Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants Characteristics
- Patients who had COVID-19 infection confirmed by reverse transcription polymerase chain reaction (RT-PCR) and required admission to the high-dependency unit (HDU).
- CRITICAL disease: Evidence of ARDS on ABGs (mild: PaO2/FiO2 > 200 but < 300 mmHg; moderate: PaO2/FiO2 > 100 but < 200 mmHg; severe: PaO2/FiO2 < 100 mmHg) + multi-organ involvement + septic shock.
- SEVERE disease: Infiltrates > 50% of total lung fields on chest X ray or HRCT chest showing extensive peripheral ground glass opacities + SpO2 < 90% + RR > 30/min + BP < 90 mmHg with tachycardia.
- Patients who did not receive steroids.
- Patients who had contraindication to use of steroids (systemic fungal infection, active concomitant tuberculosis, documented hypersensitivity reaction to intravenous steroids, uncontrolled blood pressure, and uncontrolled diabetes status).
- Severe congestive cardiac failure (EF < 25%).
- Death within 48 h of admission.
- Patients who were receiving other investigational therapies simultaneously such as tocilizumab and plasmapheresis.
2.3. Formulation and Dosages of Steroids
- Patients who received Dexamethasone 6 mg/day.
- Patients who were given Dexamethasone > 6 mg/day.
- Patients who received Methylprednisolone 500 mg/day.
2.4. Methodology
2.5. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics
- Patients receiving Dexamethasone = 6 mg/day, 40.6% (n = 176).
- Patients receiving Dexamethasone > 6 mg/day, 11.1% (n = 48).
- Patients receiving Methylprednisolone = 500 mg/day, 48.3% (n = 209).
3.2. Outcome in Related to Mode of Oxygen Delivery
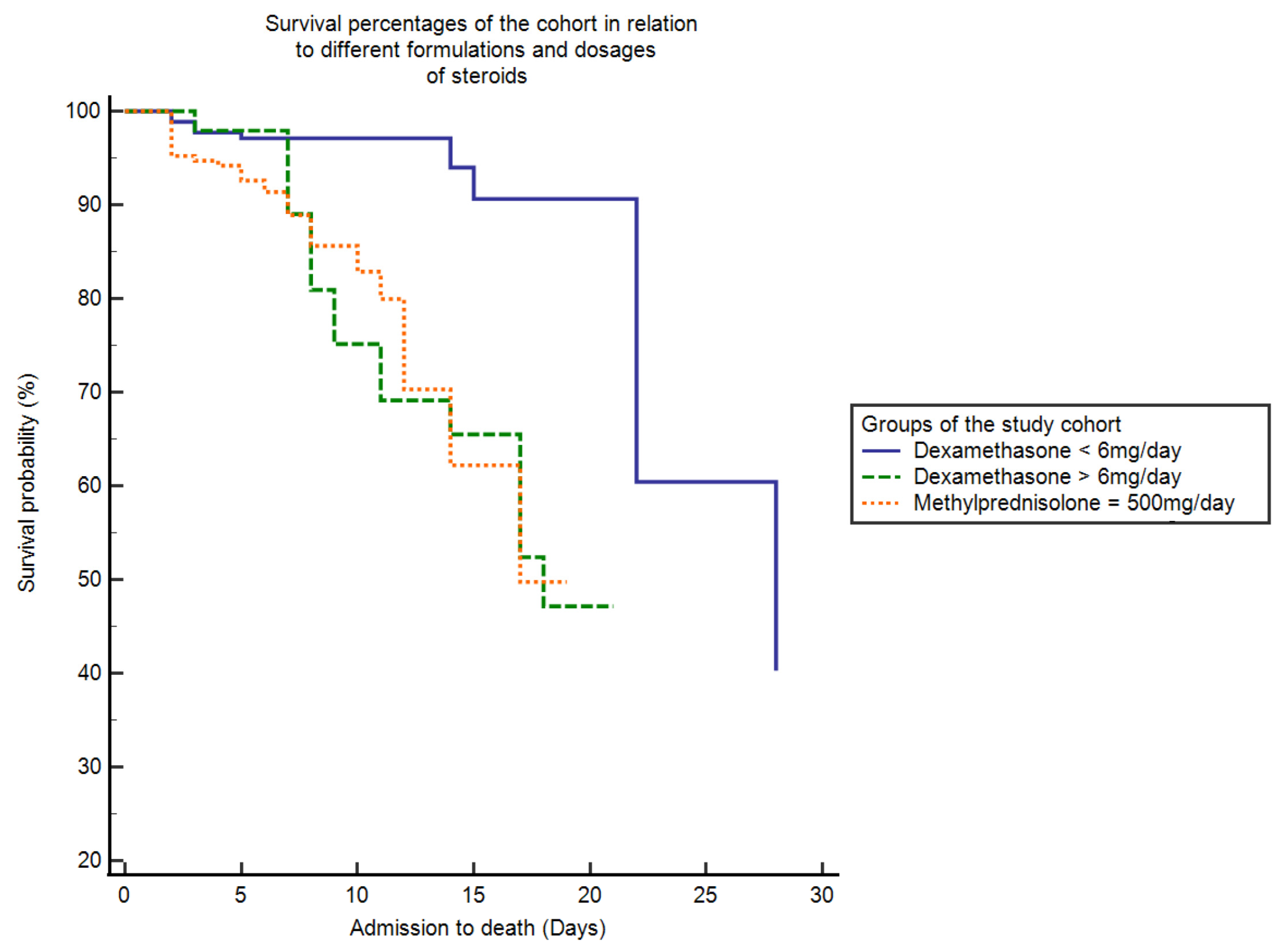
3.3. Survival Analysis
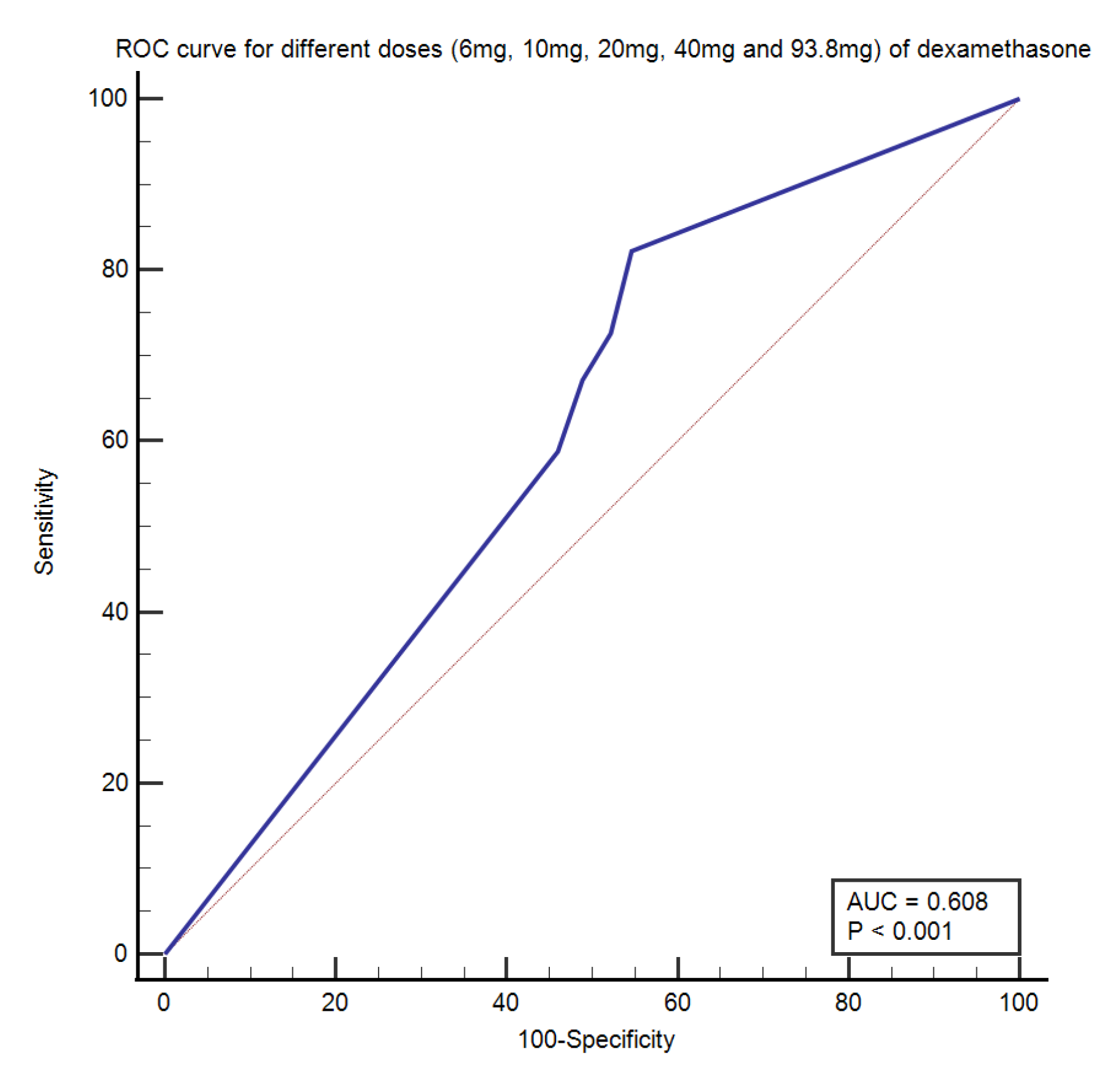
3.4. Receiver Operating Characteristic (ROC) Curve
3.5. Independent Predictors of Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dennison Himmelfarb, C.R.; Baptiste, D.J. Coronavirus Diseases (COVID-19): Implicaitons for cardiovascular and socially at risk populaiton. Cardiovasc. Nurs. 2020, 35, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscitti, P.; Berardicurti, O.; Iagnocco, A.; Giacomelli, R. Cytokine storm syndrome in severe COVID-19. Autoimmun. Rev. 2020, 19, 102562. [Google Scholar] [CrossRef] [PubMed]
- Ruch, Y.; Kaeuffer, C.; Guffroy, A.; Lefebvre, N.; Hansmann, Y.; Danion, F. Rapid Radiological Worsening and Cytokine Storm Syndrome in COVID-19 Pneumonia. Eur. J. Case Rep. Intern. Med. 2020, 7, 1822. [Google Scholar] [CrossRef]
- Kolilekas, L.; Loverdos, K.; Giannakaki, S.; Vlassi, L.; Levounets, A.; Zervas, E.; Gaga, M. Can steroids reverse the severe COVID-19 induced “cytokine storm”? J. Med. Virol. 2020, 92, 2866–2869. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer-Streit, B.; Mayr, V.; Dobrescu, A.I.; Chapman, A.; Persad, E.; Klerings, I.; Wagner, G.; Siebert, U.; Christof, C.; Zachariah, C.; et al. Quarantine alone or in combination with other public health measures to control COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020, 4, CD013574. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Mehta, Y.; Dixit, S.B.; Zirpe, K.G.; Ansari, A.S. Cytokine Storm in Novel Coronavirus Disease (COVID-19): Expert Management Considerations. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2020, 24, 429–434. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E. Basic and Clinical Pharmacology of Glucocorticosteroids. Anesth. Prog. 2013, 60, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Mueller, A.; Nothacker, M.; Kley, K.; Metzendorf, M.-I.; Fischer, A.-L.; Kopp, M.; Stegemann, M.; et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014963. [Google Scholar] [CrossRef]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Ng Gong, M.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe covid-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, F.; Ren, Y.; Wang, H.; Han, D.; Lyu, J.; Yin, H. The Role of Glucocorticoids in the Treatment of ARDS: A Multicenter Retrospective Study Based on the eICU Collaborative Research Database. Front. Med. 2021, 8, 678260. [Google Scholar] [CrossRef]
- Maláska, J.; Stašek, J.; Duška, F.; Balík, M.; Máca, J.; Hruda, J.; Vymazal, T.; Klementová, O.; Zatloukal, J.; Gabrhelík, T.; et al. Effect of dexamethasone in patients with ARDS and COVID-19—Prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 172. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, M.H.; Sohn, Y.; Cho, Y.; Baek, Y.J.; Kim, J.H.; Ahn, J.Y.; Choi, J.Y.; Yeom, J.S.; Ahn, M.Y.; et al. Effects of early corticosteroid use in patients with severe coronavirus disease 2019. BMC Infect. Dis. 2021, 21, 506. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Jeronimo, C.M.P.; Farias, M.E.L.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Safe, I.P.; Borba, M.G.S.; Netto, R.L.A.; Maciel, A.B.S.; et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin. Infect. Dis. 2021, 72, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, K.P.; Hudson, L.D.; Goodman, R.B.; Hough, C.L.; Lanken, P.N.; Hyzy, R.; Thompson, B.T.; Ancukiewicz, M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1671–1684. [Google Scholar] [CrossRef]
- Monreal, E.; Sainz de la Maza, S.; Natera-Villalba, E.; Beltrán-Corbellini, Á.; Rodríguez-Jorge, F.; Fernández-Velasco, J.I.; Walo-Delgado, P.; Muriel, A.; Zamora, J.; Alonso-Canovas, A.; et al. High versus standard doses of corticosteroids in severe COVID-19: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L. Influence of Corticosteroid Dose on Viral Shedding Duration in Patients With COVID-19. Clin. Infect. Dis. 2021, 72, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Boglione, L.; Olivieri, C.; Rostagno, R.; Poletti, F.; Moglia, R.; Bianchi, B.; Esposito, M.; Biffi, S.; Borrè, S. Role of the early short-course corticosteroids treatment in ARDS caused by COVID-19: A single-center, retrospective analysis. Adv. Med. Sci. 2021, 66, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias Arias, C.; Muñoz, B.; Quiceno, J.; Jaramillo, D.; et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Gholampoor Saadi, M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar] [CrossRef]
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S. Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef]
- WHO-2019-nCoV-Corticosteroids-2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed on 10 November 2021).
- Li, Q.; Li, W.; Jin, Y.; Xu, W.; Huang, C.; Li, L.; Huang, Y.; Fu, Q.; Chen, L. Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Infect. Dis. Ther. 2020, 9, 823–836. [Google Scholar] [CrossRef]
- van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.I.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Laracy, J.; Shoucri, S.; Dietz, D.; Zucker, J.; Patel, N.; Sobieszczyk, M.E.; Kubin, C.J.; Gomez-Simmonds, A. Clinical Outcomes Associated With Methylprednisolone in Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. 2021, 72, e367–e372. [Google Scholar] [CrossRef]
- Brook, R.; Lim, H.Y.; Ho, P.; Choy, K.W. Risk factors and early prediction of clinical deterioration and mortality in adult COVID-19 inpatients: An Australian tertiary hospital experience. Intern. Med. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
| Variables | Dexa = 6 mg/Day (N = 176) | Dexa > 6 mg/Day (N = 48) | MP = 500 mg/Day (N = 209) | p Value |
|---|---|---|---|---|
| Age (years) | 61.69 + 18.33 | 60.85 + 11.90 | 60.12 + 15.33 | 0.53 |
| Comorbidities | 33.5% (145/433) | 7.4% (32/433) | 37.4% (162/433) | 0.07 |
| PaO2/FiO2 | 163.28 + 86.88 | 193.64 + 95.82 | 179.62 + 89.51 | 0.17 |
| Hemoglobin (g/dl) | 11.43 + 2.12 | 11.16 + 2.17 | 11.22 + 2.39 | 0.98 |
| WCC × 103 cells/L | 13.00 + 5.09 | 10.85 + 5.24 | 12.84 + 8.01 | 0.21 |
| Lymphocytes (% age) | 10.64 + 0.86 | 12.23 + 0.69 | 12.09 + 0.52 | 0.65 |
| Platelets × 103 cells/L | 263.31 + 99.19 | 225.16 + 85.42 | 234.50 + 97.28 | 0.24 |
| Urea (mmol/L) | 11.60 + 9.52 | 12.75 + 3.17 | 11.01 + 7.87 | 0.31 |
| Creatinine (umol/L) | 267.60 + 35.45 | 234.57 + 22.98 | 288.02 + 59.77 | 0.34 |
| D Dimers (ng/mL) | 382.77 + 95.22 | 440.00 + 89.76 | 482.76 + 26.77 | 0.45 |
| ALT (IU/L) | 53.08 + 22.55 | 50.62 + 20.23 | 50.83 + 19.87 | 0.23 |
| Albumin (g/L) | 30.29 + 3.79 | 31.50 + 6.54 | 32.38 + 5.76 | 0.43 |
| LDH (U/L) | 492.56 + 158.06 | 503.33 + 185.32 | 444.59 + 194.76 | 0.08 |
| Ferritin (ng/mL) | 1829.78 + 168.64 | 932.21 + 103.52 | 1649.26 + 154.23 | 0.24 |
| CRP (mg/L) | 53.13 + 13.36 | 55.10 + 14.60 | 54.03 + 18.16 | 0.96 |
| ProBNP (pg/mL) | 5313.09 + 225.67 | 6067.98 + 348.97 | 6345.87 + 128.90 | 0.09 |
| Trop T (ng/mL) | 0.16 + 0.40 | 0.03 + 0.01 | 0.08 + 0.03 | 0.81 |
| CPK (U/L) | 213.57 + 28.94 | 234.55 + 23.96 | 233.00 + 24.05 | 0.46 |
| CKMB (U/L) | 36.42 + 21.89 | 34.54 + 20.65 | 27.97 + 13.36 | 0.03 |
| PaO2 | 82.93 + 42.29 | 94.43 + 49.69 | 79.76 + 39.47 | 0.04 |
| Mode of Oxygen Delivery | At the Time of Admission | Dexa = 6 mg (N = 176) | Dexa > 6 mg (N = 48) | MP = 500 mg/Day (N = 209) | Contingency Co-Efficient | p Value |
|---|---|---|---|---|---|---|
| Oxygen < 10 L/min | 1.8% (8/433) | 22.9% (99/433) | 1.4% (6/433) | 21.9% (95/433) | 48.19 | <0.0001 |
| Oxygen > 10 L/min | 75.8% (328/433) | 0 | 0.2% (1/433) | 2.8% (12/433) | ||
| NRM | 12.2% (53/433) | 5.5% (24/433) | 0.9% (4/433) | 4.8% (21/433) | ||
| NIV | 7.4% (32/433) | 6% (26/433) | 0.9% (4/433) | 7.9% (34/433) | ||
| Invasive Ventilation | 2.8% (12/433) | 6.2% (27/433) | 7.6% (33/433) | 10.9% (47/433) |
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Age | 1.05 (1.03–1.22) | 0.00 |
| Co-morbid conditions | 0.34 (0.14–0.84) | 0.02 |
| Number of days requiring oxygen | 0.80 (0.73–0.88) | 0.00 |
| Number of days on ventilator support | 0.91 (0.78–0.96) | 0.00 |
| Dexamethasone (6 mg/day) | 0.11 (0.06–0.20) | 0.00 |
| Dexamethasone > 6 mg/day | 1.08 (0.58–2.02) | 0.80 |
| Methylprednisolone (500 mg/day) | 1.02 (1.00–1.31) | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, Z.; Almajhdi, F.N.; Khalid, S.; Asghar, M.; Ahmed, J.; Waheed, Y. Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients. Antibiotics 2021, 10, 1510. https://doi.org/10.3390/antibiotics10121510
Jamil Z, Almajhdi FN, Khalid S, Asghar M, Ahmed J, Waheed Y. Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients. Antibiotics. 2021; 10(12):1510. https://doi.org/10.3390/antibiotics10121510
Chicago/Turabian StyleJamil, Zubia, Fahad N. Almajhdi, Samreen Khalid, Muhammad Asghar, Jamal Ahmed, and Yasir Waheed. 2021. "Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients" Antibiotics 10, no. 12: 1510. https://doi.org/10.3390/antibiotics10121510
APA StyleJamil, Z., Almajhdi, F. N., Khalid, S., Asghar, M., Ahmed, J., & Waheed, Y. (2021). Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients. Antibiotics, 10(12), 1510. https://doi.org/10.3390/antibiotics10121510








