Efficacy and Safety of Remdesivir over Two Waves of the SARS-CoV-2 Pandemic
Abstract
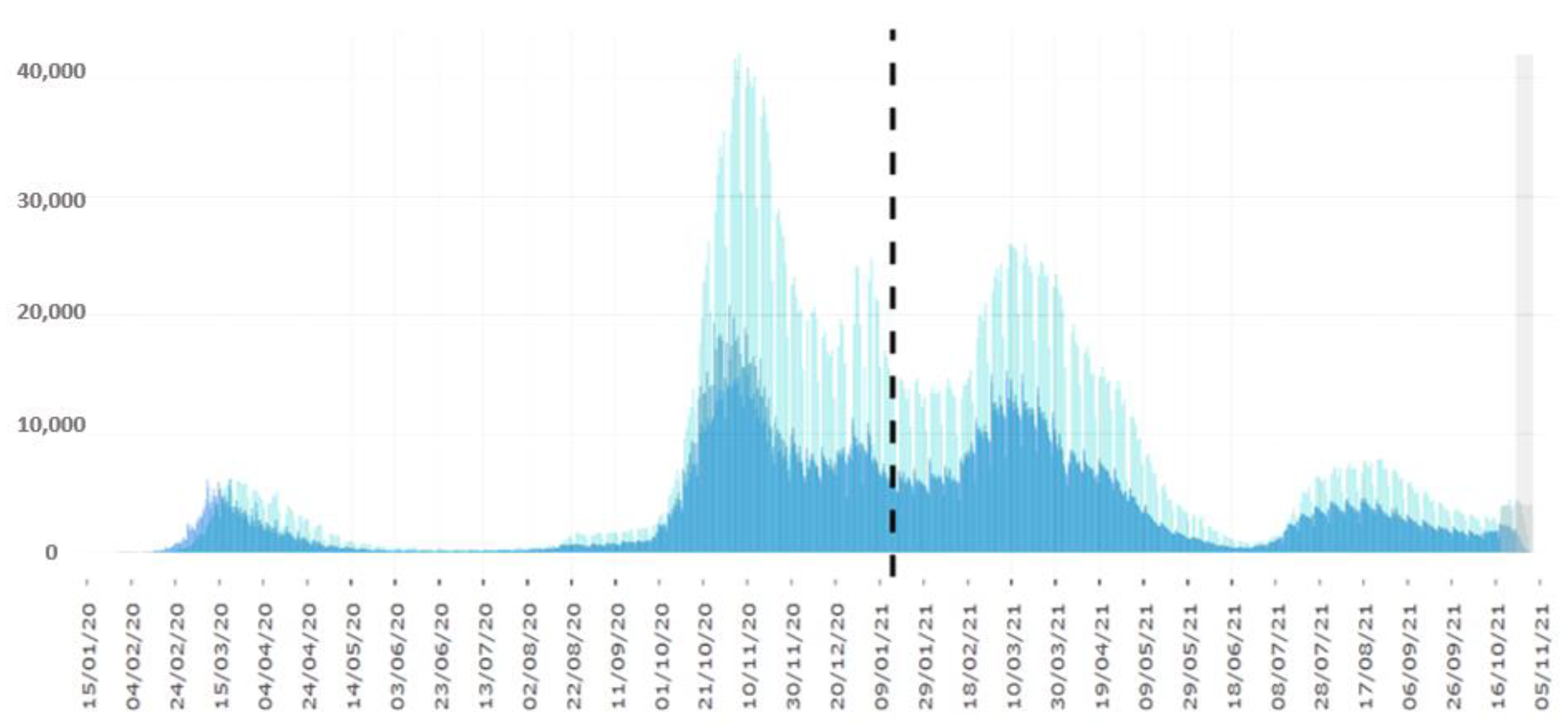
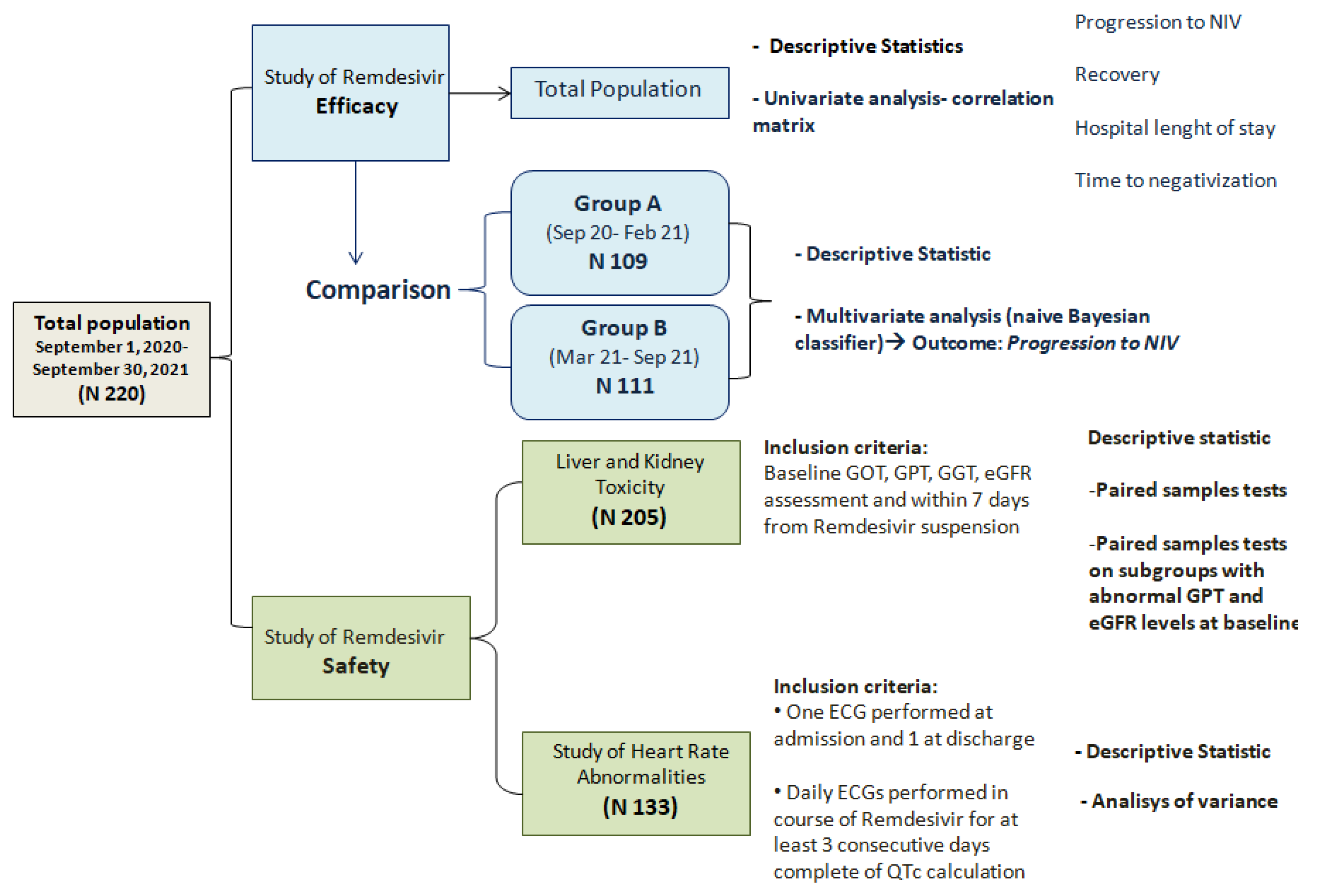
:1. Introduction
2. Results
2.1. General Characteristics of Study Population
- Group A: 109 patients admitted from 1 September 2020 to 28 February 2021;
- Group B: 111 patients admitted from 1 March to 30 September 2021.
2.2. Remdesivir Efficacy
2.3. Remdesivir Safety
2.3.1. Liver and Kidney Toxicity
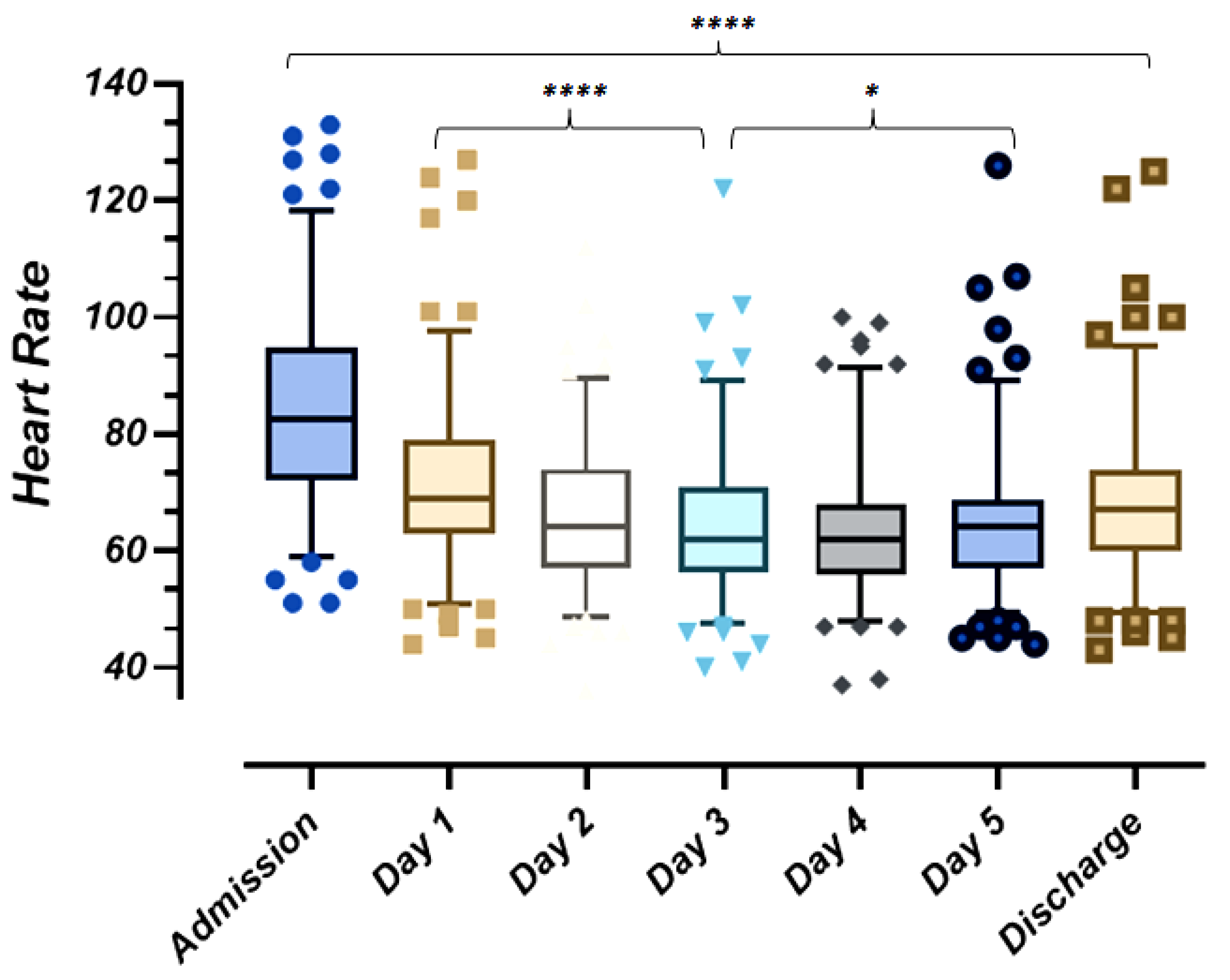
2.3.2. Heart Safety and Cardiac Rhythm Abnormalities
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Study Design and Statistical Analysis
- A primary endpoint was to report on the efficacyof the five days course treatment with Remdesivir.
- -
- Group A: patients admitted from 1 September 2020 to 28 February 2021;
- -
- Group B: patients admitted from 1 March to 30 September 2021.
- A secondary endpoint of our work was to describe the safety of Remdesivir in terms of drug-induced liver and kidney toxicity and cardiac rhythm abnormalities.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gabutti, G.; D’Anchera, E.; De Motoli, F.; Savio, M.; Stefanati, A. The Epidemiological Characteristics of the COVID-19 Pandemic in Europe: Focus on Italy. Int. J. Environ. Res. Public Health 2021, 18, 2942. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Word-Pillole ASI_2020.rtf. Available online: https://www.istat.it/ (accessed on 2 November 2021).
- COVID-19 ITALIA-Desktop. Available online: https://www.arcgis.com/index.html (accessed on 2 November 2021).
- Infografica Web-Dati Della Sorveglianza Integrata COVID-19 in Italia. Available online: https://www.iss.it/ (accessed on 3 November 2021).
- Prevalenza e Distribuzione Delle Varianti di SARS-CoV-2 di Interesse per la Sanità Pubblica in Italia. Rapporto n. 13 del 12 Novembre 2021 (Updated at 8 November 2021). Available online: https://www.iss.it/documents/20126/0/Bollettino_Varianti+n%C2%B0_13.pdf/56c935c9-0878-d5cb-b7f6-5172e559d963?t=1636729355823 (accessed on 2 November 2021).
- Available online: https://www.cdc.gov/vaccines/COVid-19/info-by-product/clinical-considerations.html (accessed on 3 November 2021).
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H., III; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.K.; Jordan, R.; Arvey, A.; Sudhamsu, J.; Shrivastava-Ranjan, P.; Hotard, A.L.; Flint, M.; McMullan, L.K.; Siegel, D.; Clarke, M.O.; et al. GS-5734 and its parent nucleoside analog inhibit flo-, pneumo-, and paramyxoviruses. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralnski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efcacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Mbaya, O.T.; Proschan, M.; Mukadi, D.; Manzo, M.L.; Nzolo, D.; Oloma, A.T.; Ibanda, A.; et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Nili, A.; Farbod, A.; Neishabouri, A.; Mozafarihashjin, M.; Tavakolpour, S.; Mahmoudi, H. Remdesivir: A beacon of hope from Ebola virus disease to COVID-19. Rev. Med. Virol. 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Reina, J. Remdesivir, la esperanza antiviral frente al SARS-CoV-2 [Remdesivir, the antiviral hope against SARS-CoV-2]. Rev. Esp. Quimioter. 2020, 33, 176–179. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; ACTT-1 Study Group Members. Remdesivir for the Treatment of COVid-19-Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578, Erratum in 2020, 395, 1694. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; López, J.R.A.; Cattelan, A.M.; Viladomiu, A.S.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Taha, H.R.; Keewan, N.; Slati, F.; Al-Sawalha, N.A. Remdesivir: A Closer Look at Its Effect in COVID-19 Pandemic. Pharmacology 2021, 106, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Kim, H.J.; Ko, M.; Jee, Y.; Kim, S. TMPRSS2 and RNA-dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID-19 caused by SARS-CoV-2 variants (B.1.1.7 and B.1.351). bioRxiv 2021. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.nih.gov/ (accessed on 3 November 2021).
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVid-19-Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Meira, F.; Cózar-Llistó, A. NCOVID19-Researcher Group. Real-life use of remdesivir in hospitalized patients with COVID-19. Rev. Esp. Quimioter. 2021, 34, 136–140. [Google Scholar] [CrossRef]
- Simioli, F.; Nicoletta, C.; Valentino, M.R.; Martino, M.; Annunziata, A.; Carannante, N.; Di Micco, P.; Fiorentino, G. Remdesivir in Severe COVID-19 and Non-Invasive Ventilation: A Real-Life Experience. Healthcare 2021, 9, 1108. [Google Scholar] [CrossRef]
- Van Laar, S.A.; de Boer, M.G.J.; Gombert-Handoko, K.B.; LUMC-COVid-19 Research Group. Liver and kidney function in patients with COVid-19 treated with remdesivir. Br. J. Clin. Pharmacol. 2021, 87, 4450–4454. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.A.; Walunas, T.L.; Martinez, E.; Perez, K.K.; Castagna, A.; Wang, S.; Kurbegov, D.; Goyal, P.; Ripamonti, D.; Balani, B.; et al. Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality. Open Forum. Infect. Dis. 2021, 8, ofab278. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; Sax, P.E.; Wohl, D.A.; Casciano, R.; et al. Remdesivir treatment in hospitalized patients with COVID-19: A comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and antiviral activity of a series of 1’-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic Med. Chem. Lett. 2021, 22, 2705–2707. [Google Scholar] [CrossRef]
- Scavone, C.; Brusco, S.; Bertini, M.; Sportiello, L.; Rafaniello, C.; Zoccoli, A.; Berrino, L.; Racagni, G.; Rossi, F.; Capuano, A. Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 2020, 177, 4813–4824. [Google Scholar] [CrossRef]
- Ferner, R.E.; Aronson, J.K. Remdesivir in covid-19. BMJ 2020, 369, m1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boshier, F.A.T.; Pang, J.; Penner, J.; Parker, M.; Alders, N.; Bamford, A.; Grandjean, L.; Grunewald, S.; Hatcher, J.; Best, T.; et al. COVID-19 Genomics UK (COG-UK) consortium. Evolution of viral variants in remdesivir-treated and untreated SARS-CoV-2-infected pediatrics patients. J. Med. Virol. 2021, 94, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Boshier, F.A.T.; Pang, P.; Penner, J.; Hughes, J.; Parker, P.; Shepherd, J.; Alders, N.; Bamford, A.; Grandjean, L.; Grunewald, S.; et al. Remdesivir induced viral RNA and subgenomic RNA suppression, and evolution of viral variants in SARS-CoV-2 infected patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. SARS-CoV-2-increased circulation of variants of concern and vaccine rollout in the EU/EEA, 14th update—15 February 15 2021. In Science Brief: Emerging SARS-CoV-2 Variants; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Gao, Y.-D.; Ding, M.; Dong, X.; Zhang, J.-J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Available online: https://files.COVid19treatmentguidelines.nih.gov/guidelines/COVid19treatmentguidelines.pdf (accessed on 3 November 2021).
- Available online: https://www.aifa.gov.it/documents/20142/1123276/remdesivir_update01_24.11.2020.pdf (accessed on 3 November 2021).
- Brunetti, N.D.; Poliseno, M.; Bottalico, I.F.; Centola, A.; Montemurro, L.; Sica, S.; Santantonio, T.; Caputo, S.L. Safety and heart rate changes in COVid-19 patients treated with Remdesivir. Int. J. Infect. Dis. 2021, 112, 254–257. [Google Scholar] [CrossRef]
- WHO Severity of Disease Classifications and COVID-19 Outcomes. Available online: https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak (accessed on 30 November 2021).
- Uso Degli Anticorpi Monoclonali per COVID-19|Agenzia Italiana del Farmaco. Available online: https://www.nih.gov/ (accessed on 3 November 2021).
- Poole, D.L.; Mackworth, A.K. Artificial Intelligence: Foundations of Computational Agents; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Bazett, J.C. An analysis of time relations of electrocardiograms. Heart 1920, 7, 353–370. [Google Scholar] [CrossRef]
- Crotti, L.; Dossena, C.; Mastantuono, E.; Dagradi, F.; Schwartz, P.J. Condizioni cliniche associate ad anomalie dell’intervallo QT: Implicazioni cliniche [Clinical conditions associated with abnormal QT interval: Clinicalimplications]. G Ital. Cardiol. (Rome) 2013, 14, 55–65. (In Italian) [Google Scholar] [CrossRef]
- 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, e51–e156.
| Variables | Overall (N = 220) |
|---|---|
| Mean (±SD) age, years | 60 (46–74) |
| Gender, n (%) | |
| Males Females | 139 (63) 81 (37) |
| Vaccinated, n (%) | 9 (4) |
| Coexisting dismetabolic conditions, n (%) | |
| Hypertension Obesity Type II diabetes | 102 (49) 54 (26) 53 (25) |
| Two or more coexisting conditions, n (%) | 70 (39) |
| Median time (IQR) from symptom onset to Hospitalization, days | 6 (3–8) |
| Median time (IQR) from symptom onset to Remdesivir, days | 7 (4–9) |
| Laboratory tests at admission, median (IQR) | |
| RPC, mg/dL IL-6, pg/mL D-dimer, ng/mL GOT, UI/mL GPT, UI/mL GGT, UI/mL eGFR, mL/min | 48 (21–108) 17 (7–37) 755 (453–1409) 28 (21–40) 28 (19–40) 37 (23–61) 91 (72–107) |
| Oxygen flow required at admission, n (%) | |
| Low flow oxygen HFNC NIV | 187 (85) 25 (11) 8 (4) |
| COVID-19 Severity of Disease, n (%) | |
| Mild Moderate Severe Critical | 15 (7) 108 (49) 79 (35) 18 (8) |
| Progression to Non Invasive Ventilation, n (%) | 60 (27) |
| Outcome, n (%) | |
| Intensive Caare Unit Admission/death Clinical Recovery | 23 (10) 197 (89) |
| Virological Recovery, n (%) | 159 (72) |
| Median time (IQR) from first positive to first negative SARS-CoV-2 PCR on nasal-pharyngeal swab, days | 20 (12–28) |
| Median (IQR) duration of hospital stay, days | 15 (11–23) |
| Variables | COVID-19 | p Value * | |
|---|---|---|---|
| Second Wave Group A (N = 109) | Third Wave Group B (N = 111) | ||
| Mean (±SD) age, years | 62 (50–74) | 58 (42–74) | 0.057 |
| Gender, n (%) | |||
| Males | 70 (32) | 69 (31) | 0.781 |
| Females | 39 (18) | 42 (19) | |
| Vaccinated, n (%) | 0 (0) | 9 (8) | 0.003 |
| Coexisting cardio metabolic conditions, n (%) | |||
| Hypertension Obesity Type II diabetes | 60 (29) 26 (12) 29 (14) | 42 (20) 28 (13) 24 (11) | 0.004 1.00 0.341 |
| Two or more coexisting conditions, n (%) | 38 (34) | 32 (28) | 0.177 |
| Median time (IQR) from symptom onset to hospitalization, days | 5 (3–8) | 6 (3–8) | 0.134 |
| Median time (IQR) from symptom onset to Remdesivir, days | 7 (5–9) | 7 (4–9) | 0.453 |
| Laboratory tests at admission, median (IQR) | |||
| RPC, mg/dL IL-6, pg/mL D-dimers, ng/mL GOT, UI/mL GPT, UI/mL GGT, UI/mL eGFR, mL/min | 36 (22–108) 17 (8–36) 735 (398–1568) 25 (20–36) 28 (20–42) 47 (23–69) 86 (69–100) | 52 (21–107) 16 (7–37) 794 (504–1222) 30 (23–42) 28 (19–40) 34 (23–63) 97 (79–111) | 0.547 0.458 0.313 0.014 0.786 0.097 0.006 |
| Oxygen flow required at admission, n (%) | |||
| Low flow oxygen | 94 (43) | 93 (13) | 0.032 |
| HFNC | 12 (5) | 13 (6) | |
| NIV | 3 (1) | 5 (2) | |
| COVID-19 Severity of disease, n (%) | |||
| Mild | 8 (4) | 7 (3) | <0.001 |
| Moderate | 68 (31) | 40 (18) | |
| Severe | 23 (10) | 56 (25) | |
| Critical | 10 (94 | 8 (4) | |
| Progression to Non Invasive Ventilation, n (%) | 15 (14) | 29 (26) | 0.028 |
| Outcome, n (%) | |||
| Intensive Care Unit admission/Death | 15(7) | 9 (4) | 0.200 |
| Clinical Recovery | 94 (42) | 102 (46) | |
| Virological Recovery, n (%) | 26 (23) | 35 (12) | 0.203 |
| Median time (IQR) from first positive to first negative SARS-CoV-2 PCR on nasal-pharyngeal swab, days | 21 (13–35) | 19 (12–24) | 0.062 |
| Median (IQR) duration of hospital stay, days | 16 (11–25) | 15 (10–22) | 0.412 |
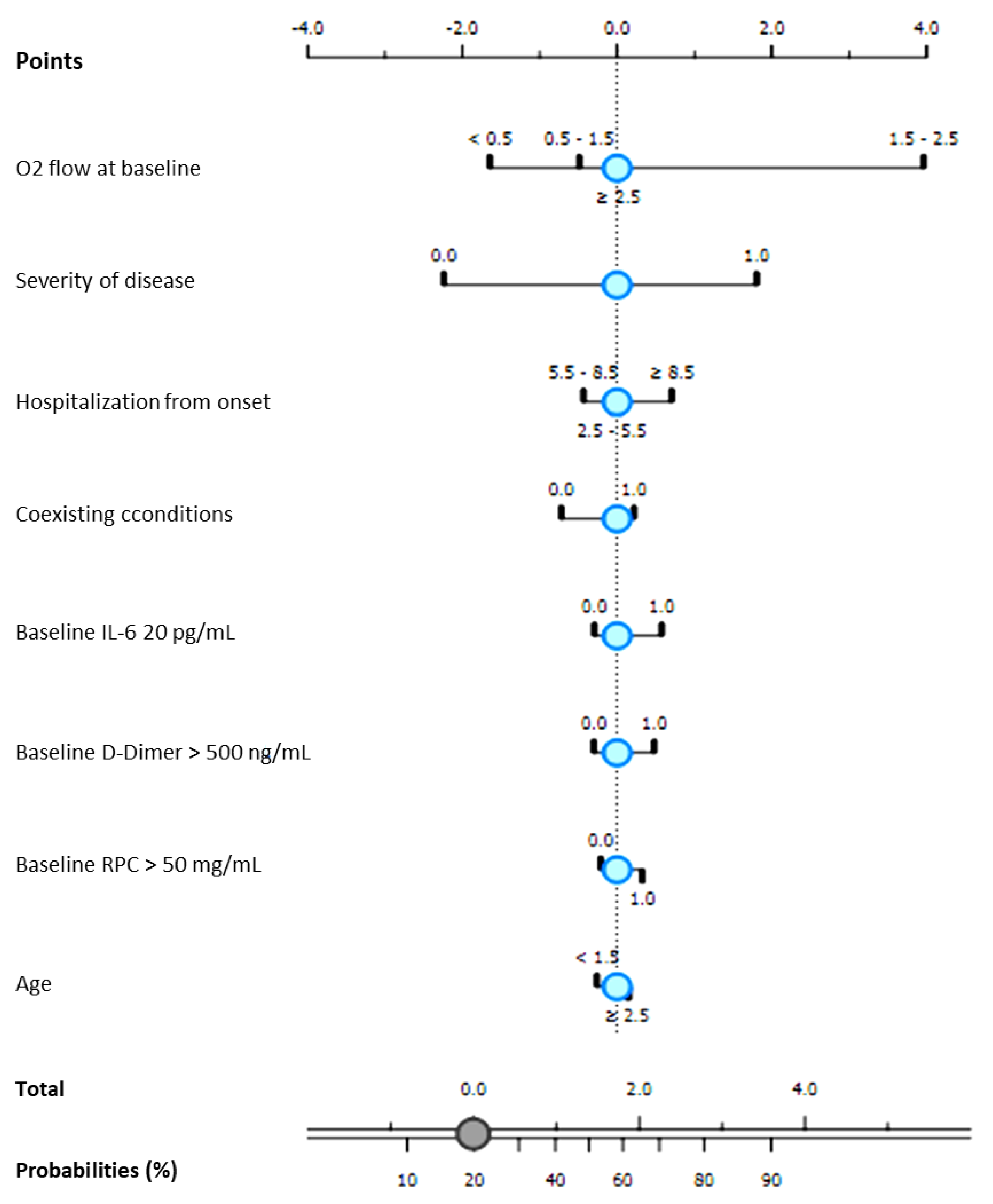
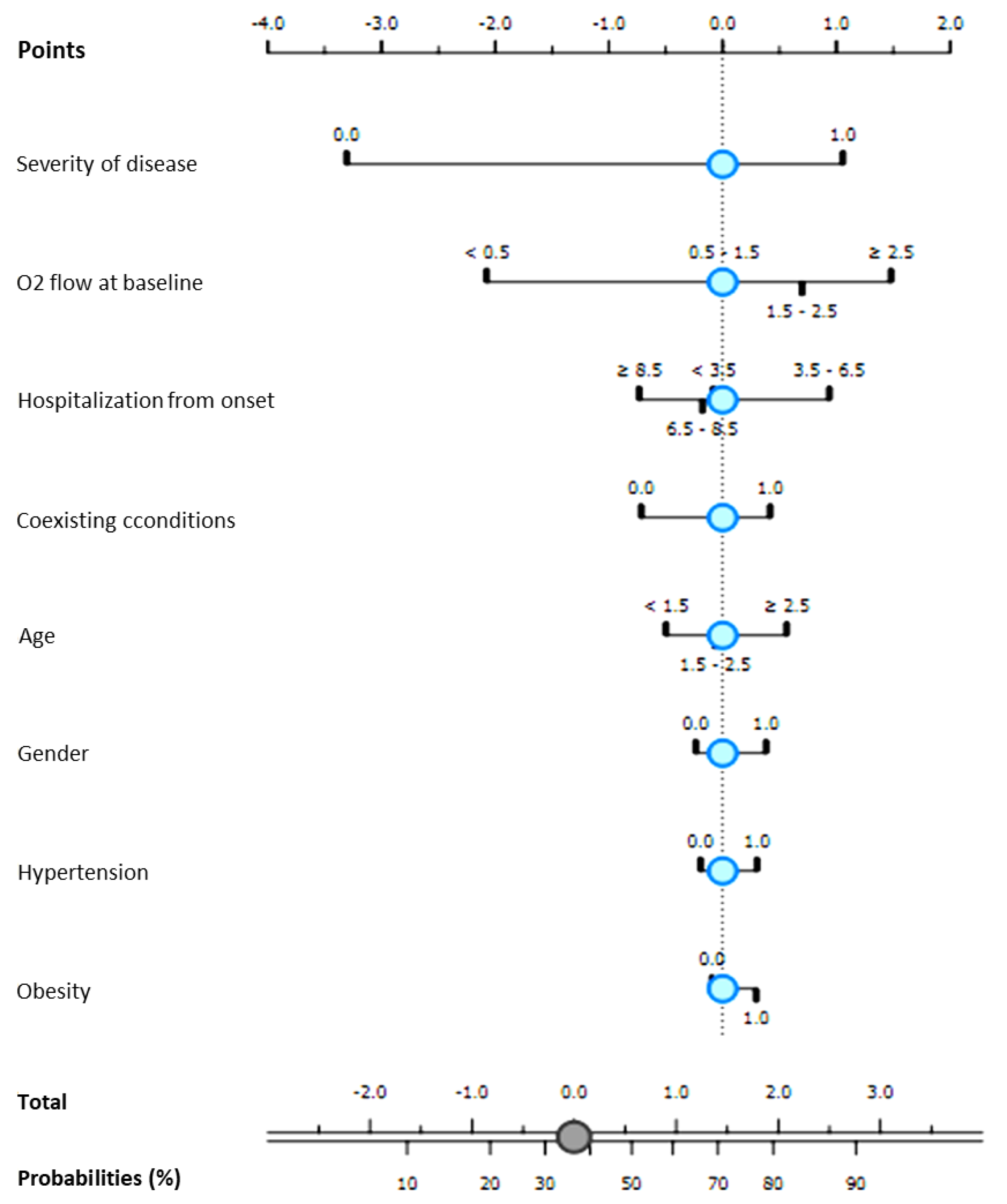
| Variables | Progression to NIV | Clinical Recovery | Hospital Length-of Stay | Time to Negativization | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age | 0.159 | 0.020 | −0.192 | 0.005 | 0.291 | <0.001 | 0.125 | 0.129 |
| Male gender | 0.062 | 0.363 | 0.045 | 0.505 | 0.089 | 0.167 | 0.085 | 0.302 |
| Coexisting conditions | 0.199 | 0.003 | −0.233 | 0.001 | 0.222 | <0.001 | 0.034 | 0.682 |
| D-dimer at admission | 0.195 | 0.004 | −0.238 | <0.001 | 0.203 | 0.003 | 0.091 | 0.276 |
| CRP at admission | 0.186 | 0.006 | −0.127 | 0.062 | 0.117 | 0.087 | 0.128 | 0.123 |
| IL-6 at admission | 0.172 | 0.014 | −0.157 | 0.025 | 0.141 | 0.045 | 0.125 | 0.141 |
| Oxygen support at admission | 0.423 | <0.001 | −0.268 | 0.001 | 0.094 | 0.066 | 0.115 | 0.164 |
| Time from onset to hospitalization | −0.010 | 0.885 | 0.113 | 0.094 | −0.103 | 0.126 | −0.011 | 0.893 |
| Time from onset to Remdesivir | −0.006 | 0.930 | 0.019 | 0.777 | −0.036 | 0.595 | 0.163 | 0.048 |
| Date of hospitalization | 0.219 | 0.001 | 0.040 | 0.560 | 0.018 | 0.791 | −0.076 | 0.357 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poliseno, M.; Gallo, C.; Cibelli, D.C.; Minafra, G.A.; Bottalico, I.F.; Bruno, S.R.; D’Errico, M.L.; Montemurro, L.; Rizzo, M.; Barbera, L.; et al. Efficacy and Safety of Remdesivir over Two Waves of the SARS-CoV-2 Pandemic. Antibiotics 2021, 10, 1477. https://doi.org/10.3390/antibiotics10121477
Poliseno M, Gallo C, Cibelli DC, Minafra GA, Bottalico IF, Bruno SR, D’Errico ML, Montemurro L, Rizzo M, Barbera L, et al. Efficacy and Safety of Remdesivir over Two Waves of the SARS-CoV-2 Pandemic. Antibiotics. 2021; 10(12):1477. https://doi.org/10.3390/antibiotics10121477
Chicago/Turabian StylePoliseno, Mariacristina, Crescenzio Gallo, Donatella Concetta Cibelli, Graziano Antonio Minafra, Irene Francesca Bottalico, Serena Rita Bruno, Maria Luca D’Errico, Laura Montemurro, Marianna Rizzo, Lucia Barbera, and et al. 2021. "Efficacy and Safety of Remdesivir over Two Waves of the SARS-CoV-2 Pandemic" Antibiotics 10, no. 12: 1477. https://doi.org/10.3390/antibiotics10121477
APA StylePoliseno, M., Gallo, C., Cibelli, D. C., Minafra, G. A., Bottalico, I. F., Bruno, S. R., D’Errico, M. L., Montemurro, L., Rizzo, M., Barbera, L., Custodero, G. E., La Marca, A., Lo Muzio, D., Miucci, A., Santantonio, T. A., & Lo Caputo, S. (2021). Efficacy and Safety of Remdesivir over Two Waves of the SARS-CoV-2 Pandemic. Antibiotics, 10(12), 1477. https://doi.org/10.3390/antibiotics10121477







