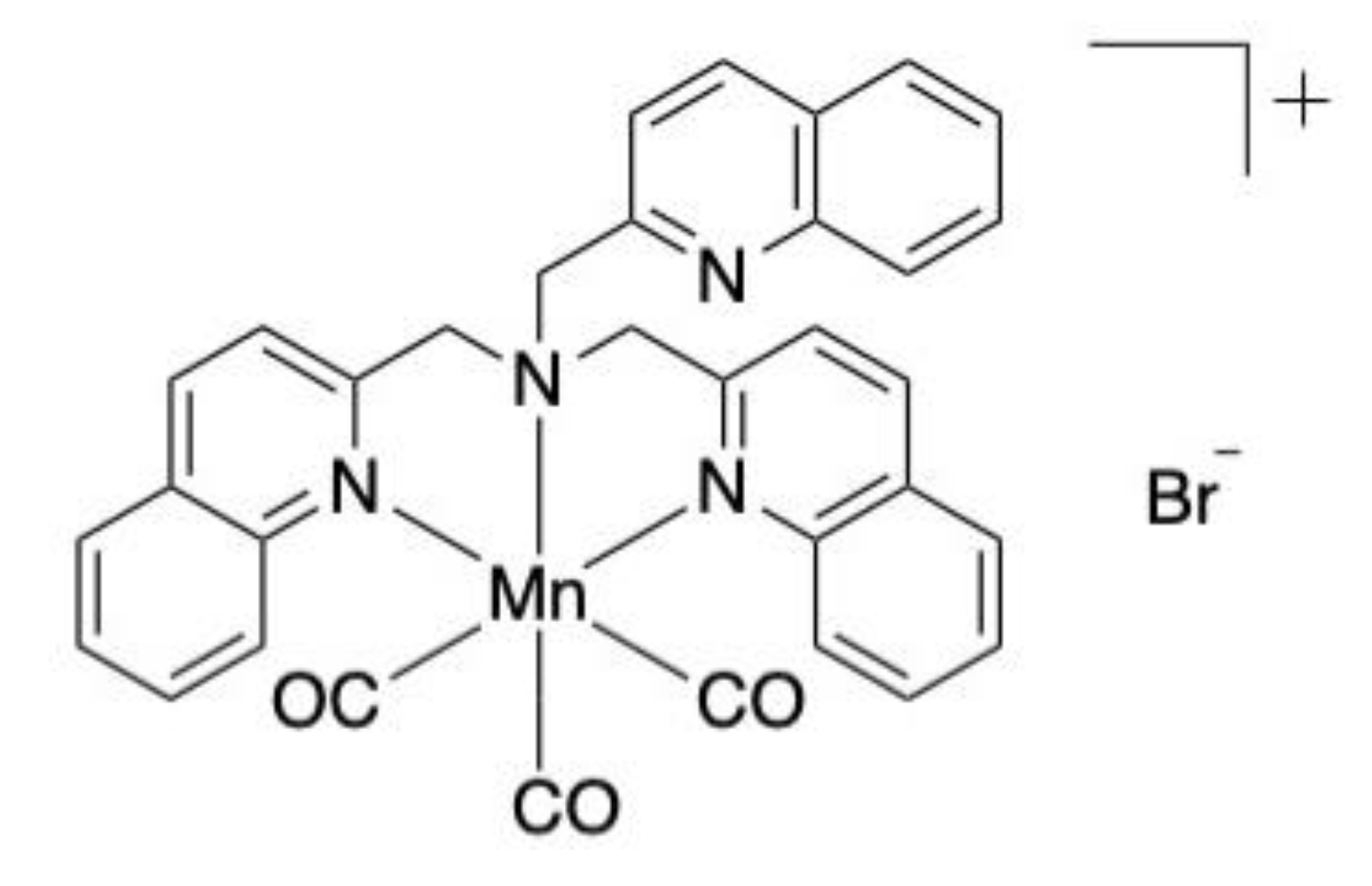
- Güntzel, P.; Nagel, C.; Weigelt, J.; Betts, J.W.; Pattrick, C.A.; Southam, H.M.; La Ragione, R.M.; Poole, R.K.; Schatzschneider, U. Biological activity of manganese(i) tricarbonyl complexes on multidrug-resistant Gram-negative bacteria: From functional studies to in vivo activity in Galleria mellonella. Metallomics 2019, 11, 2033–2042. [Google Scholar] [CrossRef]
- Wright, J.B.; Lam, K.; Hansen, D.; Burrell, R.E. Efficacy of topical silver against fungal burn wound pathogens. Am. J. Infect. Control 1999, 27, 344–350. [Google Scholar] [CrossRef]
- Thomaz, L.; Gustavo de Almeida, L.; Silva, F.R.O.; Cortez, M.; Taborda, C.P.; Spira, B. In vivo activity of silver nanoparticles against Pseudomonas aeruginosa infection in Galleria mellonella. Front. Microbiol. 2020, 11, 2798. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Zaragoza, O. Immune response of Galleria mellonella against human fungal pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, A.P.; Lee, M.; Megaw, J.; McEvoy, J.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Investigating the in vivo antimicrobial activity of a self-assembling peptide hydrogel using a Galleria mellonella infection model. ACS Omega 2019, 4, 2584–2589. [Google Scholar] [CrossRef]
- Rowan, R.; Moran, C.; McCann, M.; Kavanagh, K. Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. BioMetals 2009, 22, 461–467. [Google Scholar] [CrossRef] [Green Version]
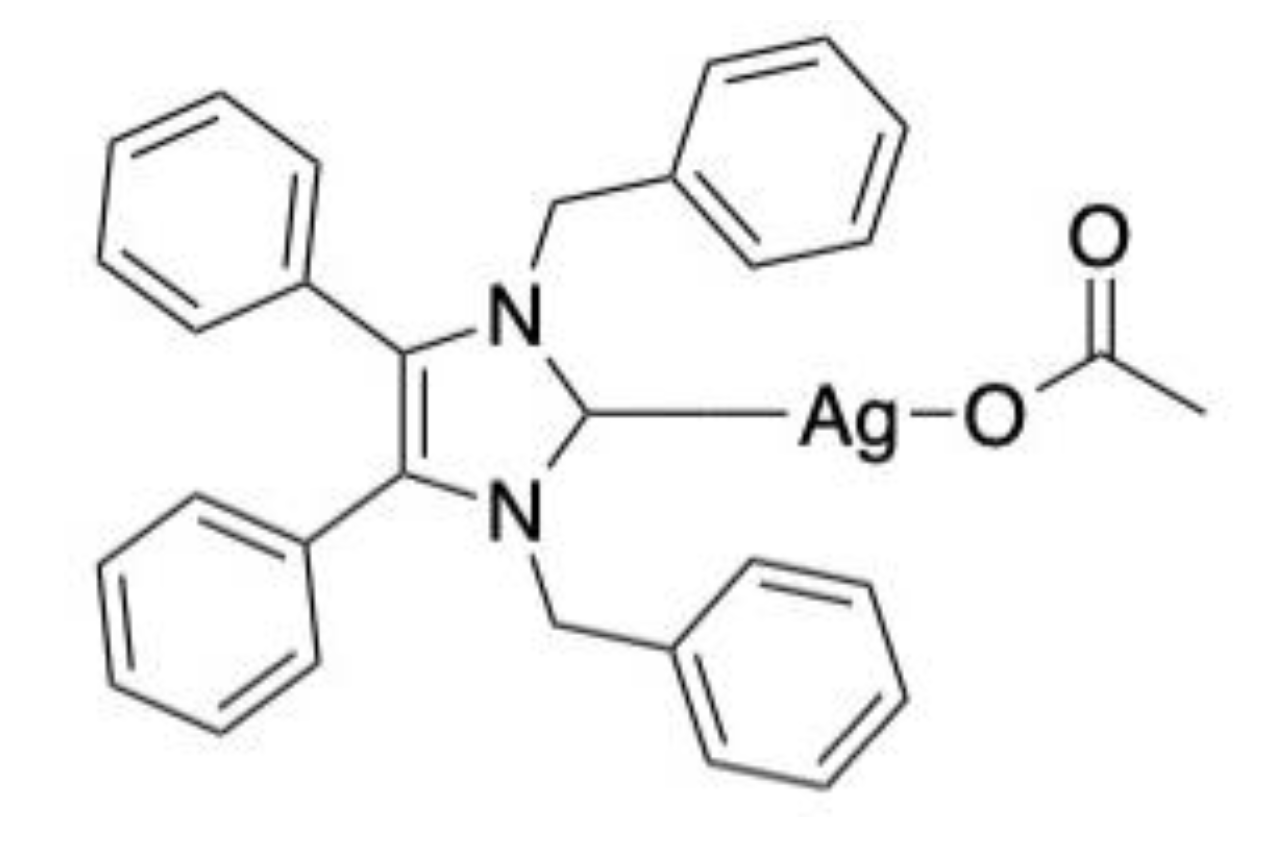
- Browne, N.; Hackenberg, F.; Streciwilk, W.; Tacke, M.; Kavanagh, K. Assessment of in vivo antimicrobial activity of the carbene silver(I) acetate derivative SBC3 using Galleria mellonella larvae. BioMetals 2014, 27, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Gandra, R.M.; McCarron, P.; Viganor, L.; Fernandes, M.F.; Kavanagh, K.; McCann, M.; Branquinha, M.H.; Santos, A.L.S.; Howe, O.; Devereux, M. In vivo activity of copper(II), manganese(II), and silver(I) 1,10-phenanthroline chelates against Candida haemulonii using the Galleria mellonella model. Front. Microbiol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.; Melse, Y.; Konings, M.; Phat Duong, H.; Eadie, K.; Laleu, B.; Perry, B.; Todd, M.H.; Ioset, J.R.; van de Sande, W.W.J. Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLoS Negl. Trop. Dis. 2018, 12, e0006437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, K.; Yadav, A.; Prasher, P.; Mishra, S.; Singh, B.; Singh, P.; Komath, S.S. Identification of an indole-triazole-amino acid conjugate as a highly effective antifungal agent. Medchemcomm 2015, 6, 1352–1359. [Google Scholar] [CrossRef]
- Aneja, B.; Irfan, M.; Kapil, C.; Jairajpuri, M.A.; Maguire, R.; Kavanagh, K.; Rizvi, M.M.A.; Manzoor, N.; Azam, A.; Abid, M. Effect of novel triazole-amino acid hybrids on growth and virulence of Candida species: In vitro and in vivo studies. Org. Biomol. Chem. 2016, 14, 10599–10619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangalli-Leite, F.; Scorzoni, L.; Alves de Paula e Silva, A.C.; de Fátima da Silva, J.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; Moraes da Silva, R.; Regasini, L.O.; Siqueira da Silva, D.H.; et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int. J. Antimicrob. Agents 2016, 48, 504–511. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 31161, Pedalitin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pedalitin (accessed on 5 November 2021).
- Pratt, I.S. Global harmonisation of classification and labelling of hazardous chemicals. Toxicol. Lett. 2002, 128, 5–15. [Google Scholar] [CrossRef]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella larvae as an in vivo model for assessing the relative toxicity of food preservative agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef]
- Wu, X.; Tong, Z.-H.; Li, L.-L.; Yu, H.-Q. Toxic effects of imidazolium-based ionic liquids on Caenorhabditis elegans: The role of reactive oxygen species. Chemosphere 2013, 93, 2399–2404. [Google Scholar] [CrossRef]
- Boškin, A.; Tran, C.D.; Franko, M. Oxidation of organophosphorus pesticides with chloroperoxidase enzyme in the presence of an ionic liquid as co-solvent. Environ. Chem. Lett. 2009, 7, 267–270. [Google Scholar] [CrossRef]
- Luo, Y.-R.; San-Hu, W.; Li, X.-Y.; Yun, M.-X.; Wang, J.-J.; Sun, Z.-J. Toxicity of ionic liquids on the growth, reproductive ability, and ATPase activity of earthworm. Ecotoxicol. Environ. Saf. 2010, 73, 1046–1050. [Google Scholar] [CrossRef]
- Bakshi, K.; Mitra, S.; Sharma, V.K.; Jayadev, M.S.K.; Sakai, V.G.; Mukhopadhyay, R.; Gupta, A.; Ghosh, S.K. Imidazolium-based ionic liquids cause mammalian cell death due to modulated structures and dynamics of cellular membrane. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183103. [Google Scholar] [CrossRef] [PubMed]
- Megaw, J.; Thompson, T.P.; Lafferty, R.A.; Gilmore, B.F. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 2015, 139, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Suay-García, B.; Alemán-López, P.A.; Bueso-Bordils, J.I.; Falcó, A.; Antón-Fos, G.; Pérez-Gracia, M.T. New solvent options for in vivo assays in the Galleria mellonella larvae model. Virulence 2019, 10, 776–782. [Google Scholar] [CrossRef] [Green Version]
- Bulcão, R.P.; Freitas, F.A.; Venturini, C.G.; Dallegrave, E.; Durgante, J.; Göethel, G.; Cerski, C.T.S.; Zielinsky, P.; Pohlmann, A.R.; Guterres, S.S.; et al. Acute and subchronic toxicity evaluation of poly(ε-caprolactone) lipid-core nanocapsules in rats. Toxicol. Sci. 2013, 132, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Cé, R.; Silva, R.C.; Trentin, D.S.; De Marchi, J.G.B.; Paese, K.; Guterres, S.S.; Macedo, A.J.; Pohlmann, A.R. Galleria mellonella larvae as an in vivo model to evaluate the toxicity of polymeric nanocapsules. J. Nanosci. Nanotechnol. 2019, 20, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Dixon, A.; Kavanagh, K. Utilization of Galleria mellonella larvae to characterize the development of Staphylococcus aureus infection. Microbiology 2019, 165, 863–875. [Google Scholar] [CrossRef]
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Molne, L.; Verdrengh, M.; Tarkowski, A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 2000, 68, 6162–6167. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; Deleo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009, 23, 3393–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomer, L.; Schneewind, O.; Missiakas, D. Pathogenesis of Staphylococcus aureus bloodstream infections. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 343–364. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Altincicek, B.; Hain, T.; Domann, E.; Vilcinskas, A.; Chakraborty, T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. 2010, 76, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Drevets, D.A. Listeria monocytogenes virulence factors that stimulate endothelial cells. Infect. Immun. 1998, 66, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Hain, T.; Fischer, R.; Chakraborty, T.; Vilcinskas, A. Brain infection and activation of neuronal repair mechanisms by the human pathogen Listeria monocytogenes in the lepidopteran model host Galleria mellonella. Virulence 2013, 4, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, N.K.; Mazumdar, K.; Seok, S.H.; Park, J.H. The anti-inflammatory drug Diclofenac retains anti-listerial activity in vivo. Lett. Appl. Microbiol. 2008, 47, 106–111. [Google Scholar] [CrossRef]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.M.; Kolbe, T.; Stulnig, T.M.; Hörl, W.H.; Hengstschläger, M.; et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Insua, J.L.; Llobet, E.; Moranta, D.; Pérez-Gutiérrez, C.; Tomás, A.; Garmendia, J.; Bengoechea, J.A. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect. Immun. 2013, 81, 3552–3565. [Google Scholar] [CrossRef] [Green Version]
- Cortés, G.; Borrell, N.; De Astorza, B.; Gómez, C.; Sauleda, J.; Albertí, S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 2002, 70, 2583–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, G.; Clarke, G.; Kavanagh, K. Characterisation of the cellular and proteomic response of Galleria mellonella larvae to the development of invasive aspergillosis. BMC Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.G.; Greco, W.R.; Brun, Y.; Youn, R.; Slocum, H.K.; Bernacki, R.J.; Lewis, R.; Wiederhold, N.; Holland, S.M.; Petraitiene, R.; et al. Effect of amphotericin B and micafungin combination on survival, histopathology, and fungal burden in experimental aspergillosis in the p47 phox-/- mouse model of chronic granulomatous disease. Antimicrob. Agents Chemother. 2006, 50, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, J.; Xin, Z.; Wu, X. Aspergillus fumigatus triggers innate immune response via NOD1 signaling in human corneal epithelial cells. Exp. Eye Res. 2014, 127, 170–178. [Google Scholar] [CrossRef]
- Alekseeva, L.; Huet, D.; Féménia, F.; Mouyna, I.; Abdelouahab, M.; Cagna, A.; Guerrier, D.; Tichanné-Seltzer, V.; Baeza-Squiban, A.; Chermette, R.; et al. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol. 2009, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Clow, L.A.; Raftos, D.A.; Gross, P.S.; Smith, L.C. The sea urchin complement homologue, SpC3, functions as an opsonin. J. Exp. Biol. 2004, 207, 2147–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological Mediators of Insect Immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.P.; Bailey, A.M.; Cobb, B.; Vilcinskas, A. Fungi as elicitors of insect immune responses. Arch. Insect Biochem. Physiol. 2000, 44, 49–68. [Google Scholar] [CrossRef]
- Vasco, P.; Herriko, E.; Rementeria, A.; López-molina, N.; Ludwig, A. Genes and molecules involved in Aspergillus fumigatus virulence. Rev. Iberoam Micol. 2005, 22, 1–23. [Google Scholar]
- Namvar, S.; Warn, P.; Farnell, E.; Bromley, M.; Fraczek, M.; Bowyer, P.; Herrick, S. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin. Exp. Allergy 2015, 45, 982–993. [Google Scholar] [CrossRef]
- Ahmed, A.O.A.; Van Leeuwen, W.; Fahal, A.; Van De Sande, W.; Verbrugh, H.; Van Belkum, A. Mycetoma caused by Madurella mycetomatis: A neglected infectious burden. Lancet Infect. Dis. 2004, 4, 566–574. [Google Scholar] [CrossRef]
- Ibrahim, A.I.; El Hassan, A.M.; Fahal, A.; van de Sande, W.W. A histopathological exploration of the Madurella mycetomatis grain. PLoS ONE 2013, 8, e57774. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, G.; Konings, M.; Lim, W.; Fahal, A.; Kavanagh, K.; van de Sande, W.W.J. Proteomic analysis of the processes leading to Madurella mycetomatis grain formation in Galleria mellonella larvae. PLoS Negl. Trop. Dis. 2020, 14, e0008190. [Google Scholar] [CrossRef] [Green Version]
- Seitz, V.; Clermont, A.; Wedde, M.; Hummel, M.; Vilcinskas, A.; Schlatterer, K.; Podsiadlowski, L. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev. Comp. Immunol. 2003, 27, 207–215. [Google Scholar] [CrossRef]
- De Klerk, N.; De Vogel, C.; Fahal, A.; Van Belkum, A.; Van De Sande, W.W.J. Fructose-bisphosphate aldolase and pyruvate kinase, two novel immunogens in Madurella mycetomatis. Med. Mycol. 2012, 50, 143–151. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Barnoy, S.; Gancz, H.; Zhu, Y.; Honnold, C.L.; Zurawski, D.V.; Venkatesan, M.M. The Galleria mellonella larvae as an in vivo model for evaluation of Shigella virulence. Gut Microbes 2017, 8, 335–350. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. https://doi.org/10.3390/antibiotics10121545
Piatek M, Sheehan G, Kavanagh K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics. 2021; 10(12):1545. https://doi.org/10.3390/antibiotics10121545
Chicago/Turabian StylePiatek, Magdalena, Gerard Sheehan, and Kevin Kavanagh. 2021. "Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions" Antibiotics 10, no. 12: 1545. https://doi.org/10.3390/antibiotics10121545
APA StylePiatek, M., Sheehan, G., & Kavanagh, K. (2021). Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics, 10(12), 1545. https://doi.org/10.3390/antibiotics10121545




