Screening for Tuberculosis in Migrants: A Survey by the Global Tuberculosis Network
Abstract
1. Introduction
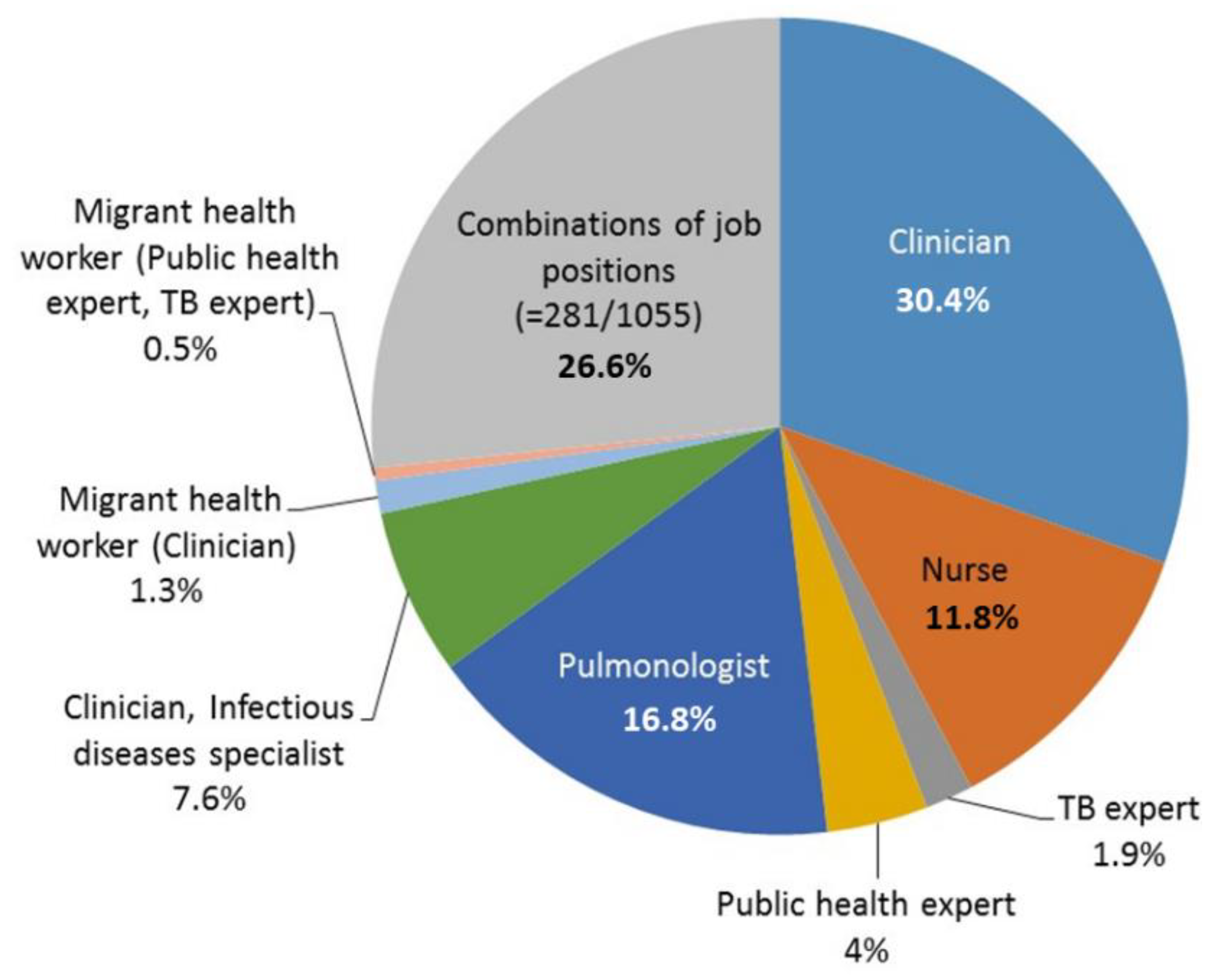
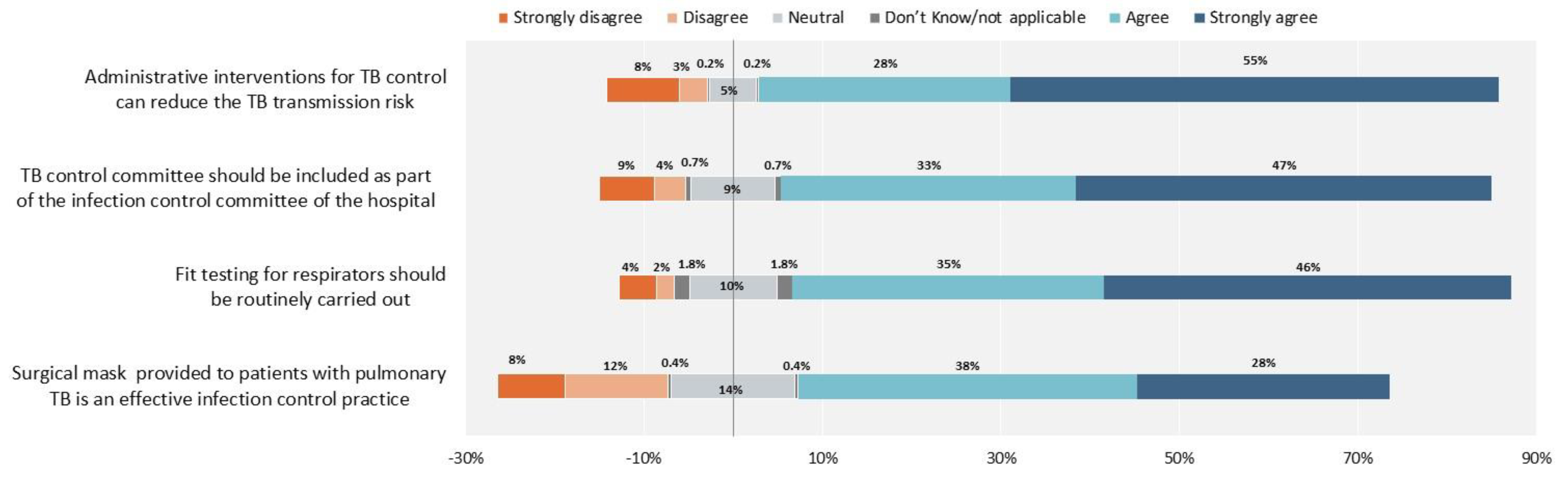
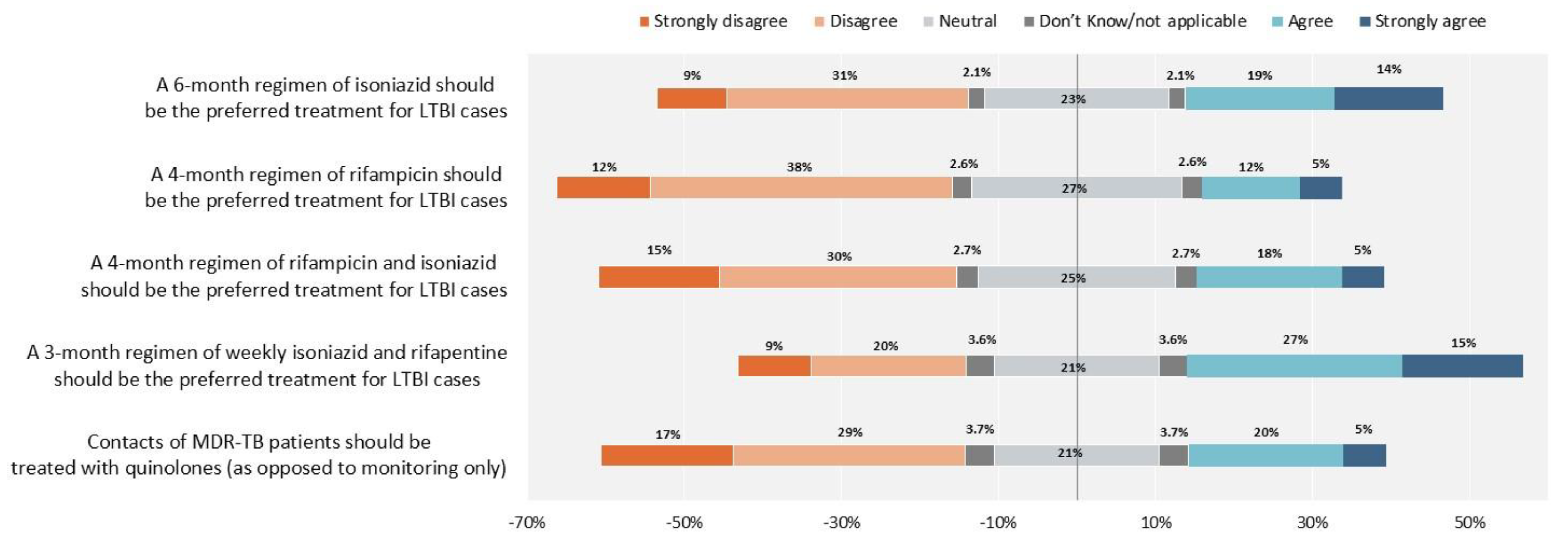
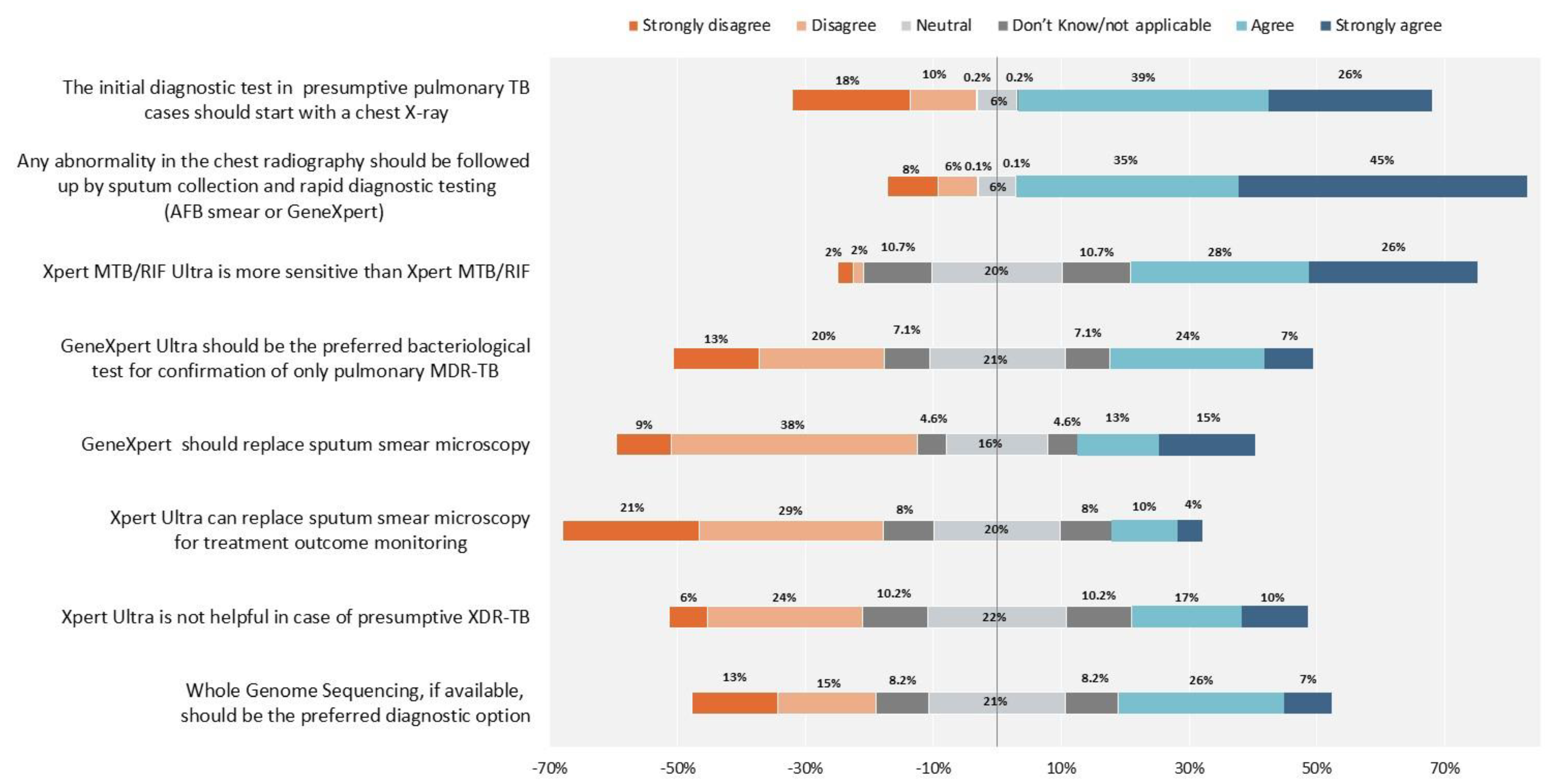
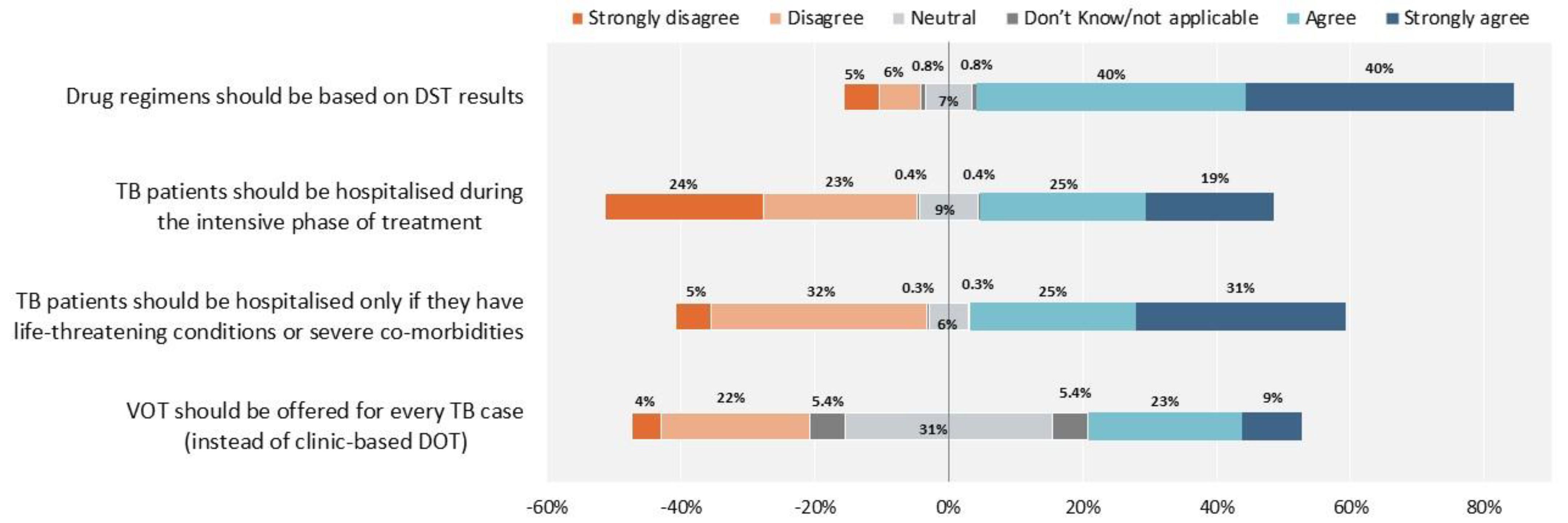
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Questionnaire Translation, Distribution and Data Collection
4.3. Data Analysis
4.4. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 13 September 2021).
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Reestimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- Migliori, G.B.; Tiberi, S.; Zumla, A.; Petersen, E.; Chakaya, J.M.; Wejse, C.; Muñoz Torrico, M.; Duarte, R.; Alffenaar, J.W.; Schaaf, H.S.; et al. MDR/XDR-TB management of patients and contacts: Challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. Int. J. Infect. Dis. 2020, 92S, S15–S25. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe. Roadmap to Implement the Tuberculosis Action Plan for the WHO European Region 2016–2020: Towards Ending Tuberculosis and Multidrug-Resistant Tuberculosis; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2016; Available online: https://www.euro.who.int/__data/assets/pdf_file/0020/318233/50148-WHO-TB-Plan_May17_web.pdf (accessed on 13 September 2021).
- Dara, M.; Gushulak, B.D.; Posey, D.L.; Zellweger, J.-P.; Migliori, G.B. The history and evolution of immigration medical screening for tuberculosis. Expert Rev. Anti infect. Ther. 2013, 11, 137–146. [Google Scholar] [CrossRef]
- Dara, M.; Solovic, I.; Sotgiu, G.; D’Ambrosio, L.; Centis, R.; Tran, R.; Goletti, D.; Duarte, R.; Aliberti, S.; De Benedictis, F.M.; et al. Tuberculosis care among refugees arriving in Europe: A ERS/WHO Europe Region survey of current practices. Eur. Respir. J. 2016, 48, 808–817. [Google Scholar] [CrossRef]
- Dara, M.; Solovic, I.; Sotgiu, G.; D’Ambrosio, L.; Centis, R.; Goletti, D.; Duarte, R.; Aliberti, S.; De Benedictis, F.M.; Bothamley, G.; et al. Statement of the European Respiratory Society (ERS) and the European Region of the International Union Against TB and Lung Disease (The Union): Call for urgent actions to ensure access to early diagnosis and care of tuberculosis among refugees. Eur. Respir. J. 2016, 47, 1345–1347. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control; WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2021–2019 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/tuberculosis-surveillance-monitoring-Europe-2021.pdf (accessed on 13 September 2021).
- Pareek, M.; Greenaway, C.; Noori, T.; Munoz, J.; Zenner, D. The impact of migration on tuberculosis epidemiology and control in high-income countries: A review. BMC Med. 2016, 14, 48. [Google Scholar] [CrossRef]
- Aldridge, R.W.; Zenner, D.; White, P.J.; Williamson, E.J.; Muzyamba, M.C.; Dhavan, P.; Mosca, D.; Thomas, H.L.; Lalor, M.K.; Abubakar, I.; et al. Tuberculosis in migrants moving from high-incidence to low-incidence countries: A population-based cohort study of 519 955 migrants screened before entry to England, Wales, and Northern Ireland. Lancet 2016, 388, 2510–2518. [Google Scholar] [CrossRef]
- Villa, S.; Kasaeva, T.; Raviglione, M.C. A Multisectoral Approach to Tuberculosis Control and Elimination in the Era of the United Nations Sustainable Development Goals. In Essential Tuberculosis; Migliori, G.B., Raviglione, M.C., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 349–358. ISBN 978-3-030-66703-0. [Google Scholar] [CrossRef]
- Denholm, J.T.; Millan-Marcelo, J.C.; Fiekert, K. Latent tuberculosis infection and the EndTB Strategy: Ethical tensions and imperatives. Int. J. Tuberc. Lung Dis. 2020, 24, 21–26. [Google Scholar] [CrossRef]
- Gullón-Blanco, J.-A.; Rodrigo-Sanz, T.; Álvarez-Navascues, F.; Tabernero-Huguet, E.; Sabría-Mestres, J.; García-García, J.-M. Completion of treatment for latent TB infection in a low prevalence setting. Int. J. Tuberc. Lung Dis. 2021, 25, 321–323. [Google Scholar] [CrossRef]
- Lonnroth, K.; Migliori, G.B.; Abubakar, I.; D’Ambrosio, L.; de Vries, G.; Diel, R.; Douglas, P.; Falzon, D.; Gaudreau, M.-A.; Goletti, D.; et al. Towards tuberculosis elimination: An action framework for low-incidence countries. Eur. Respir. J. 2015, 45, 928–952. [Google Scholar] [CrossRef]
- Matteelli, A.; Rendon, A.; Tiberi, S.; Al-Abri, S.; Voniatis, C.; Carvalho, A.C.C.; Centis, R.; D’Ambrosio, L.; Visca, D.; Spanevello, A.; et al. Tuberculosis elimination: Where are we now? Eur. Respir. Rev. 2018, 27, 180035. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Europe. Tuberculosis Elimination in the WHO European Region: Review of Key Actions, with a Special Focus on Tuberculosis Infection Management; Licence: CC BY-NC-SA 3.0 IGO; WHO Regional Office for Europe: Copenhagen, Denmark, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/336973/9789289055314-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. In Module 2: Screening—Systematic Screening for Tuberculosis Disease; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/bitstream/handle/10665/340243/9789240022713-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- World Health Organization. WHO Operational Handbook on Tuberculosis. In Module 2: Screening—Systematic Screening for Tuberculosis Disease; 2021. Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/bitstream/handle/10665/340256/9789240022614-eng.pdf (accessed on 13 September 2021).
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. In Module 1: Prevention—Tuberculosis Preventive Treatment; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/331170/9789240001503-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- World Health Organization. WHO Operational Handbook on Tuberculosis. In Module 1: Prevention—Tuberculosis Preventive Treatment; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/331525/9789240002906-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants within the EU/EEA; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, December 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Public%20health%20guidance%20on%20screening%20and%20vaccination%20of%20migrants%20in%20the%20EU%20EEA.pdf (accessed on 13 September 2021).
- Dobler, C.C.; Fox, G.J.; Douglas, P.; Viney, K.A.; Ahmad Khan, F.; Temesgen, Z.; Marais, B.J. Screening for tuberculosis in migrants and visitors from high-incidence settings: Present and future perspectives. Eur. Respir. J. 2018, 52, 1800591. [Google Scholar] [CrossRef] [PubMed]
- Seedat, F.; Hargreaves, S.; Nellums, L.B.; Ouyang, J.; Brown, M.; Friedland, J.S. How effective are approaches to migrant screening for infectious diseases in Europe? A systematic review. Lancet Infect. Dis. 2018, 18, e259–e271. [Google Scholar] [CrossRef]
- Greenaway, C.; Pareek, M.; Abou Chakra, C.-N.; Walji, M.; Makarenko, I.; Alabdulkarim, B.; Hogan, C.; McConnell, T.; Scarfo, B.; Christensen, R.; et al. The effectiveness and cost-effectiveness of screening for latent tuberculosis among migrants in the EU/EEA: A systematic review. Eurosurveillance 2018, 23, 17–00543. [Google Scholar] [CrossRef]
- Collin, S.M.; de Vries, G.; Lönnroth, K.; Migliori, G.B.; Abubakar, I.; Anderson, S.R.; Zenner, D. Tuberculosis in the European Union and European Economic Area: A survey of national tuberculosis programmes. Eur. Respir. J. 2018, 52, 1801449. [Google Scholar] [CrossRef]
- Collin, S.M.; Wurie, F.; Muzyamba, M.C.; de Vries, G.; Lönnroth, K.; Migliori, G.B.; Abubakar, I.; Anderson, S.R.; Zenner, D. Effectiveness of interventions for reducing TB incidence in countries with low TB incidence: A systematic review of reviews. Eur. Respir. Rev. 2019, 28, 180107. [Google Scholar] [CrossRef]
- Migliori, G.B.; Nardell, E.; Yedilbayev, A.; D’Ambrosio, L.; Centis, R.; Tadolini, M.; van den Boom, M.; Ehsani, S.; Sotgiu, G.; Dara, M. Reducing tuberculosis transmission: A consensus document from the World Health Organization Regional Office for Europe. Eur. Respir. J. 2019, 53, 1900391. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe. Guiding Principles to Reduce Tuberculosis Transmission in the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/342227/9789289053419-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- Sosa, L.E.; Njie, G.J.; Lobato, M.N.; Bamrah Morris, S.; Buchta, W.; Casey, M.L.; Goswami, N.D.; Gruden, M.; Hurst, B.J.; Khan, A.R.; et al. Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 439–443. [Google Scholar] [CrossRef]
- Dobler, C.C.; Farah, W.H.; Alsawas, M.; Mohammed, K.; Breeher, E.L.; Murad, M.H.; Molella, R.G. Tuberculin Skin Test Conversions and Occupational Exposure Risk in US Healthcare Workers. Clin. Infect. Dis. 2018, 66, 706–711. [Google Scholar] [CrossRef]
- World Health Organization. WHO consolidated guidelines on tuberculosis. In Module 4: Treatment—Drug-Resistant Tuberculosis Treatment; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/332397/9789240007048-eng.pdf?sequence=1&isAllowed=y (accessed on 13 September 2021).
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Cegielski, J.P.; Chen, L.; Daley, C.L.; et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e93–e142, Erratum in Am. J. Respir. Crit. Care Med. 2020, 201, 500–501. [Google Scholar] [CrossRef]
- Méchaï, F.; Figoni, J.; Wyplosz, B.; Aoun, O.; Bouchaud, O.; Robert, J. Survey of French physician practices in treatment and control of transmission of smear-positive tuberculosis. Int. J. Tuberc. Lung Dis. 2015, 19, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Fraisse, P.; Cordel, H.; Charlois, C.; Méchaï, F.; Ibanez, G.; Hargreaves, S.; Mechain, M.; Nicolas Vignier, N. Screening for active and latent tuberculosis among migrants in France: A national study of practice. Int. J. Tuberc. Lung Dis. 2021, in press. [Google Scholar] [CrossRef]
- Gutsfeld, C.; Olaru, I.D.; Vollrath, O.; Lange, C. Attitudes about Tuberculosis Prevention in the Elimination Phase: A Survey among Physicians in Germany. PLoS ONE 2014, 9, e112681. [Google Scholar] [CrossRef]
- Pareek, M.; Abubakar, I.; White, P.J.; Garnett, G.P.; Lalvani, A. Tuberculosis screening of migrants to low-burden nations: Insights from evaluation of UK practice. Eur. Respir. J. 2011, 37, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Dobler, C.C.; Codecasa, L.R. Tuberculosis and Migration. In Essential Tuberculosis; Migliori, G.B., Raviglione, M.C., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 349–358. ISBN 978-3-030-66703-0. [Google Scholar] [CrossRef]
- Kaushik, N.; Lowbridge, C.; Scandurra, G.; Dobler, C.C. Post-migration follow-up programme for migrants at increased risk of developing tuberculosis: A cohort study. ERJ Open Res. 2018, 4, 00008–02018. [Google Scholar] [CrossRef]
- Chan, I.H.Y.; Kaushik, N.; Dobler, C.C. Post-migration follow-up of migrants identified to be at increased risk of developing tuberculosis at pre-migration screening: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 770–779. [Google Scholar] [CrossRef]
- Scandurra, G.; Degeling, C.; Douglas, P.; Dobler, C.C.; Marais, B. Tuberculosis in migrants—Screening, surveillance and ethics. Pneumonia 2020, 12, 9. [Google Scholar] [CrossRef]
- Lakhani, P.; Sundaram, B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017, 284, 574–582. [Google Scholar] [CrossRef]
- Dara, M.; Sulis, G.; Centis, R.; D’Ambrosio, L.; de Vries, G.; Douglas, P.; Garcia, D.; Jansen, N.; Zuroweste, E.; Migliori, G.B. Cross-border collaboration for improved tuberculosis prevention and care: Policies, tools and experiences. Int. J. Tuberc. Lung Dis. 2017, 21, 727–736. [Google Scholar] [CrossRef]
- D’Ambrosio, L.; Dara, M.; Tadolini, M.; Centis, R.; Sotgiu, G.; van der Werf, M.J.; Gaga, M.; Cirillo, D.; Spanevello, A.; Raviglione, M.; et al. Tuberculosis elimination: Theory and practice in Europe. Eur. Respir. J. 2014, 43, 1410–1420. [Google Scholar] [CrossRef]
- Dobler, C.C. Screening strategies for active tuberculosis: Focus on cost-effectiveness. Clin. Outcomes Res. 2016, 8, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Shete, P.B.; Boccia, D.; Dhavan, P.; Gebreselassie, N.; Lönnroth, K.; Marks, S.; Matteelli, A.; Posey, D.L.; Van Der Werf, M.J.; Winston, C.A.; et al. Defining a migrant-inclusive tuberculosis research agenda to end TB. Int. J. Tuberc. Lung Dis. 2018, 22, 835–843, Erratum in Int. J. Tuberc. Lung Dis. 2018, 22, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lönnroth, K.; Mor, Z.; Erkens, C.; Bruchfeld, J.; Nathavitharana, R.R.; Van Der Werf, M.J.; Lange, C. Tuberculosis in migrants in low-incidence countries: Epidemiology and intervention entry points. Int. J. Tuberc. Lung Dis. 2017, 21, 624–636. [Google Scholar] [CrossRef]
- Öhd, J.N.; Lönnroth, K.; Abubakar, I.; Aldridge, R.W.; Erkens, C.; Jonsson, J.; Marchese, V.; Matteelli, A.; Menezes, D.; Zenner, D.; et al. Building a European database to gather multi-country evidence on active and latent TB screening for migrants. Int. J. Infect. Dis. 2019, 80S, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Amicosante, M.; D’Ambrosio, L.; Munoz, M.; Mello, F.C.D.Q.; Tebruegge, M.; Chegou, N.N.; Seghrouchni, F.; Centis, R.; Goletti, D.; Bothamley, G.; et al. Current use and acceptability of novel diagnostic tests for active tuberculosis: A worldwide survey. J. Bras. Pneumol. 2017, 43, 380–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Migliori, G.B.; Marx, F.M.; Ambrosino, N.; Zampogna, E.; Schaaf, H.S.; van der Zalm, M.M.; Allwood, B.; Byrne, A.L.; Mortimer, K.; Wallis, R.S.; et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int. J. Tuberc. Lung Dis. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; Sotgiu, G.; Rosales-Klintz, S.; Centis, R.; D’Ambrosio, L.; Abubakar, I.; Bothamley, G.; Caminero, J.A.; Cirillo, D.M.; Dara, M.; et al. ERS/ECDC Statement: European Union standards for tuberculosis care, 2017 update. Eur. Respir. J. 2018, 51, 1702678. [Google Scholar] [CrossRef]
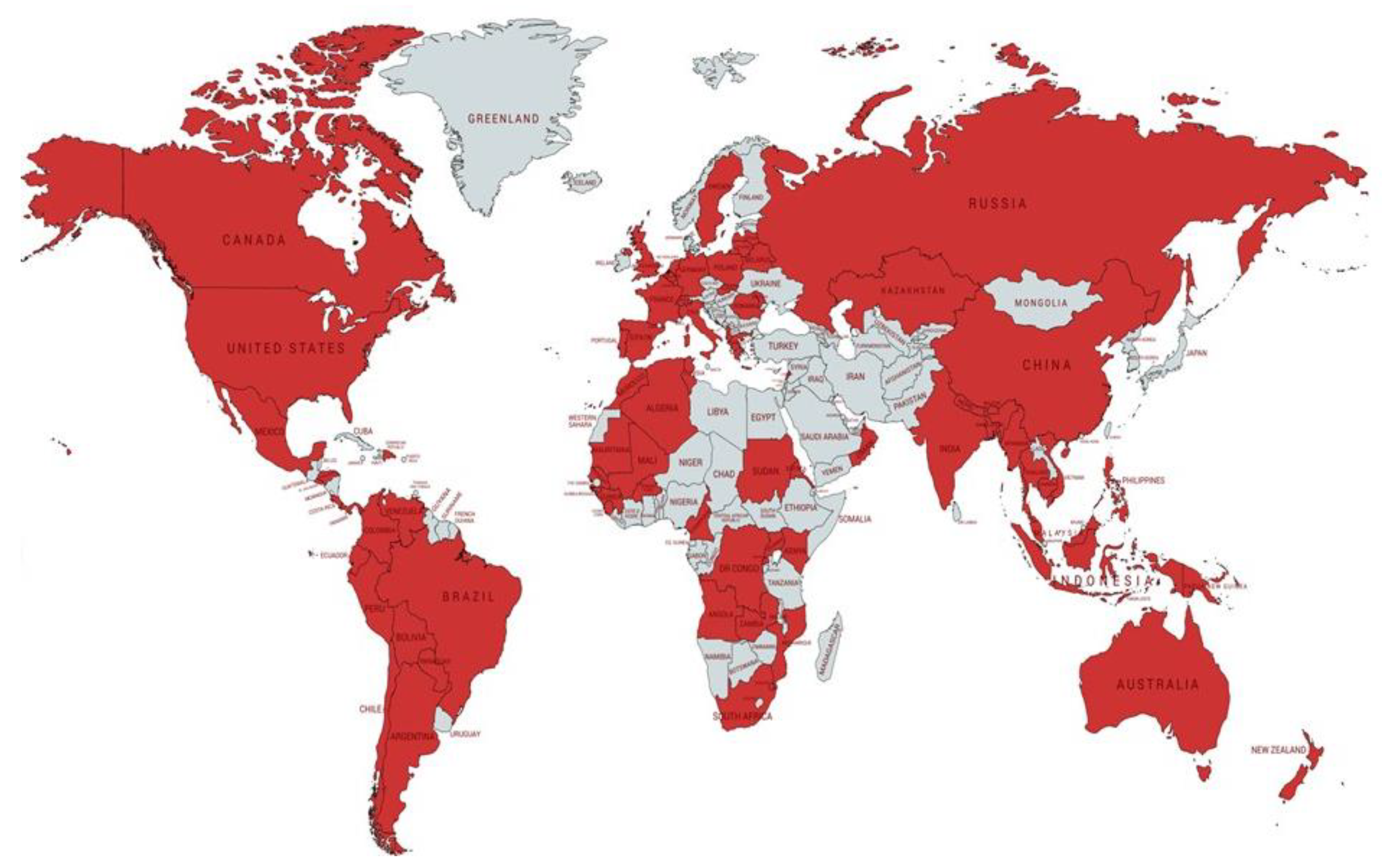


| Country You Spend the Majority of Your Work Relating to TB | ||
|---|---|---|
| Albania 1 | Greece 7 | Peru 21 |
| Algeria 8 | Guinea 1 | Philippines 1 |
| Angola 1 | Guinea Bissau 1 | Poland 1 |
| Argentina 20 | Honduras 1 | Portugal 7 |
| Australia 20 | India 5 | R. of Moldova 1 |
| Bangladesh 2 | Indonesia 3 | Romania 2 |
| Belarus 1 | Italy 29 | Russian Federation 297 |
| Belgium 1 | Kazakhstan 1 | Rwanda 1 |
| Bhutan 1 | Kenya 1 | Senegal 3 |
| Bolivia 1 | Latvia 1 | Sierra Leone 1 |
| Brazil 188 | Lebanon 3 | Slovakia 10 |
| Burkina Faso 1 | Lithuania 18 | South Africa 4 |
| Cambodia 2 | Luxembourg 1 | Spain 20 |
| Cameroon 2 | Malaysia 1 | Sudan 5 |
| Chile 7 | Mali 1 | Sweden 2 |
| China 20 | Mauritania 1 | Switzerland 5 |
| Colombia 4 | Mexico 24 | Thailand 1 |
| Congo 2 | Morocco 1 | Timor-Este 1 |
| Canada 2 | Mozambique 3 | Tunisia 13 |
| Costa Rica 1 | Myanmar 1 | Uganda 1 |
| Dominican Republic 3 | Nepal 4 | United Kingdom 2 |
| Ecuador 53 | Netherlands 8 | USA 11 |
| El Salvador 1 | New Zealand 1 | Vanuatu Islands 1 |
| Eritrea 1 | Oman 16 | Venezuela 2 |
| Eswatini 1 | Panama 1 | Vietnam 2 |
| France 155 | Papua New Guinea 1 | Zambia 1 |
| Germany 2 | Paraguay 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, L.; Centis, R.; Dobler, C.C.; Tiberi, S.; Matteelli, A.; Denholm, J.; Zenner, D.; Al-Abri, S.; Alyaquobi, F.; Arbex, M.A.; et al. Screening for Tuberculosis in Migrants: A Survey by the Global Tuberculosis Network. Antibiotics 2021, 10, 1355. https://doi.org/10.3390/antibiotics10111355
D’Ambrosio L, Centis R, Dobler CC, Tiberi S, Matteelli A, Denholm J, Zenner D, Al-Abri S, Alyaquobi F, Arbex MA, et al. Screening for Tuberculosis in Migrants: A Survey by the Global Tuberculosis Network. Antibiotics. 2021; 10(11):1355. https://doi.org/10.3390/antibiotics10111355
Chicago/Turabian StyleD’Ambrosio, Lia, Rosella Centis, Claudia C. Dobler, Simon Tiberi, Alberto Matteelli, Justin Denholm, Dominik Zenner, Seif Al-Abri, Fatma Alyaquobi, Marcos Abdo Arbex, and et al. 2021. "Screening for Tuberculosis in Migrants: A Survey by the Global Tuberculosis Network" Antibiotics 10, no. 11: 1355. https://doi.org/10.3390/antibiotics10111355
APA StyleD’Ambrosio, L., Centis, R., Dobler, C. C., Tiberi, S., Matteelli, A., Denholm, J., Zenner, D., Al-Abri, S., Alyaquobi, F., Arbex, M. A., Belilovskiy, E., Blanc, F.-X., Borisov, S., Carvalho, A. C. C., Chakaya, J. M., Cocco, N., Codecasa, L. R., Dalcolmo, M. P., Dheda, K., ... Migliori, G. B. (2021). Screening for Tuberculosis in Migrants: A Survey by the Global Tuberculosis Network. Antibiotics, 10(11), 1355. https://doi.org/10.3390/antibiotics10111355















