Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline
Abstract
1. Introduction
2. Fluoroquinolones
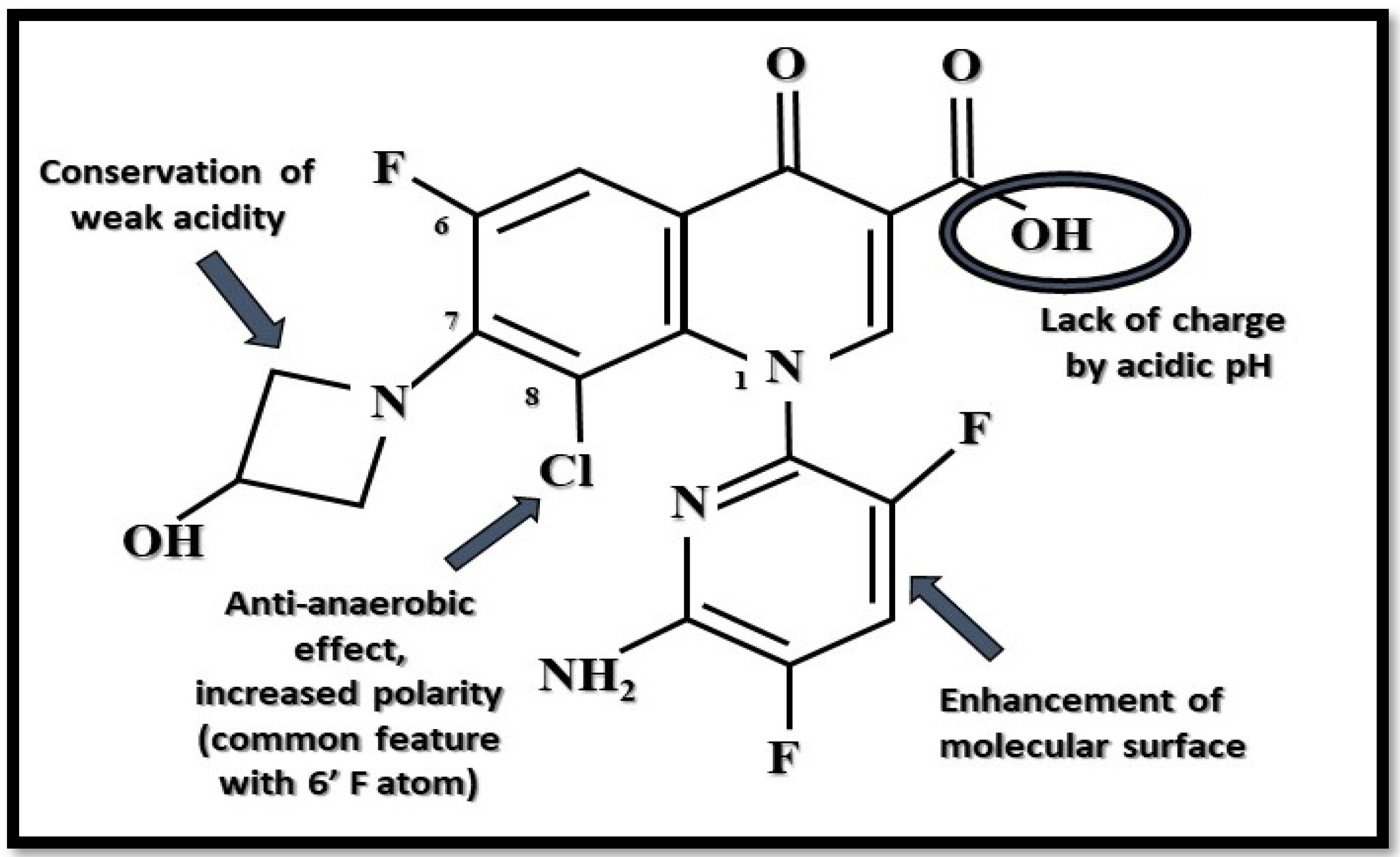
3. Delafloxacin
| Novel Fluoroquinolones | Delafloxacin | Finafloxacin | Zabofloxacin | Reference |
|---|---|---|---|---|
| Chemical structure | Unique anionic (non-zwitterionic) structure, with special substituents and augmented polarity. | Zwitterionic chemical structure of fluoroquinolones supplemented with substituents. | Zwitterionic chemical structure of fluoroquinolones supplemented with substituents (two forms are available). | [16,18,29] |
| Bioavailability | 58.8% | 75% (by oral use) | No data available. | [29,31] |
| Protein binding | Approximately 84% | No data available. | No data available. | [24] |
| Mechanism of action | Dual-targeting of DNA gyrase and topoisomerase IV enzymes of gram-positives and gram-negatives with equal affinity. Increased bactericidal effect in acidic pH | Dual-targeting (weaker effect compared to other group members) of DNA gyrase and topoisomerase IV enzymes of gram-positives and gram-negatives with equal affinity. Increased bactericidal effect in acidic pH. | Dual-targeting of DNA gyrase and topoisomerase IV enzymes, predominantly of community-acquired respiratory tract pathogen gram-positives, and some gram-negatives. Ineffective against major nosocomial gram-negatives. | [26,27,28,49,50] |
| Approved Indication | Acute bacterial skin and skin-structure infections (ABSSSI) of adults caused by MRSA, MSSA, S.haemolyticus, S. lugdunensis, S. agalactiae, Streptococcus anginosus Group, S. pyogenes, E. faecalis, E. coli, E. cloacae, K.pneumoniae, and P.aeruginosa. Community-Acquired Bacterial Pneumonia of adults caused by S.pneumoniae, MSSA, K. pneumoniae, P.aeruginosa, H. influenzae, H. parainfluenzae, C. pneumoniae, L. pneumophila, and M. pneumoniae. | Otic suspension for acute otitis externa caused by P. aeruginosa and S. aureus in patients age one year and older. | Oral administration for acute bacterial exacerbation of chronic obstructive pulmonary disease (COPD). | [18,43,44,45,51] |
| Novel Fluoroquinolones | Delafloxacin | Finafloxacin | Zabofloxacin | Reference |
|---|---|---|---|---|
| Further possible clinical applications | P. aeruginosa-mediated lung infections in patients with cystic fibrosis. Infection by multidrug-resistant H.pylori. | Complicated and non-complicated urinary-tract infections. Zoonoses, e.g., Y.pestis and B.anthracis. Prophylaxis and treatment of B. pseudomallei infections. | Community-acquired bacterial pneumonia. | [18,42,46,50] |
| Contraindication and side effects | Well-tolerated; lack of teratogenic effect, photosensitivity and cardiotoxicity. Diarrhoea, vomiting and other fluoroquinolone-specific adverse affects may occur. | Ophthalmic use is contraindicated. In animal studies, showed teratogenic ability and fluoroquinolone-specific adverse effects (per os). Hypersensitivity and pruritus. | Well-tolerated; lack of long QT-syndrome; in animal studies, subacute toxicity (atrophy of endocrine organs with vomitus by dogs) was found. Mainly gastrointestinal adverse effects were reported. | [16,18,24,30,34,35,36,37,51] |
| Resistance mechanisms | Multiple mutations by bacterial topoisomerase IV enzymes. Single mutations with efflux pumps. Generally fluoroquinolone-resistant strains are susceptible to to Delafloxacin (cross-resistance is also known). | Multiple mutations in bacterial topoisomerase IV enzymes. Cross-resistance with other fluoroquinolones was reported. | Multiple mutations in bacterial Topoisomerase IV enzymes. Generally fluoroquinolone-resistant strains are susceptible to Zabofloxacin. | [18,26,28,30,39,47,48,51] |
| Novel Fluoroquinolones | Delafloxacin | Finafloxacin | Zabofloxacin | Reference | |||
|---|---|---|---|---|---|---|---|
| MIC90 (mg/L) | MIC Range | MIC90 (mg/L) | MIC Range | MIC90 (mg/L) | MIC Range | ||
| E. faecalis | 1 | ≤0.004 to 2 | 16 | 0.25–16 | 2 | 0.008 ≥ 4 | [18,24,38,52] |
| E. faecium | >4 | 0.008 to > 4 | No data available. | 0.5–32 | 16 | 2–32 | [18,24,38,52] |
| MRSA | 0.5 | ≤ 0.004 to 4 | 0.125 | 0.06–0.125 | 32 | 0.016–0.64 | [18,24,38,52] |
| MSSA | 0.008 | No data available. | No data available. | [24,38] | |||
| E. coli | >4 | 0.008 to > 4 | 32 | 2–64 | 1 | 0.015–64 | [18,24,38,52] |
| K.pneumoniae | 0.06 to > 4 | 0.5 | 0.008–1 | 0.06–8 | [24,38,52] | ||
| P. aeruginosa | 0.015 to > 4 | 2 | 0.25–8 | 8 | 0.125–32 | [24,38,52] | |
| A. baumannii/ A. calcoaceticus | No data available. | 4 | 0.008–8 | [30] | |||
| S. maltophilia | 2 | 0.12–16 | 1 | 0.125–16 | No data available. | [30,52] | |
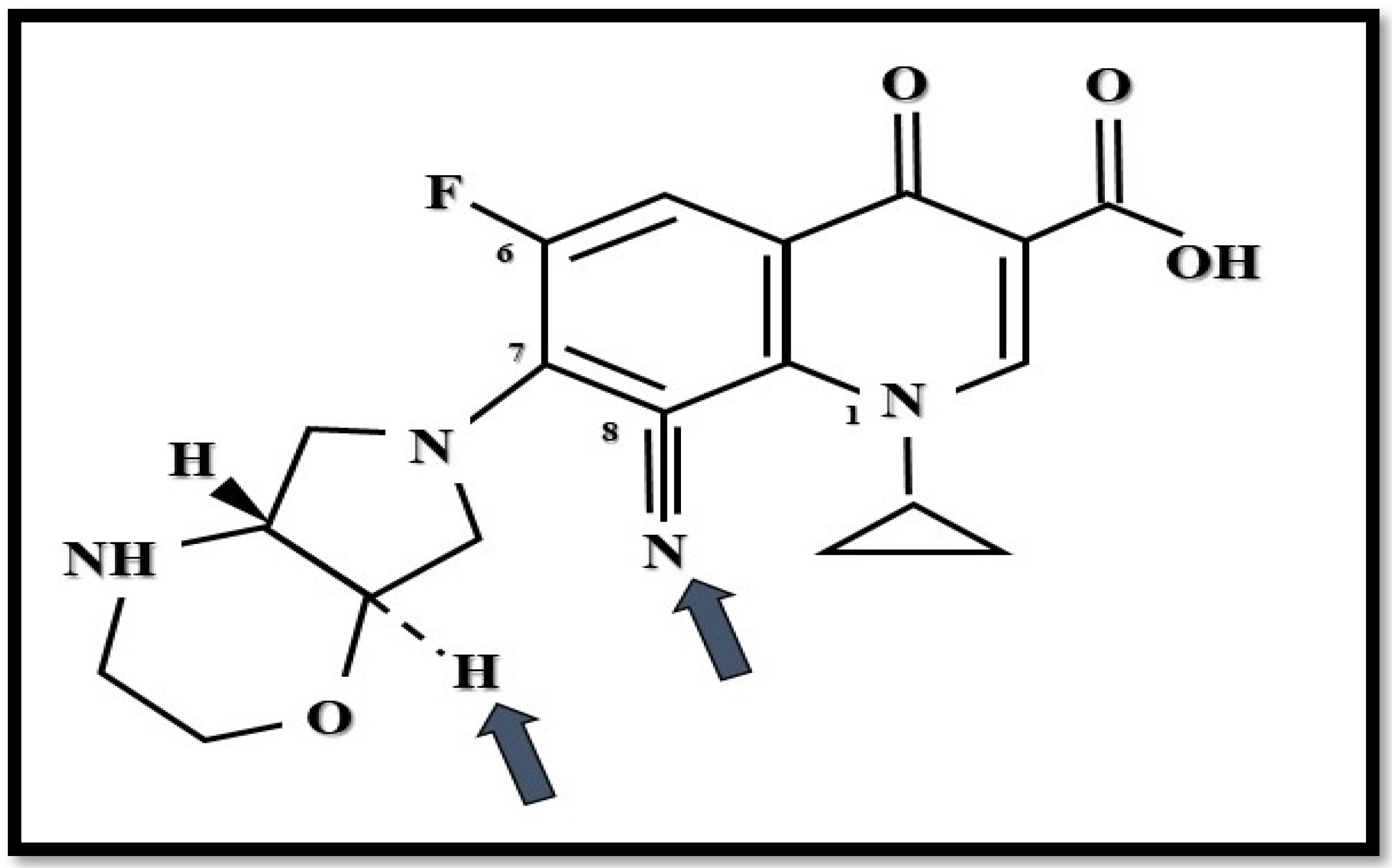
4. Finafloxacin
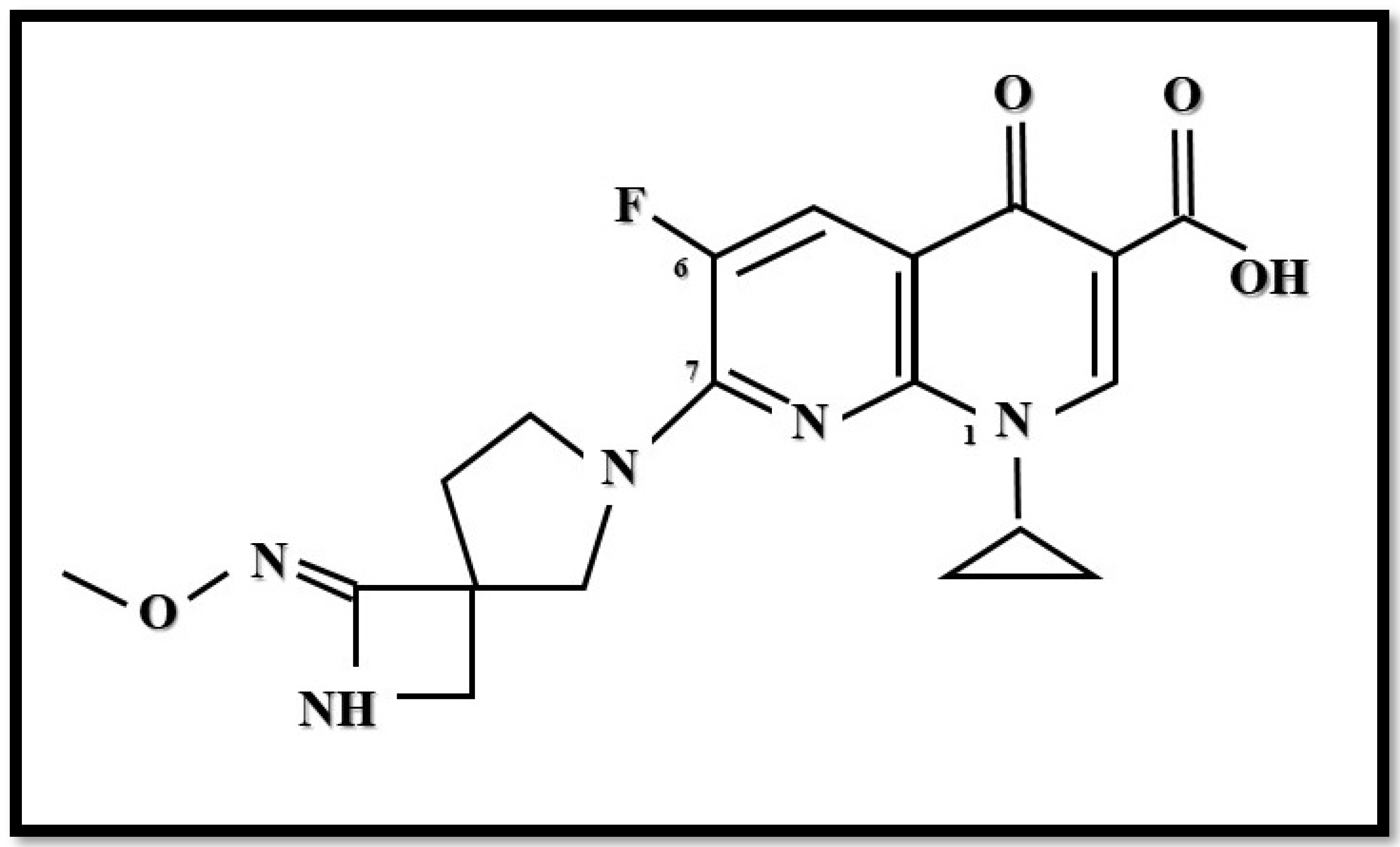
5. Zabofloxacin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control. 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. European survey of carbapenemase-producing enterobacteriaceae (EuSCAPE) working group. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [PubMed]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018, 7, 212527. [Google Scholar] [CrossRef]
- Livermore, D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009, 64 (Suppl. S1), i29–i36. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates 2015, 21–22, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa Mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 26–47. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E. Plazomicin: A new aminoglycoside. Clin. Infect. Dis. 2020, 70, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S538–S543. [Google Scholar] [CrossRef] [PubMed]
- Petty, L.A.; Henig, O.; Patel, T.S.; Pogue, J.M.; Kaye, K.S. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect. Drug Resist. 2018, 11, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Interplay between beta-lactamases and new beta-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. BAXDELA (Delafloxacin) Prescribing Information and Medication Guide. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf (accessed on 8 December 2018).
- Rusu, A.; Lungu, I.A.; Moldovan, O.L.; Tanase, C.; Hancu, G. Structural characterization of the millennial antibacterial (fluoro) quinolones-shaping the fifth generation. Pharmaceutics 2021, 13, 1289. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase inhibitors: Fluoroquinolone mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Vinué, L.; Sater, M.R.A.; Herriott, I.C.; Huntley, M.H.; Wang, M.; Jacoby, G.A.; Hooper, D.C. Plasmids and genes contributing to high-level quinolone resistance in Escherichia coli. Int. J. Antimicrob. Agents. 2020, 56, 105987. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.M.; Machuca, J.; Cano, M.E.; Calvo, J.; Martínez-Martínez, L.; Pascual, A. Plasmid-mediated quinolone resistance: Two decades on. Drug Resist. Updates 2016, 29, 13–29. [Google Scholar] [CrossRef]
- Kocsis, B.; Szmolka, A.; Szabo, O.; Gulyas, D.; Kristóf, K.; Göcző, I.; Szabo, D. Ciprofloxacin promoted qnrD expression and phylogenetic analysis of qnrD harboring plasmids. Microb. Drug Resist. 2019, 25, 501–508. [Google Scholar] [CrossRef]
- Gulyás, D.; Kocsis, B.; Szabó, D. Plasmid copy number and qnr gene expression in selection of fluoroquinolone-resistant Escherichia coli. Acta Microbiol. Immunol. Hung. 2019, 66, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, P.M.; Van Bambeke, F.; Zinner, S.H. Profile of a novel anionic fluoroquinolone-delafloxacin. Clin. Infect. Dis. 2019, 68 (Suppl. S3), S213–S222. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Quinolone- and Fluoroquinolone-Containing Medicinal Products: Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 8 December 2018).
- Nilius, A.M.; Shen, L.L.; Hensey-Rudloff, D.; Almer, L.S.; Beyer, J.M.; Balli, D.J.; Cai, Y.; Flamm, R.K. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob. Agents Chemother. 2003, 47, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Harnett, S.J.; Fraise, A.P.; Andrews, J.M.; Jevons, G.; Brenwald, N.P.; Wise, R. Comparative study of the in vitro activity of a new fluoroquinolone, ABT-492. J. Antimicrob. Chemother. 2004, 53, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Almer, L.S.; Hoffrage, J.B.; Keller, E.L.; Flamm, R.K.; Shortridge, V.D. In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against Gram-positive and Gram-negative organisms. Antimicrob. Agents Chemother. 2004, 48, 2771. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E. Delafloxacin: A new anti–methicillin-resistant Staphylococcus aureus Fluoroquinolone. Clin. Infect. Dis. 2019, 68, 1058–1062. [Google Scholar] [CrossRef]
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.B.H.; Kufel, W.D. Clinical review of delafloxacin: A novel anionic fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451. [Google Scholar] [CrossRef]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin. Ther. 2016, 38, 53–65. [Google Scholar] [CrossRef]
- Hoover, R.; Marbury, T.C.; Preston, R.A.; Quintas, M.; Lawrence, L.E.; Paulson, S.K.; Luke, R.D.; Cammarata, S.K. Clinical pharmacology of delafloxacin in patients with hepatic impairment. J. Clin. Pharmacol. 2017, 57, 328–335. [Google Scholar] [CrossRef]
- Hoover, R.; Lawrence, L.; Smith, C.; Longcor, J. Pharmacokinetics (PK) of delafloxacin (DLX) in patients with varying degrees of renal impairment. In Proceedings of the Fifty-Third Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, USA, 10–13 September 2013; Poster A-017e. American Society for Microbiology: Washington, DC, USA, 2013. [Google Scholar]
- Center for Drug Evaluation and Research: Delafloxacin NDA Briefing. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000Approv.pdf (accessed on 10 September 2021).
- O’Riordan, W.; Mehra, P.; Manos, P.; Kingsley, J.; Lawrence, L.; Cammarata, S. A randomized Phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int. J. Infect. Dis. 2015, 30, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Camm, J. Cardiotoxicity of fluoroquinolones. J. Antimicrob. Chemother. 2002, 49, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sandrock, C.E.; Meehan, J.; Theriault, N. Community-acquired bacterial pneumonia-changing epidemiology, resistance patterns, and newer antibiotics: Spotlight on delafloxacin. Clin. Drug Investig. 2020, 40, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sader, H.S.; Rhomberg, P.R.; Flamm, R.K. In vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrob. Agents Chemother. 2018, 62, e02803-17. [Google Scholar] [CrossRef] [PubMed]
- Soge, O.O.; Salipante, S.J.; No, D.; Duffy, E.; Roberts, M.C. In vitro activity of delafloxacin against clinical Neisseria gonorrhoeae isolates and selection of gonococcal delafloxacin resistance. Antimicrob. Agents Chemother. 2016, 60, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, M.R.; Roblin, P.M. The in vitro activity of a new fluoroquinolone, ABT-492, against recent clinical isolates of Chlamydia pneumoniae. J. Antimicrob. Chemother. 2004, 54, 281–282. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B. Comparative in vitro susceptibilities and bactericidal activities of investigational fluoroquinolone ABT-492 and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob. Agents Chemother. 2003, 47, 3973–3975. [Google Scholar] [CrossRef]
- Boyanova, L.; Markovska, R.; Medeiros, J.; Gergova, G.; Mitov, I. Delafloxacin against Helicobacter pylori, a potential option for improving eradication success? Diagn. Microbiol. Infect. Dis. 2020, 96, 114980. [Google Scholar] [CrossRef]
- Melinta Therapeutics. Baxdela (Delafloxacin) Tablets, for Oral Use; Baxdela (Delafloxacin) for Injection, for Intravenous Use: US Prescribing Information. 2019. Available online: https://baxdela.com/docs/baxdela-prescribing-information.pdf (accessed on 16 March 2020).
- European Medicines Agency. Quofenix (Delafloxacin): Summary of Product Characteristics. 2019. Available online: https://www.ema.europa.eu/ (accessed on 16 March 2020).
- Scott, L.J. Delafloxacin: A review in acute bacterial skin and skin structure infections. Drugs 2020, 80, 1247–1258. [Google Scholar] [CrossRef]
- Millar, B.C.; McCaughan, J.; Rendall, J.C.; Moore, J.E. Delafloxacin—A novel fluoroquinolone for the treatment of ciprofloxacin-resistant Pseudomonas aeruginosa in patients with cystic fibrosis. Clin. Respir. J. 2021, 15, 116–120. [Google Scholar] [CrossRef]
- Remy, J.M.; Tow-Keogh, C.A.; McConnell, T.S.; Dalton, J.M.; DeVito, J.A. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: Resistance selection and characterization. J. Antimicrob. Chemother. 2012, 67, 2814–2820. [Google Scholar] [CrossRef]
- Iregui, A.; Khan, Z.; Malik, S.; Landman, D.; Quale, J. Emergence of delafloxacin-resistant Staphylococcus aureus in Brooklyn, New York. Clin. Infect. Dis. 2020, 70, 1758–1760. [Google Scholar] [CrossRef]
- Patel, H.; Andresen, A.; Vente, A.; Heilmann, H.D.; Stubbings, W.; Seiberling, M.; Lopez-Lazaro, L.; Pokorny, R.; Labischinski, H. Human pharmacokinetics and safety profile of finafloxacin, a new fluoroquinolone antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 2011, 55, 4386–4393. [Google Scholar] [CrossRef][Green Version]
- Wagenlehner, F.; Nowicki, M.; Bentley, C.; Lückermann, M.; Wohlert, S.; Fischer, C.; Vente, A.; Naber, K.; Dalhoff, A. Explorative randomized phase II clinical study of the efficacy and safety of finafloxacin versus ciprofloxacin for treatment of complicated urinary tract infections. Antimicrob. Agents Chemother. 2018, 62, e02317-17. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. XTORO Prescribing Information and Medication Guide 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206307s000lbl.pdf (accessed on 20 August 2021).
- Stubbings, W.; Leow, P.; Yong, G.C.; Goh, F.; Körber-Irrgang, B.; Kresken, M.; Endermann, R.; Labischinski, H. In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob. Agents Chemother. 2011, 55, 4394–4397. [Google Scholar] [CrossRef]
- Lemaire, S.; van Bambeke, F.; Tulkens, P.M. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int. J. Antimicrob. Agents. 2011, 38, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Emrich, N.C.; Heisig, A.; Stubbings, W.; Labischinski, H.; Heisig, P. Antibacterial activity of finafloxacin under different pH conditions against isogenic strains of Escherichia coli expressing combinations of defined mechanisms of fluoroquinolone resistance. J. Antimicrob. Chemother. 2010, 65, 2530–2533. [Google Scholar] [CrossRef]
- Dalhoff, A.; Stubbings, W.; Schubert, S. Comparative in vitro activities of the novel antibacterial finafloxacin against selected Gram-positive and Gram-negative bacteria tested in Mueller-Hinton broth and synthetic urine. Antimicrob. Agents Chemother. 2011, 55, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Vente, A.; Bentley, C.; Lückermann, M.; Tambyah, P.; Dalhoff, A. Early clinical assessment of the antimicrobial activity of finafloxacin compared to ciprofloxacin in subsets of microbiologically characterized isolates. Antimicrob. Agents Chemother. 2018, 62, e02325-17. [Google Scholar] [CrossRef]
- Taubert, M.; Lückermann, M.; Vente, A.; Dalhoff, A.; Fuhr, U. Population pharmacokinetics of finafloxacin in healthy volunteers and patients with complicated urinary tract infections. Antimicrob. Agents Chemother. 2018, 62, e02328-17. [Google Scholar] [CrossRef]
- Bartoletti, R.; Cai, T.; Perletti, G.; Wagenlehner, F.M.E.; Bjerklund Johansen, T.E. Finafloxacin for the treatment of urinary tract infections. Expert Opin. Investig. Drugs 2015, 24, 957–963. [Google Scholar] [CrossRef]
- Peyrusson, F.; Whelan, A.O.; Hartley, M.G.; Norville, I.H.; Harding, S.V.; Van Bambeke, F. Intracellular activity of antibiotics against Coxiella burnetii in a model of activated human THP-1 cells. Antimicrob. Agents Chemother. 2021, 65, e01061-21. [Google Scholar] [CrossRef]
- Chalhoub, H.; Harding, S.V.; Tulkens, P.M.; Van Bambeke, F. Influence of pH on the activity of finafloxacin against extracellular and intracellular Burkholderia thailandensis, Yersinia pseudotuberculosis and Francisella philomiragia and on its cellular pharmacokinetics in THP-1 monocytes. Clin. Microbiol. Infect. 2020, 26, 1254.e1–1254.e8. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.B.; Zumbrun, S.D.; Halasohoris, S.A.; Desai, P.D.; Miller, L.L.; Richards, M.I.; Russell, P.; Bentley, C.; Harding, S.V. Demonstration of the broad spectrum in vitro activity of finafloxacin against pathogens of biodefence interest. Antimicrob. Agents Chemother. 2019, 63, e01470-19. [Google Scholar] [CrossRef]
- Barnes, K.B.; Hamblin, K.A.; Richards, M.I.; Laws, T.R.; Vente, A.; Atkins, H.S.; Harding, S.V. The fluoroquinolone finafloxacin protects BALB/c Mice against an intranasal infection with francisella tularensis strain SchuS4. Front. Microbiol. 2019, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.B.; Georgi, E.; Genzel, G.H.; Schweizer, H.P. Finafloxacin overcomes Burkholderia pseudomallei efflux-mediated fluoroquinolone resistance. J. Antimicrob Chemother. 2017, 72, 1258–1260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barnes, K.B.; Hamblin, K.A.; Richards, M.I.; Laws, T.R.; Vente, A.; Atkins, H.S.; Harding, S.V. Demonstrating the protective efficacy of the novel fluoroquinolone finafloxacin against an inhalational exposure to burkholderia pseudomallei. Antimicrob. Agents Chemother. 2017, 61, e00082-17. [Google Scholar] [CrossRef]
- Barnes, K.B.; Richards, M.I.; Laws, T.R.; Núñez, A.; Thwaite, J.E.; Bentley, C.; Harding, S.V. finafloxacin is an effective treatment for inhalational tularemia and plague in mouse models of infection. Antimicrob. Agents Chemother. 2021, 65, e02294-20. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, H.J.; Seol, M.J.; Choi, D.R.; Choi, E.C.; Kwak, J.H. In vitro and in vivo antibacterial activities of DW-224a, a new fluoronaphthyridone. Antimicrob. Agent Chemother. 2006, 50, 2261–2264. [Google Scholar] [CrossRef]
- Kocsis, B.; Szabo, D. Zabofloxacin for chronic bronchitis. Drugs Today 2016, 52, 495–500. [Google Scholar] [CrossRef]
- Dong Wha Obtains Approval for Zabolante from MFDS (Press Release 20 March 2015). Available online: https://www.dong-wha.co.kr/english/customer/dnews/content.asp?t_idx=856 (accessed on 20 August 2021).
- Dong Wha Pharm’s Quinolone Antibacterial Agent, “Zabolante,”Wins at the 19th KNDA (Press Release 28 February 2018). Available online: https://www.dong-wha.co.kr/english/customer/dnews/content.asp?t_idx=1139 (accessed on 20 August 2021).
- Van Bambeke, F. Renaissance of antibiotics against difficult infections: Focus on oritavancin and new ketolides and quinolones. Ann. Med. 2014, 46, 512–529. [Google Scholar] [CrossRef][Green Version]
- Kwon, A.R.; Min, Y.H.; Ryu, J.M.; Choi, D.R.; Shim, M.J.; Choi, E.C. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J. Antimicrob. Chemother. 2006, 58, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Oh, S.-H.; Kim, H.-S.; Choi, D.-R.; Kwak, J.-H. Antimicrobial activity of zabofloxacin against clinically isolated Streptococcus pneumoniae. Molecules 2016, 21, 1562. [Google Scholar] [CrossRef]
- Jones, R.N.; Biedenbach, D.J.; Ambrose, P.G.; Wikler, M.A. Zabofloxacin (DW-224a) activity against Neisseria gonorrhoeae including quinolone-resistant strains. Diagn. Microbiol. Infect. Dis. 2008, 62, 110–112. [Google Scholar] [CrossRef]
- Han, H.K.; Kim, S.E.; Shin, K.H.; Lim, C.; Lim, K.S.; Yu, K.S.; Cho, J.Y. Comparison of pharmacokinetics between new quinolone antibiotics: The zabofloxacin hydrochloride capsule and the zabofloxacin aspartate tablet. Curr. Med. Res. Opin. 2013, 29, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.K.; Chang, J.H.; Choi, E.G.; Kim, H.K.; Kwon, Y.S.; Kyung, S.Y.; Lee, J.-H.; Park, M.J.; Yoo, K.H.; Oh, Y.M. Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: A multicenter, double-blind, double-dummy, randomized, controlled, phase III, non-inferiority trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. https://doi.org/10.3390/antibiotics10121506
Kocsis B, Gulyás D, Szabó D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics. 2021; 10(12):1506. https://doi.org/10.3390/antibiotics10121506
Chicago/Turabian StyleKocsis, Béla, Dániel Gulyás, and Dóra Szabó. 2021. "Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline" Antibiotics 10, no. 12: 1506. https://doi.org/10.3390/antibiotics10121506
APA StyleKocsis, B., Gulyás, D., & Szabó, D. (2021). Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics, 10(12), 1506. https://doi.org/10.3390/antibiotics10121506






