In Vitro Activities of Ceftazidime–Avibactam and Aztreonam–Avibactam at Different Inoculum Sizes of Extended-Spectrum β-Lactam-Resistant Enterobacterales Blood Isolates
Abstract
:1. Introduction
2. Results
2.1. Susceptibilities of E. coli and K. pneumoniae Isolates
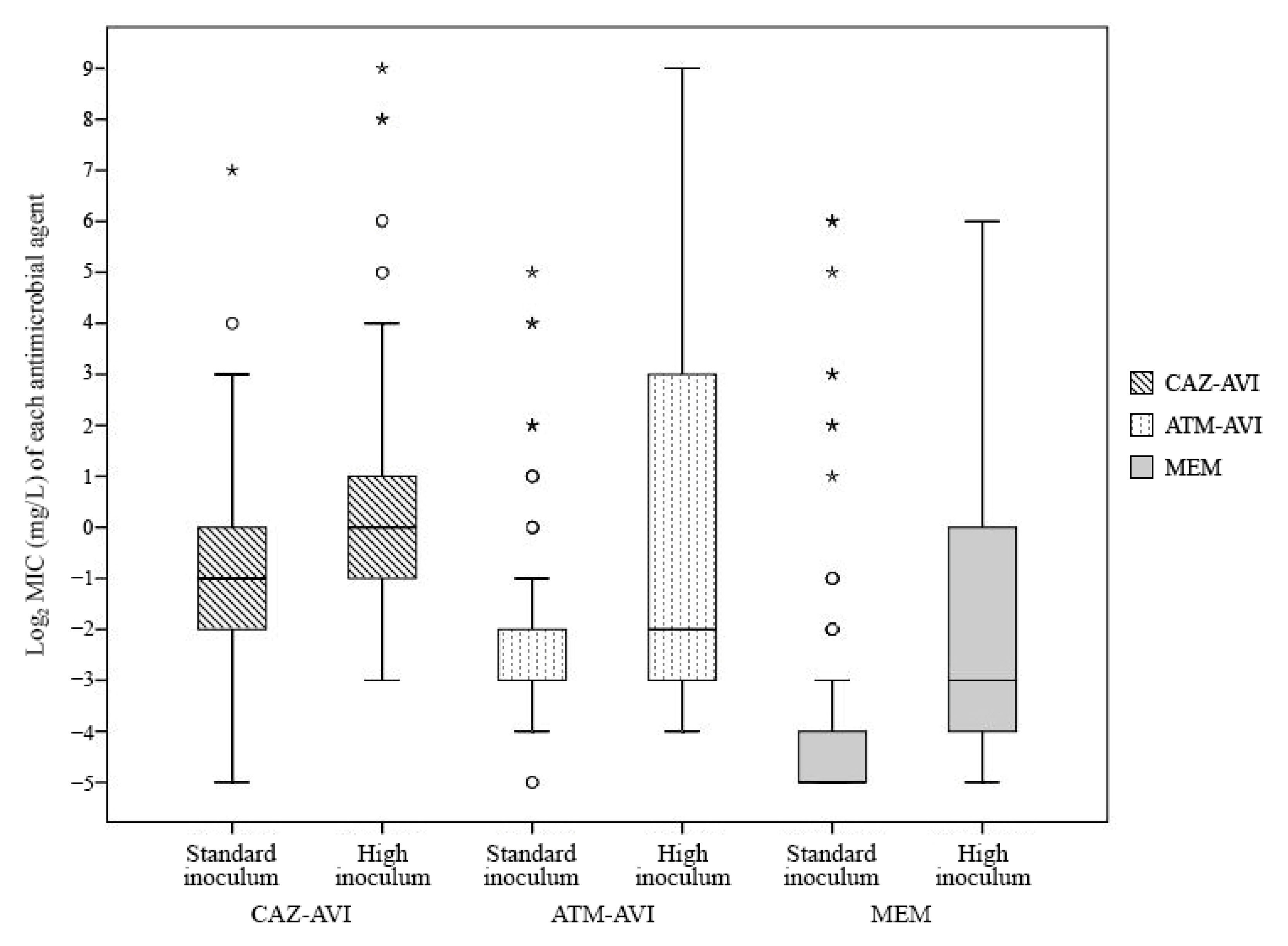
2.2. The Factors Affecting MIC Values and Inoculum Effect
2.3. Antimicrobial Susceptibilities and Inoculum Effects Stratified According to Resistance Mechanism
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial Susceptibility Test and Inoculum Effect
4.3. Investigation of Resistance
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Jean, S.S.; Coombs, G.; Ling, T.; Balaji, V.; Rodrigues, C.; Mikamo, H.; Kim, M.J.; Rajasekaram, D.G.; Mendoza, M.; Tan, T.Y.; et al. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-pacific region: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010–2013. Int. J. Antimicrob. Agents 2016, 47, 328–334. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Advincula, M.R.; Malczynski, M.; Qi, C.; Bolon, M.; Scheetz, M.H. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 5131–5133. [Google Scholar] [CrossRef] [Green Version]
- Mózes, J.; Ebrahimi, F.; Gorácz, O.; Miszti, C.; Kardos, G. Effect of carbapenem consumption patterns on the molecular epidemiology and carbapenem resistance of Acinetobacter baumannii. J. Med. Microbiol. 2014, 63, 1654–1662. [Google Scholar] [CrossRef] [Green Version]
- Tamma, P.D.; Han, J.H.; Rock, C.; Harris, A.D.; Lautenbach, E.; Hsu, A.J.; Avdic, E.; Cosgrove, S.E. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin. Infect. Dis. 2015, 60, 1319–1325. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gutiérrez, B.; Pérez-Galera, S.; Salamanca, E.; de Cueto, M.; Calbo, E.; Almirante, B.; Viale, P.; Oliver, A.; Pintado, V.; Gasch, O.; et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016, 60, 4159–4169. [Google Scholar] [CrossRef] [Green Version]
- Son, S.K.; Lee, N.R.; Ko, J.H.; Choi, J.K.; Moon, S.Y.; Joo, E.J.; Peck, K.R.; Park, D.A. Clinical effectiveness of carbapenems versus alternative antibiotics for treating ESBL-producing Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 2631–2642. [Google Scholar] [CrossRef]
- Issakhanian, L.; Behzadi, P. Antimicrobial agents and urinary tract infections. Curr. Pharm. Des. 2019, 25, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of piperacillin-tazobactam vs. meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docobo-Pérez, F.; López-Cerero, L.; López-Rojas, R.; Egea, P.; Domínguez-Herrera, J.; Rodríguez-Baño, J.; Pascual, A.; Pachón, J. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob. Agents Chemother. 2013, 57, 2109–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulitta, J.B.; Ly, N.S.; Yang, J.C.; Forrest, A.; Jusko, W.J.; Tsuji, B.T. Development and qualification of a pharmacodynamic model for the pronounced inoculum effect of ceftazidime against Pseudomonas aeruginosa. Antimicrob. Agents. Chemother. 2009, 53, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumbe, N.; Louie, A.; Leary, R.; Liu, W.; Deziel, M.R.; Tam, V.H.; Bachhawat, R.; Freeman, C.; Kahn, J.B.; Bush, K.; et al. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Investig. 2003, 112, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Drusano, G.L. From lead optimization to NDA approval for a new antimicrobial: Use of pre-clinical effect models and pharmacokinetic/pharmacodynamic mathematical modeling. Bioorganic Med. Chem. 2016, 24, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.E.; Jahić, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Kern, G.; Walkup, G.K.; Fisher, S.L. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 11663–11668. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, S.D.; Johnstone, M.R.; Ross, P.L.; McLaughlin, R.E.; Olivier, N.B.; Alm, R.A. Avibactam and class C beta-lactamases: Mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob. Agents Chemother. 2014, 58, 5704–5713. [Google Scholar] [CrossRef] [Green Version]
- Shirley, M. Ceftazidime-avibactam: A review in the treatment of serious Gram-negative bacterial infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef]
- Lin, W.T.; Lai, C.C.; Cheong, C.U. Novel β-lactam/β-lactamase combination versus meropenem for treating nosocomial pneumonia. Antibiotics 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; De Rosa, F.G.; Puente, F.D.; Mastroianni, C.M.; Cascio, A.; et al. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant Gram-negative bacteria other than carbapenem-resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, M.; Alfieri, A.; Di Franco, S.; Pace, M.C.; Simeon, V.; Ingoglia, G.; Cortegiani, A. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: A systematic review and network meta-analysis. Antibiotics 2020, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Barker, K.; Hope, R.; Warner, M.; Woodford, N. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104). J. Antimicrob. Chemother. 2012, 67, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Mischnik, A.; Baumert, P.; Hamprecht, A.; Rohde, A.; Peter, S.; Feihl, S.; Knobloch, J.; Golz, H.; Kola, A.; Obermann, B.; et al. Susceptibility to cephalosporin combinations and aztreonam/avibactam among third-generation cephalosporin-resistant Enterobacteriaceae recovered on hospital admission. Int. J. Antimicrob. Agents 2017, 49, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, e00472-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, F.; Zhao, C.; Wang, Z.; Nichols, W.W.; Testa, R.; Li, H.; Chen, H.; He, W.; Wang, Q.; et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob. Agents Chemother. 2014, 58, 1774–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Estabrook, M.; Jacoby, G.A.; Nichols, W.W.; Testa, R.T.; Bush, K. In vitro susceptibility of characterized beta-lactamase-producing strains tested with avibactam combinations. Antimicrob. Agents Chemother. 2015, 59, 1789–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kang, C.I.; Joo, E.J.; Ha, Y.E.; Wi, Y.M.; Chung, D.R.; Peck, K.R.; Lee, N.Y.; Song, J.H. Risk factors for multidrug resistance in nosocomial bacteremia caused by extended-spectrum β-lactamase-producing Escherichia Coli Kleb. Pneumoniae. Microb Drug Resist. 2012, 18, 518–524. [Google Scholar] [CrossRef]
- Tam, V.H.; Ledesma, K.R.; Chang, K.T.; Wang, T.Y.; Quinn, J.P. Killing of Escherichia coli by beta-lactams at different inocula. Diagn. Microbiol. Infect. Dis. 2009, 64, 166–171. [Google Scholar] [CrossRef]
- Harada, Y.; Morinaga, Y.; Kaku, N.; Nakamura, S.; Uno, N.; Hasegawa, H.; Izumikawa, K.; Kohno, S.; Yanagihara, K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2014, 20, O831–O839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, K.S. Controversies about extended-spectrum and AmpC beta-lactamases. Emerg. Infect. Dis. 2001, 7, 333–336. [Google Scholar] [CrossRef]
- Lahiri, S.D.; Mangani, S.; Durand-Reville, T.; Benvenuti, M.; De Luca, F.; Sanyal, G.; Docquier, J.D. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: Avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC beta-lactamases. Antimicrob. Agents Chemother. 2013, 57, 2496–2505. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M.; Issakhanian, L. Metallo-ß-lactamases: A review. Mol. Biol. Rep. 2020, 47, 6281–6294. [Google Scholar] [CrossRef] [PubMed]
- Canovas, J.; Petitjean, G.; Chau, F.; Le Monnier, A.; Fantin, B.; Lefort, A. Expression of CTX-M-15 limits the efficacy of ceftolozane/tazobactam against Escherichia coli in a high-inoculum murine peritonitis model. Clin. Microbiol. Infect. 2020, 26, 1416.e5–1416.e9. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.C.; Bae, M.; Sung, H.; Kim, M.N.; Jung, J.; Kim, M.J.; Kim, S.H.; Lee, S.O.; Choi, S.H.; et al. In vitro activities and inoculum effects of ceftazidime-avibactam and aztreonam-avibactam against carbapenem-resistant Enterobacterales isolates from South Korea. Antibiotics 2020, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institue (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2019. [Google Scholar]
- Clinical and Laboratory Standards Institue (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2018. [Google Scholar]
- Kang, C.I.; Cha, M.K.; Kim, S.H.; Wi, Y.M.; Chung, D.R.; Peck, K.R.; Lee, N.Y.; Song, J.H. Extended-spectrum cephalosporins and the inoculum effect in tests with CTX-M-type extended-spectrum β-lactamase-producing Escherichia coli: Potential clinical implications of the revised CLSI interpretive criteria. Int. J. Antimicrob. Agents 2014, 43, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.S.; Moland, E.S. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2001, 45, 3548–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzelepi, E.; Giakkoupi, P.; Sofianou, D.; Loukova, V.; Kemeroglou, A.; Tsakris, A. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 2000, 38, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.Y.; Ng, L.S.; He, J.; Koh, T.H.; Hsu, L.Y. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 146–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Antimicrobial Agent | Inoculum Size | Number of Isolates (Cumulative %) with Indicated MICs (mg/L) | MIC (mg/L) a | S (%) b | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ≥512 | MIC50 | MIC90 | |||
| Ceftazidime | Standard | 5 (2.2) | 11 (7.0) | 12 (12.3) | 21 (21.5) | 22 (31.1) | 34 (46.1) | 21 (55.3) | 22 (64.9) | 24 (75.4) | 56 (100) | 64 | ≥512 | 12.3 | |||||
| High | 1 (0.4) | 3 (1.8) | 7 (4.8) | 7 (7.9) | 12 (13.2) | 14 (19.3) | 13 (25.0) | 16 (32.0) | 26 (43.4) | 129 (100) | ≥512 | ≥512 | 4.8 | ||||||
| Aztreonam | Standard | 4 (1.8) | 5 (3.9) | 5 (6.1) | 16 (13.2) | 20 (21.9) | 25 (32.9) | 37 (49.1) | 33 (63.6) | 83 (100) c | 128 | ≥256 | 6.1 | ||||||
| High | 1 (0.4) | 1 (0.9) | 4 (2.6) | 7 (5.7) | 7 (8.8) | 23 (18.9) | 185 (100) c | ≥256 | ≥256 | 0.9 | |||||||||
| Ceftazidime–avibactam | Standard | 1 (0.4) | 8 (3.9) | 65 (32.5) | 72 (64.0) | 47 (84.6) | 22 (94.3) | 9 (98.2) | 2 (99.1) | 1 (99.6) | 1 (100) | 0.5 | 2 | 99.1 | |||||
| High | 1 (0.4) | 35 (15.8) | 73 (47.8) | 39 (64.9) | 27 (76.8) | 20 (85.5) | 15 (92.1) | 9 (96.1) | 4 (97.8) | 2 (98.7) | 2 (99.6) | 1 (100) | 1 | 8 | 92.1 | ||||
| Aztreonam–avibactam | Standard | 2 (0.9) | 42 (19.3) | 109 (67.1) | 48 (88.2) | 9 (92.1) | 6 (94.7) | 6 (97.4) | 3 (98.7) | 2 (99.6) | 1 (100) | 0.125 | 0.5 | N/A | |||||
| High | 18 (7.9) | 80 (43.0) | 40 (60.5) | 12 (65.8) | 4 (67.5) | 4 (69.3) | 6 (71.9) | 30 (85.1) | 5 (87.3) | 4 (89.0) | 4 (90.8) | 4 (92.5) | 7 (95.6) | 10 (100) | 0.25 | 64 | N/A | ||
| Meropenem | Standard | 143 (62.7) | 49 (84.2) | 12 (89.5) | 10 (93.9) | 5 (96.1) | 1 (96.5) | 2 (97.4) | 2 (98.2) | 1 (98.7) | 3 c (100) | ≤0.03 | 0.25 | 96.1 | |||||
| High | 29 (12.7) | 70 (43.4) | 23 (53.5) | 7 (56.6) | 36 (72.4) | 16 (79.4) | 13 (85.1) | 9 (89.0) | 2 (89.9) | 9 (93.9) | 4 (95.6) | 10 c (100) | 0.125 | 16 | 79.4 | ||||
| Estimate | SE | Lower | Upper | p-Value | |
|---|---|---|---|---|---|
| Intercept | −4.96 | 0.12 | −5.19 | −4.72 | <0.0001 |
| Inoculum size | |||||
| Standard | (reference) | ||||
| High | 2.13 | 0.18 | 1.77 | 2.49 | <0.0001 |
| Antimicrobial agent | |||||
| Meropenem | (reference) | ||||
| ATM-AVI | 1.67 | 0.12 | 1.43 | 1.91 | <0.0001 |
| CAZ-AVI | 3.49 | 0.12 | 3.26 | 3.71 | <0.0001 |
| Species | |||||
| E. coli | (reference) | ||||
| K. pneumonia | 1.16 | 0.13 | 0.89 | 1.42 | <0.0001 |
| β-lactamase | |||||
| ESBL | (reference) | ||||
| AmpC | 0.94 | 0.41 | 0.13 | 1.74 | 0.02 |
| ESBL + AmpC | 1.94 | 0.36 | 1.24 | 2.64 | <0.0001 |
| CPE | 4.88 | 0.58 | 3.74 | 6.01 | <0.0001 |
| Inoculum size*MEM | (reference) | ||||
| Inoculum size*ATM-AVI | −0.20 | 0.23 | −0.65 | 0.26 | 0.39 |
| Inoculum size*CAZ-AVI | −1.49 | 0.19 | −1.86 | −1.11 | <0.0001 |
| Inoculum size*E. coli | (reference) | ||||
| Inoculum size*K. pnemoniae | 0.82 | 0.21 | 0.42 | 1.23 | <0.0001 |
| Inoculum size*ESBL | (reference) | ||||
| Inoculum size*AmpC | 0.37 | 0.63 | −0.88 | 1.61 | 0.56 |
| Inoculum size*ESBL + AmpC | −0.01 | 0.55 | −1.10 | 1.08 | 0.99 |
| Inoculum size*CPE | −1.10 | 0.89 | −2.86 | 0.67 | 0.22 |
| Number of Isolates (%) with Positive Inoculum Effect b | Agreement on Inoculum Effects a | ||||
|---|---|---|---|---|---|
| Species | Ceftazidime–Avibactam | Aztreonam–Avibactam | Meropenem | p-Value a for McNemar’s Test | Strength of Agreement, Kappa (95% CI) |
| E. coli | 10 (8.3) | 12 (10.0) | 15 (12.5) | 0.63 | 0.80 (0.61–0.99) |
| K. pneumoniae | 21 (20.2) c,d | 54 (51.9) c,e | 69 (66.3) d,e | <0.001 | 0.31 (0.17–0.45) |
| Total | 31 (13.8) f,g | 66 (29.5) f,h | 84 (37.5) g,h | <0.001 | 0.48 (0.36–0.61) |
| β-Lactamase (n) | Antimicrobial Agent | Inoculum Size | MIC (mg/L) | S (n (%)) | No. of Isolates (%) with Inoculum Effect | ||
|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||||
| ESBL | CAZ-AVI | Standard | 0.5 | 2 | ≤0.03 to 16 | 210 (99.5) | 28 (13.3) |
| (211) | High | 0.5 | 8 | 0.125 to ≥512 | 198 (93.8) c | ||
| ATM-AVI | Standard | 0.125 | 0.25 | ≤0.03 to 16 | N/A | 61 (28.9) | |
| High | 0.25 | 32 | 0.06 to ≥512 | N/A | |||
| MEM | Standard | ≤0.03 | 0.125 | ≤0.03 to 64 | 207 (98.1) d | 77 (36.7) b | |
| High | 0.125 | 4 | ≤0.03 to 64 | 175 (87.2) e | |||
| AmpC | CAZ-AVI | Standard | 1 | 4 | 0.25 to 4 | 6 (100) | 1 (16.7) |
| (6) | High | 2 | 256 | 0.5 to 256 | 4 (66.7) c | ||
| ATM-AVI | Standard | 1 | 4 | 0.25 to 4 | N/A | 3 (50.0) | |
| High | 8 | ≥512 | 0.25 to ≥512 | N/A | |||
| MEM | Standard | 0.06 | 0.25 | ≤0.03 to 0.25 | 6 (100) d | 3 (50.0) | |
| High | 1 | 4 | ≤0.03 to 4 | 4 (66.7) e | |||
| ESBL+ | CAZ-AVI | Standard | 2 | 8 | 0.25 to 8 | 8 (100) | 3 (37.5) |
| AmpC | High | 2 | 64 | 0.25 to 64 | 6 (75.0) c | ||
| (8) | ATM-AVI | Standard | 1 | 32 | 0.125 to 32 | N/A | 4 (50.0) |
| High | 2 | ≥512 | 0.125 to ≥512 | N/A | |||
| MEM | Standard | 0.25 | 32 | ≤0.03 to 32 | 6 (75.0) d | 4 (57.1) b | |
| High | 2 | 64 | 0.5 to 64 | 2 (25.0) e | |||
| CRE a | CAZ-AVI | Standard | 2 | 16 | 0.25 to 128 | 17 (89.5) | 3 (15.8) |
| (19) | High | 2 | 256 | 0.25 to 256 | 16 (84.2) | ||
| ATM-AVI | Standard | 0.5 | 16 | 0.06 to 32 | N/A | 4 (21.1) | |
| High | 0.5 | ≥512 | 0.06 to ≥512 | N/A | |||
| MEM | Standard | 0.25 | ≥64 | ≤0.03 to ≥64 | 11 (57.9) | 3 (20.0) b | |
| High | 2 | ≥64 | ≤0.03 to ≥64 | 7 (36.8) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Kim, T.; Park, J.H.; Bae, S.; Sung, H.; Kim, M.-N.; Jung, J.; Kim, M.J.; Kim, S.-H.; Lee, S.-O.; et al. In Vitro Activities of Ceftazidime–Avibactam and Aztreonam–Avibactam at Different Inoculum Sizes of Extended-Spectrum β-Lactam-Resistant Enterobacterales Blood Isolates. Antibiotics 2021, 10, 1492. https://doi.org/10.3390/antibiotics10121492
Bae M, Kim T, Park JH, Bae S, Sung H, Kim M-N, Jung J, Kim MJ, Kim S-H, Lee S-O, et al. In Vitro Activities of Ceftazidime–Avibactam and Aztreonam–Avibactam at Different Inoculum Sizes of Extended-Spectrum β-Lactam-Resistant Enterobacterales Blood Isolates. Antibiotics. 2021; 10(12):1492. https://doi.org/10.3390/antibiotics10121492
Chicago/Turabian StyleBae, Moonsuk, Taeeun Kim, Joung Ha Park, Seongman Bae, Heungsup Sung, Mi-Na Kim, Jiwon Jung, Min Jae Kim, Sung-Han Kim, Sang-Oh Lee, and et al. 2021. "In Vitro Activities of Ceftazidime–Avibactam and Aztreonam–Avibactam at Different Inoculum Sizes of Extended-Spectrum β-Lactam-Resistant Enterobacterales Blood Isolates" Antibiotics 10, no. 12: 1492. https://doi.org/10.3390/antibiotics10121492
APA StyleBae, M., Kim, T., Park, J. H., Bae, S., Sung, H., Kim, M.-N., Jung, J., Kim, M. J., Kim, S.-H., Lee, S.-O., Choi, S.-H., Kim, Y. S., & Chong, Y. P. (2021). In Vitro Activities of Ceftazidime–Avibactam and Aztreonam–Avibactam at Different Inoculum Sizes of Extended-Spectrum β-Lactam-Resistant Enterobacterales Blood Isolates. Antibiotics, 10(12), 1492. https://doi.org/10.3390/antibiotics10121492






