Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm
Abstract
:1. Introduction
2. Results
2.1. Ceftazidime/Avibactam
2.2. Meropenem/Vaborbactam
2.3. Cefiderocol
| Antibiotic | Mechanism of Action | In-Vitro Activity | Emergence of Resistance | Use in Combination | Disadvantages | Comments |
|---|---|---|---|---|---|---|
| Ceftazidime/avibactam | β-lactam/β-lactamase inhibitor Avibactam is a non-β-lactam inhibitor through a reversible mechanism that regenerates an intact molecule of avibactam, allowing for inhibition of further enzymes | -Enterobacterales • ESBL • CTX-M • CPE (KPC, OXA-48) -MDR/XDR P. aeruginosa -S. maltophilia Synergism with carbapenems, tigecycline and fosfomycin against KPC-producing K. pneumoniae [80,81] Synergism with fosfomycin against P. aeruginosa | Yes (point mutations in the Ω-loop of lactamase; mutations in Omp35 and Omp36 porin channels and efflux pumps overexpression) Risk of in vivo resistance: -CRRT -pneumonia -septic thrombosis -delayed/absence of source control | Used as monotherapy or in combination No definite evidence on superiority of monotherapy over combination therapy In real-life studies, mostly used in combination | -No activity against MBLs -No activity against CRAB -Emergence of resistance | Decreasing susceptibility to this agent during treatment represents a significant concern Combination with aztreonam restores activity against MBL ELF concentration is 30% compared to plasma concentration [42] |
| Meropenem/vaborbactam | β-lactam/β-lactamase inhibitor Vaborbactam is a competitive inhibitor with a boron-ring structure with high activity towards KPC (Kiapp = 69 nM) | -Enterobacterales • ESBL • CTX-M • CPE (KPC) | Yes (increased KPC production and porin mutations) Less emergence of resistance than ceftazidime/avibactam | Mostly used as monotherapy | -No activity against MBL -No activity against OXA-48 -No activity against XDR-P. aeruginosa -No activity against CRAB | Reduced emergence of resistance as compared to ceftazidime/avibactam Combination with aztreonam restores activity against MBL ELF concentrations are 65% (meropenem) and 79% (vaborbactam) compared to plasma concentration |
| Cefiderocol | Siderophore-conjugated cephalosporin actively taken up by iron transporter | -Enterobacterales • ESBL • CTX-M • CPE (KPC, OXA-48, MBL) -MDR/XDR-Pa -S. maltophilia -CRAB -B. cepaciae | Yes (mutations in iron transporters) Risk of in vivo resistance due to increased copy numbers of bla NDM genes | Used as monotherapy or in combination | Suboptimal activity against NDM | Combination treatment may probably be necessary in high inoculum infections Due to suboptimal activity against NDM, combination with aztreonam should be considered Anti-biofilm activity |
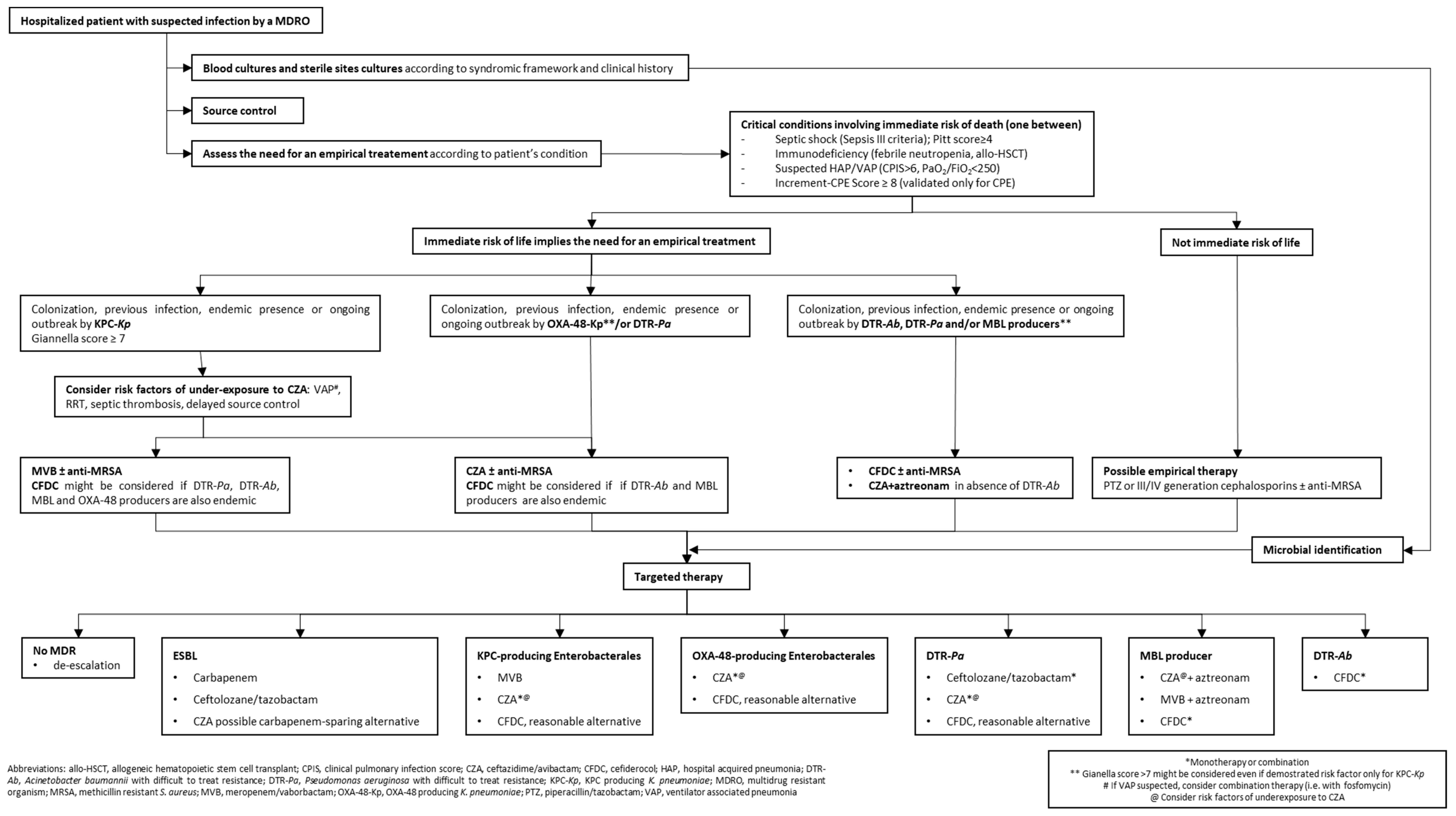
3. Algorithm Construction
3.1. Sample Collection, Source Control, Severity of Infection and Risk of Death
3.2. Risk Factors, Colonization, and Ecology
3.3. Identification, De-Escalation, and Combination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Website, Newsroom, Antibiotic Resistance 31 July 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 20 October 2021).
- European Centre for Disease Prevention and Control. Carbapenem-Resistant Enterobacteriaceae. Second Update—26 September 2019. ECDC: Stockholm. 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/carbapenem-resistant-Enterobacteriaceae-second-update#no-link (accessed on 20 October 2021).
- Haller, S.; Kramer, R.; Becker, K.; Bohnert, J.A.; Eckmanns, T.; Hans, J.B.; Hecht, J.; Heidecke, C.D.; Hübner, N.O.; Kramer, A.; et al. Extensively drug-resistant Klebsiella pneumoniae ST307 outbreak, north-eastern Germany, June to October 2019. Eur. Surveill. 2019, 24, 1900734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Cangini, A.; Fortinguerra, F.; Di Filippo, A.; Pierantozzi, A.; Da Cas, R.; Villa, F.; Trotta, F.; Moro, M.L.; Gagliotti, C. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br. J. Clin. Pharmacol. 2021, 87, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassetti, M.; Labate, L.; Russo, C.; Vena, A.; Giacobbe, D.R. Therapeutic options for difficult-to-treat Acinetobacter baumannii infections: A 2020 perspective. Expert Opin. Pharmacother. 2021, 22, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mammina, C.; Palma, D.M.; Bonura, C.; Plano, M.R.A.; Monastero, R.; Sodano, C.; Calà, C.; Tetamo, R. Outbreak of infection with Klebsiella pneumoniae sequence type 258 producing Klebsiella pneumoniae Carbapenemase 3 in an intensive care unit in Italy. J. Clin. Microbiol. 2010, 48, 1506–1507. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Quirino, A.; Giordano, M.; Marano, V.; Rizzo, C.; Liberto, M.C.; Focà, A.; Pavia, M. Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC Infect. Dis. 2016, 16, 747. [Google Scholar] [CrossRef] [Green Version]
- Agodi, A.; Barchitta, M.; Auxilia, F.; Brusaferro, S.; D’Errico, M.M.; Montagna, M.T.; Pasquarella, C.; Tardivo, S.; Arrigoni, C.; Fabiani, L. Collaborators. Epidemiology of intensive care unit-acquired sepsis in Italy: Results of the SPIN-UTI network. Ann. Ig. 2018, 5 (Suppl. S2), 15–21. [Google Scholar] [CrossRef]
- Moniz, P.; Coelho, L.; Póvoa, P. Antimicrobial Stewardship in the Intensive Care Unit: The Role of Biomarkers, Pharmacokinetics, and Pharmacodynamics. Adv. Ther. 2021, 38, 164–179. [Google Scholar] [CrossRef]
- Soman, R.; Bakthavatchalam, Y.D.; Nadarajan, A.; Dwarakanathan, H.T.; Venkatasubramanian, R.; Veeraraghavan, B. Is it time to move away from polymyxins? Evidence and alternatives. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 461–475. [Google Scholar] [CrossRef]
- WHO Website, News, WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 27 February 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 20 October 2021).
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S565–S575. [Google Scholar] [CrossRef] [Green Version]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Papp-Wallace, K.M.; Mack, A.R.; Taracila, M.A.; Bonomo, R.A. Resistance to Novel β-Lactam-β-Lactamase Inhibitor Combinations: The “Price of Progress”. Infect. Dis. Clin. North Am. 2020, 34, 773–819. [Google Scholar] [CrossRef]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef]
- EMA Website, European Medicines Agency: Zavicefta Medicine Overview. Last Updated 20 November 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zavicefta (accessed on 20 October 2021).
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [Green Version]
- Agyeman, A.A.; Bergen, P.J.; Rao, G.G.; Nation, R.L.; Landersdorfer, C.B. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents 2020, 55, 105833. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients with Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, C.; Zygoulis, P.; Papadonta, M.E.; Petinaki, E.; et al. Ceftazidime-Avibactam to Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically Ill Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 2020, 64, e02320-19. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Daikos, G.L.; Gkoufa, A.; Adamis, G.; Stefos, A.; Symbardi, S.; Chrysos, G.; Filiou, E.; Basoulis, D.; Mouloudi, E.; et al. Hellenic Ceftazidime/Avibactam Registry Study Group. Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: Experience from a national registry study. J. Antimicrob. Chemother. 2021, 76, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-avibactam use for KPC-Kp infections: A retrospective observational multicenter study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef]
- Aktaş, Z.; Kayacan, C.; Oncul, O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2012, 39, 86–89. [Google Scholar] [CrossRef] [PubMed]
- De la Calle, C.; Rodríguez, O.; Morata, L.; Marco, F.; Cardozo, C.; García-Vidal, C.; Del Río, A.; Feher, C.; Pellicè, M.; Puerta-Alcalde, P.; et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int. J. Antimicrob. Agents 2019, 53, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.B.; Brigman, H.V.; Zucchi, P.C.; Chen, A.; Anderson, J.C.; Eliopoulos, G.M.; Cheung, N.; Gilbertsen, A.; Hunter, R.C.; Emery, C.L.; et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against β-lactam-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Enterobacterales clinical isolates from U.S. medical centres. J. Glob. Antimicrob. Resist. 2020, 22, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Duncan, L.R.; Mendes, R.E. Antimicrobial activities of ceftazidime/avibactam, ceftolozane/tazobactam, imipenem/relebactam, meropenem/vaborbactam, and comparators against Pseudomonas aeruginosa from patients with skin and soft tissue infections [published online ahead of print, 2021 Oct 17]. Int. J. Infect. Dis. 2021, 113, 279–281. [Google Scholar] [CrossRef]
- Kristóf, K.; Adámková, V.; Adler, A.; Gospodarek-Komkowska, E.; Rafila, A.; Billová, S.; Możejko-Pastewka, B.; Kiss, F. In vitro activity of ceftazidime-avibactam and comparators against Enterobacterales and Pseudomonas aeruginosa isolates from Central Europe and Israel, 2014–2017 and 2018. Diagn. Microbiol. Infect. Dis. 2021, 101, 115420. [Google Scholar] [CrossRef]
- EMA Website. European Medicines Agency. GLOBAL RECALL: Zerbaxa (Ceftolozane/Tazobactam) 1 g/0.5 g Powder for Concentrate for Solution for Infusion. 22 December 2020. Available online: https://www.ema.europa.eu/en/medicines/dhpc/global-recall-zerbaxa-ceftolozane-tazobactam-1-g05-g-powder-concentrate-solution-infusion (accessed on 20 October 2021).
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metallo-β-lactamase-Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Menichetti, F.; Cattaneo, D.; Tiseo, G.; Baldelli, S.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Di Paolo, A.; Pai, M.P. Pragmatic options for dose optimization of ceftazidime/avibactam with aztreonam in complex patients. J. Antimicrob. Chemother. 2021, 76, 1025–1031. [Google Scholar] [CrossRef]
- Lin, Q.; Zou, H.; Chen, X.; Wu, M.; Ma, D.; Yu, H.; Niu, S.; Huang, S. Avibactam potentiated the activity of both ceftazidime and aztreonam against S. maltophilia clinical isolates in vitro. BMC Microbiol. 2021, 21, 60. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Giacobbe, D.R.; Maraolo, A.E.; Viaggi, V.; Luzzati, R.; Bassetti, M.; Luzzaro, F.; Principe, L. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: A systematic review of observational clinical studies. J. Glob. Antimicrob. Resist. 2021, 25, 268–281. [Google Scholar] [CrossRef]
- Carattoli, A.; Arcari, G.; Bibbolino, G.; Sacco, F.; Tomolillo, D.; Di Lella, F.M.; Trancassini, M.; Faino, L.; Venditti, M.; Antonelli, G.; et al. Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates. Antimicrob. Agents Chemother. 2021, 2, AAC0057421. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; De Rosa, F.G.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatti, M.; Pea, F. Antimicrobial Dose Reduction in Continuous Renal Replacement Therapy: Myth or Real Need? A Practical Approach for Guiding Dose Optimization of Novel Antibiotics. Clin. Pharmacokinet. 2021, 60, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P.; Siew, L.; Armstrong, J.; Li, J.; Edeki, T.; Learoyd, M.; Das, S. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J. Antimicrob. Chemother. 2015, 70, 2862–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkhout, J.; Melchers, M.J.; van Mil, A.C.; Seyedmousavi, S.; Lagarde, C.M.; Nichols, W.W.; Mouton, J.W. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob. Agents Chemother. 2015, 59, 2299–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, A.; Curtolo, A.; Volpicelli, L.; Cogliati Dezza, F.; De Angelis, M.; Cairoli, S.; Dell’Utri, D.; Goffredo, B.M.; Raponi, G.; Venditti, M. Synergistic Meropenem/Vaborbactam Plus Fosfomycin Treatment of KPC Producing K. pneumoniae Septic Thrombosis Unresponsive to Ceftazidime/Avibactam: From the Bench to the Bedside. Antibiotics 2021, 10, 781. [Google Scholar] [CrossRef]
- Tiseo, G.; Falcone, M.; Leonildi, A.; Giordano, C.; Barnini, S.; Arcari, G.; Carattoli, A.; Menichetti, F. Meropenem-Vaborbactam as Salvage Therapy for Ceftazidime-Avibactam-, Cefiderocol-Resistant ST-512 Klebsiella pneumoniae-Producing KPC-31, a D179Y Variant of KPC-3. Open Forum Infect. Dis. 2021, 8, ofab141. [Google Scholar] [CrossRef]
- Pogue, J.M.; Bonomo, R.A.; Kaye, K.S. Ceftazidime/Avibactam, Meropenem/Vaborbactam, or Both? Clinical and Formulary Considerations. Clin. Infect. Dis. 2019, 68, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzler, E.; Scoble, P.J. An Appraisal of the Pharmacokinetic and Pharmacodynamic Properties of Meropenem-Vaborbactam. Infect. Dis. Ther. 2020, 9, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Huband, M.D.; Mendes, R.E.; Flamm, R.K. Meropenem-Vaborbactam Tested against Contemporary Gram-Negative Isolates Collected Worldwide during 2014, Including Carbapenem-Resistant, KPC-Producing, Multidrug-Resistant, and Extensively Drug-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e00567-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, G.W.; Cain, R.; Tyrrell, J.M.; Hinchliffe, P.; Calvopiña, K.; Tooke, C.L.; Widlake, E.; Dowson, C.G.; Spencer, J.; Walsh, T.R.; et al. Profiling interactions of vaborbactam with metallo-β-lactamases. Bioorg. Med. Chem. Lett. 2019, 29, 1981–1984. [Google Scholar] [CrossRef]
- Hackel, M.A.; Lomovskaya, O.; Dudley, M.N.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of Meropenem-Vaborbactam against Clinical Isolates of KPC-Positive Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 62, e01904-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquex, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA 2018, 319, 788–799. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Giacobbe, D.R.; Patel, N.; Tillotson, G.; Massey, J. Efficacy and Safety of Meropenem-Vaborbactam versus Best Available Therapy for the Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Patients without Prior Antimicrobial Failure: A Post Hoc Analysis. Adv. Ther. 2019, 36, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- EMA Website, European Medicines Agency: Vaborem Medicine Overview. Last Updated 20 September 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/vabomere-epar-public-assessment-report_en.pdf (accessed on 20 October 2021).
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e01694-17. [Google Scholar] [CrossRef] [Green Version]
- Ackley, R.; Roshdy, D.; Meredith, J.; Minor, S.; Anderson, W.E.; Capraro, G.A.; Polk, C. Meropenem-Vaborbactam versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2020, 64, e02313-19. [Google Scholar] [CrossRef]
- Bhowmick, T.; Weinstein, M.P. Microbiology of Meropenem-Vaborbactam: A Novel Carbapenem Beta-Lactamase Inhibitor Combination for Carbapenem-Resistant Enterobacterales Infections. Infect. Dis. Ther. 2020, 9, 757–767. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S538–S543. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Valverde, M.; Conejo, M.D.C.; Serrano, L.; Fernández-Cuenca, F.; Pascual, Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2020, 75, 1840–1849. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ko, W.C.; Lee, W.S.; Lu, P.L.; Chen, Y.H.; Cheng, S.H.; Lu, M.C.; Lin, C.Y.; Wu, T.S.; Yen, M.Y.; et al. In-vitro activity of cefiderocol, cefepime/zidebactam, cefepime/enmetazobactam, omadacycline, eravacycline and other comparative agents against carbapenem-nonsusceptible Enterobacterales: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2017–2020. Int. J. Antimicrob. Agents 2021, 58, 06377. [Google Scholar] [CrossRef]
- Candel, F.J.; Henriksen, A.S.; Longshaw, C.; Yamano, Y.; Oliver, A. In vitro activity of the novel siderophore cephalosporin, cefiderocol, in Gram-negative pathogens in Europe by site of infection. Clin. Microbiol. Infect. 2021. [CrossRef]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In Vitro Antibacterial Activity of Cefiderocol against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0264620. [Google Scholar] [CrossRef]
- Pybus, C.A.; Felder-Scott, C.; Obuekwe, V.; Greenberg, D.E. Cefiderocol Retains Antibiofilm Activity in Multidrug-Resistant Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2021, 65, e01194-20. [Google Scholar] [CrossRef]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef]
- Zalacain, M.; Lozano, C.; Llanos, A.; Sprynski, N.; Valmont, T.; De Piano, C.; Davies, D.; Leiris, S.; Sable, C.; Ledoux, A.; et al. Novel Specific Metallo-β-Lactamase Inhibitor ANT2681 Restores Meropenem Activity to Clinically Effective Levels against NDM-Positive Enterobacterales. Antimicrob. Agents Chemother. 2021, 65, e00203-21. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive Development of Cefiderocol Resistance in Escherichia coli During Therapy Is Associated with Increased blaNDM-5 Copy Number and Gene Expression. Clin. Infect. Dis. 2021, ciab888. [Google Scholar] [CrossRef]
- Portsmouth, S.; van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Heil, E.L.; Tamma, P.D. Cefiderocol: The Trojan horse has arrived but will Troy fall? Lancet Infect. Dis. 2021, 21, 153–155. [Google Scholar] [CrossRef]
- Dickstein, Y.; Lellouche, J.; Ben Dalak Amar, M.; Schwartz, D.; Nutman, A.; Daitch, V.; Yahav, D.; Leibovici, L.; Skiada, A.; Antoniadou, A.; et al. AIDA Study Group. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin. Infect. Dis. 2019, 69, 769–776. [Google Scholar] [CrossRef]
- Piperaki, E.T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Oliva, A.; Ceccarelli, G.; De Angelis, M.; Sacco, F.; Miele, M.C.; Mastroianni, C.M.; Venditti, M. Cefiderocol for compassionate use in the treatment of complicated infections caused by extensively and pan-resistant Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2020, 23, 292–296. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 2021, 72, 2021–2024. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A descriptive case series of PK/PD target attainment and microbiological outcome in critically ill patients with documented severe XDR Acinetobacter baumannii BSI and/or VAP treated with cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef]
- Siméon, S.; Dortet, L.; Bouchand, F.; Roux, A.L.; Bonnin, R.A.; Duran, C.; Decousser, J.W.; Bessis, S.; Davido, B.; Sorriaux, G.; et al. Compassionate Use of Cefiderocol to Treat a Case of Prosthetic Joint Infection Due to Extensively Drug-Resistant Enterobacter hormaechei. Microorganisms 2020, 8, 1236. [Google Scholar] [CrossRef]
- Mabayoje, D.A.; NicFhogartaigh, C.; Cherian, B.P.; Tan, M.G.M.; Wareham, D.W. Compassionate use of cefiderocol for carbapenem-resistant Acinetobacter baumannii prosthetic joint infection. JAC Antimicrob. Resist. 2021, 3 (Suppl. S1), i21–i24, Erratum in JAC Antimicrob Resist. 2021, 3, dlab109. Erratum in JAC Antimicrob. Resist. 2021, 3, dlab110. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Vialichka, A.; Jurkovic, M.; Wu, T.; Shajee, A.; Lee, M.; Patel, S.; Mendes, R.E.; Wenzler, E. Activity of Cefiderocol Alone and in Combination with Levofloxacin, Minocycline, Polymyxin B, or Trimethoprim-Sulfamethoxazole against Multidrug-Resistant Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2020, 64, e00559-20. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Lewis, R.E.; Volpe, S.L.; Giannella, M.; Campoli, C.; Landini, M.P.; Viale, P.; Re, M.C.; Ambretti, S. In vitro interaction of ceftazidime-avibactam in combination with different antimicrobials against KPC-producing Klebsiella pneumoniae clinical isolates. Int. J. Infect. Dis. 2017, 65, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanelli, F.; De Robertis, A.; Carone, G.; Dalfino, L.; Stufano, M.; Del Prete, R.; Mosca, A. In Vitro Activity of Ceftazidime/Avibactam Alone and in Combination with Fosfomycin and Carbapenems Against KPC-producing Klebsiella pneumoniae. New Microbiol. 2020, 43, 136–138. [Google Scholar] [PubMed]
- Molina, J.; Peñalva, G.; Gil-Navarro, M.V.; Praena, J.; Lepe, J.A.; Pérez-Moreno, M.A.; Ferràndiz, C.; Aldabò, T.; Aguilar, M.; Olbrich, P.; et al. PRIOAM team. Long-Term Impact of an Educational Antimicrobial Stewardship Program on Hospital-Acquired Candidemia and Multidrug-Resistant Bloodstream Infections: A Quasi-Experimental Study of Interrupted Time-Series Analysis. Clin. Infect. Dis. 2017, 65, 1992–1999. [Google Scholar] [CrossRef] [Green Version]
- Butt, A.A.; Al Kaabi, N.; Saifuddin, M.; Krishnanreddy, K.M.; Khan, M.; Jasim, W.H.; Khan, T.; Sara, M.; Pitout, M.; Weber, S. Impact of Infectious Diseases Team Consultation on Antimicrobial Use, Length of Stay and Mortality. Am. J. Med. Sci. 2015, 350, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aguilar, P.; López-Cortés, L.E.; Rodríguez-Baño, J. Impact of infectious diseases consultation on the outcome of patients with bacteraemia. Ther. Adv. Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Pintado, V.; et al. Investigators from the REIPI/ESGBIS/INCREMENT Group. A Predictive Model of Mortality in Patients with Bloodstream Infections due to Carbapenemase-Producing Enterobacteriaceae. Mayo Clin. Proc. 2016, 91, 1362–1371, Erratum in Mayo Clin Proc. 2016, 91, 1843. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Corcia-Palomo, Y.; Amaya-Villar, R.; Martin-Villen, L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect. Dis. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Tran, A.; Cheng, W.; Klompas, M.; Kyeremanteng, K.; Mehta, S.; English, S.W.; Muscedere, J.; Cook, D.J.; Torres, A.; et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Karakuzu, Z.; Iscimen, R.; Akalin, H.; Kelebek Girgin, N.; Kahveci, F.; Sinirtas, M. Prognostic Risk Factors in Ventilator-Associated Pneumonia. Med. Sci. Monit. 2018, 24, 1321–1328. [Google Scholar] [CrossRef] [Green Version]
- Falcone, M.; Bassetti, M.; Tiseo, G.; Giordano, C.; Nencini, E.; Russo, A.; Graziano, E.; Tagliaferri, E.; Leonildi, A.; Barnini, S.; et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 2020, 24, 29. [Google Scholar] [CrossRef] [Green Version]
- Alosaimy, S.; Lagnf, A.M.; Morrisette, T.; Scipione, M.R.; Zhao, J.J.; Jorgensen, S.C.J.; Mynatt, R.; Carlson, T.J.; Jo, J.; Garey, K.W.; et al. Real-world, Multicenter Experience With Meropenem-Vaborbactam for Gram-Negative Bacterial Infections Including Carbapenem-Resistant Enterobacterales and Pseudomonas aeruginosa. Open Forum Infect. Dis. 2021, 8, ofab371. [Google Scholar] [CrossRef] [PubMed]
- Henderson, H.; Luterbach, C.L.; Cober, E.; Richter, S.S.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; Kaye, K.S.; Evans, S.; et al. The Pitt Bacteremia Score Predicts Mortality in Nonbacteremic Infections. Clin. Infect. Dis. 2020, 70, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Baddour, L.M. Resilience of the Pitt Bacteremia Score: 3 Decades and Counting. Clin. Infect. Dis. 2020, 70, 1834–1836, Erratum in Clin. Infect. Dis. 2019, 69, 2238. [Google Scholar] [CrossRef]
- Recio, R.; Mancheño, M.; Viedma, E.; Villa, J.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Predictors of Mortality in Bloodstream Infections Caused by Pseudomonas aeruginosa and Impact of Antimicrobial Resistance and Bacterial Virulence. Antimicrob. Agents Chemother. 2020, 64, e01759-19. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, Y.; Zhang, W.; Yu, Y.; He, X.; Tao, J.; Hou, X.; Wang, H.; Deng, M.; Zhou, M.; et al. Risk factors and outcomes of bloodstream infections caused by Acinetobacter baumannii: A case-control study. Diagn. Microbiol. Infect. Dis. 2021, 99, 115229. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Ye, Q.F.; Yuan, H. Multidrug-resistant Gram-negative bacteria in solid organ transplant recipients with bacteremias. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Alrstom, A.; Alsuliman, T.; Daher, N.; Abouharb, R. The Impact of Modifying Empirical Antibiotic Therapy Based on Intestinal Colonization Status on Clinical Outcomes of Febrile Neutropenic Patients. Infect. Chemother. 2021, 53, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, F.; Cigana, C.; Massimo, D.; Lazzarotto, D.; Geromin, A.; Isola, M.; Battista, M.L.; Medeot, M.; Cerno, M.; Sperotto, A.; et al. Risk Factors and Outcomes of Infections by Multidrug-Resistant Gram-Negative Bacteria in Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 333–339, Erratum in 2017, 23, 1040. [Google Scholar] [CrossRef] [Green Version]
- Micozzi, A.; Gentile, G.; Santilli, S.; Minotti, C.; Capria, S.; Moleti, M.L.; Barberi, W.; Cartoni, C.; Trisolini, S.M.; Testi, A.M.; et al. Reduced mortality from KPC-K. pneumoniae bloodstream infection in high-risk patients with hematological malignancies colonized by KPC-K. pneumoniae. BMC Infect. Dis. 2021, 21, 1079. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; De Rosa, F.G.; Del Bono, V.; Grossi, P.A.; Pea, F.; Petrosillo, N.; Rossolini, G.M.; Tascini, C.; Tumbarello, M.; Viale, P.; et al. Ceftobiprole: Drug evaluation and place in therapy. Expert Rev. Anti Infect. Ther. 2019, 17, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Burillo, A.; Muñoz, P.; Bouza, E. Risk stratification for multidrug-resistant Gram-negative infections in ICU patients. Curr. Opin. Infect. Dis. 2019, 32, 626–637. [Google Scholar] [CrossRef]
- Leal, H.F.; Azevedo, J.; Silva, G.E.O.; Amorim, A.M.L.; de Roma, L.R.C.; Palmeira Arraes, A.C.; Lins Gouveia, E.; Reis, M.G.; Mendes, A.V.; de Oliveira Silva, M.; et al. Bloodstream infections caused by multidrug-resistant gram-negative bacteria: Epidemiological, clinical, and microbiological features. BMC Infect. Dis. 2019, 19, 609. [Google Scholar] [CrossRef]
- Catry, B.; Latour, K.; Jans, B.; Vandendriessche, S.; Preal, R.; Mertens, K.; Denis, O. Risk factors for methicillin resistant Staphylococcus aureus: A multi-laboratory study. PLoS ONE 2014, 9, e89579. [Google Scholar] [CrossRef] [Green Version]
- Keighley, C.L.; Pope, A.; Marriott, D.J.E.; Chapman, B.; Bak, N.; Daveson, K.; Hajkowicz, K.; Halliday, C.; Kennedy, K.; Kidd, S.; et al. Risk factors for candidaemia: A prospective multi-centre case-control study. Mycoses 2021, 64, 257–263. [Google Scholar] [CrossRef]
- Detsis, M.; Karanika, S.; Mylonakis, E. ICU Acquisition Rate, Risk Factors, and Clinical Significance of Digestive Tract Colonization with Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, 705–714. [Google Scholar] [CrossRef]
- Giannella, M.; Trecarichi, E.M.; De Rosa, F.G.; Del Bono, V.; Bassetti, M.; Lewis, R.E.; Losito, A.R.; Corcione, S.; Saffioti, C.; Bartoletti, M.; et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin. Microbiol. Infect. 2014, 20, 1357–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, A.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Perèz-Nadales, E.; Causse, M.; Castòn, J.J.; Guzman-Puche, J.; Torre-Gimenèz, J.; Kindelàn, L.; et al. Risks of Infection and Mortality among Patients Colonized with Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, P.Y.; Sengupta, S.; Kukreja, A.; Ponnampalavanar, S.S.; Ng, O.T.; Marimuthu, K. The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Antimicrob. Resist. Infect. Control. 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliara, G.; Di Paolo, C.; Barbato, D.; Baccolini, V.; Salerno, C.; Nardi, A.; Alessandri, F.; Giordano, A.; Tufi, D.; Marinelli, L.; et al. Multimodal surveillance of healthcare associated infections in an intensive care unit of a large teaching hospital. Ann. Ig. 2019, 31, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, G.; Arena, F.; Borrè, S.; Campanile, F.; Falcone, M.; Mussini, C.; Pea, F.; Sganga, G.; Stefani, S.; Venditti, M. Diagnostic stewardship based on patient profiles: Differential approaches in acute versus chronic infectious syndromes. Expert Rev. Anti Infect. Ther. 2021, 19, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of Piperacillin-Tazobactam vs. Meropenem on 30-Day Mortality for Patients with E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994, Erratum in 2019, 321, 2370. [Google Scholar] [CrossRef] [Green Version]
- Karaiskos, I.; Giamarellou, H. Carbapenem-Sparing Strategies for ESBL Producers: When and How. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Maraki, S.; Mavromanolaki, V.E.; Moraitis, P.; Stafylaki, D.; Kasimati, A.; Magkafouraki, E.; Scoulica, E. Ceftazidime-avibactam, meropenen-vaborbactam, and imipenem-relebactam in combination with aztreonam against multidrug-resistant, metallo-β-lactamase-producing Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1755–1759. [Google Scholar] [CrossRef]
- Meini, S.; Viaggi, B.; Tascini, C. Mono vs. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: Insights from the literature. Infection 2021, 49, 411–421, Erratum in 2021, 49, 423–425. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Khawcharoenporn, T.; Chuncharunee, A.; Maluangnon, C.; Taweesakulvashra, T.; Tiamsak, P. Active monotherapy and combination therapy for extensively drug-resistant Pseudomonas aeruginosa pneumonia. Int. J. Antimicrob. Agents 2018, 52, 828–834. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpicelli, L.; Venditti, M.; Ceccarelli, G.; Oliva, A. Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm. Antibiotics 2021, 10, 1475. https://doi.org/10.3390/antibiotics10121475
Volpicelli L, Venditti M, Ceccarelli G, Oliva A. Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm. Antibiotics. 2021; 10(12):1475. https://doi.org/10.3390/antibiotics10121475
Chicago/Turabian StyleVolpicelli, Lorenzo, Mario Venditti, Giancarlo Ceccarelli, and Alessandra Oliva. 2021. "Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm" Antibiotics 10, no. 12: 1475. https://doi.org/10.3390/antibiotics10121475
APA StyleVolpicelli, L., Venditti, M., Ceccarelli, G., & Oliva, A. (2021). Place in Therapy of the Newly Available Armamentarium for Multi-Drug-Resistant Gram-Negative Pathogens: Proposal of a Prescription Algorithm. Antibiotics, 10(12), 1475. https://doi.org/10.3390/antibiotics10121475







