Newborns with Bloody Stools—At the Crossroad between Efficient Management of Necrotizing Enterocolitis and Antibiotic Stewardship
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Exposure
2.2. Outcome of Delayed Antibiotic Exposure
2.3. Mortality
2.4. Feeding Regimes
2.5. Analysis of Risk Factors for NEC
3. Discussion
4. Materials and Methods
4.1. Inclusion/Exclusion Criteria and Patient Stratification
4.2. Setting and Three Different Time Periods of Antibiotic Stewardship
4.3. Outcomes and Objectives
4.4. Data Acquisition and Study Variables
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, P.W.; Stoll, B.J. Necrotising Enterocolitis. Lancet 2006, 368, 13. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, C.R.; Knell, J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Trends in Incidence and Outcomes of Necrotizing Enterocolitis over the Last 12 Years: A Multicenter Cohort Analysis. J. Pediatr. Surg. 2020, 55, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of Necrotizing Enterocolitis Expressed by Birth Weight Categories. J. Pediatr. Surg. 2009, 44, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Wong, A.L. Lower Gastrointestinal Bleeding in Children. Pediatr. Emerg. Care 2002, 18, 319–323. [Google Scholar] [CrossRef]
- Romano, C.; Oliva, S.; Martellossi, S.; Miele, E.; Arrigo, S.; Graziani, M.G.; Cardile, S.; Gaiani, F.; de’Angelis, G.L.; Torroni, F. Pediatric Gastrointestinal Bleeding: Perspectives from the Italian Society of Pediatric Gastroenterology. World J. Gastroenterol. 2017, 23, 1328. [Google Scholar] [CrossRef]
- Pant, C.; Olyaee, M.; Sferra, T.J.; Gilroy, R.; Almadhoun, O.; Deshpande, A. Emergency Department Visits for Gastrointestinal Bleeding in Children: Results from the Nationwide Emergency Department Sample 2006–2011. Curr. Med. Res. Opin. 2015, 31, 347–351. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Szajewska, H.; Shamir, R.; Nowak-Węgrzyn, A. Non-IgE-Mediated Gastrointestinal Food Allergies in Children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef]
- Arvola, T. Rectal Bleeding in Infancy: Clinical, Allergological, and Microbiological Examination. Pediatrics 2006, 117, e760–e768. [Google Scholar] [CrossRef] [Green Version]
- Rees, C.M.; Eaton, S.; Pierro, A. National Prospective Surveillance Study of Necrotizing Enterocolitis in Neonatal Intensive Care Units. J. Pediatr. Surg. 2010, 45, 1391–1397. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Alsaied, A.; Islam, N.; Thalib, L. Global Incidence of Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K.; The Canadian Neonatal Network. Incidence and Timing of Presentation of Necrotizing Enterocolitis in Preterm Infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [Green Version]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of Neonatal Necrotising Enterocolitis in High-Income Countries: A Systematic Review. Arch. Dis. Child. -Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef]
- Holman, R.C.; Stoll, B.J.; Curns, A.T.; Yorita, K.L.; Steiner, C.A.; Schonberger, L.B. Necrotising Enterocolitis Hospitalisations among Neonates in the United States. Paediatr. Perinat. Epidemiol. 2006, 20, 498–506. [Google Scholar] [CrossRef]
- Samuels, N.; van de Graaf, R.A.; de Jonge, R.C.J.; Reiss, I.K.M.; Vermeulen, M.J. Risk Factors for Necrotizing Enterocolitis in Neonates: A Systematic Review of Prognostic Studies. BMC Pediatr. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal Necrotizing Enterocolitis: Therapeutic Decisions Based upon Clinical Staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Walsh, M.C. Neonatal Necrotizing Enterocolitis: Pathogenesis, Classification, and Spectrum of Illness. Curr. Probl. Pediatr. 1987, 17, 219–288. [Google Scholar] [CrossRef]
- Neu, J. Necrotizing enterocolitis. Pediatr. Clin. N. Am. 1996, 43, 409–432. [Google Scholar] [CrossRef]
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining Necrotizing Enterocolitis: Current Difficulties and Future Opportunities. Pediatr. Res. 2020, 88, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non–IgE-Mediated Gastrointestinal Food Allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef]
- Cordova, J.; Sriram, S.; Patton, T.; Jericho, H.; Gokhale, R.; Weinstein, D.; Sentongo, T. Manifestations of Cow’s-Milk Protein Intolerance in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 140–144. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A. Food Protein-Induced Enterocolitis Syndrome and Allergic Proctocolitis. Allergy Asthma Proc. 2015, 36, 172–184. [Google Scholar] [CrossRef]
- Lazare, F.B.; Brand, D.A.; Marciano, T.A.; Daum, F. Rapid Resolution of Milk Protein Intolerance in Infancy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Lenfestey, M.W.; de la Cruz, D.; Neu, J. Food Protein–Induced Enterocolitis Instead of Necrotizing Enterocolitis? A Neonatal Intensive Care Unit Case Series. J. Pediatr. 2018, 200, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Lambert, D.K.; Gordon, P.V.; Baer, V.L.; Gerday, E.; Henry, E. Neonates Presenting with Bloody Stools and Eosinophilia Can Progress to Two Different Types of Necrotizing Enterocolitis. J. Perinatol. 2012, 32, 874–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktaş, S.; Ergenekon, E.; Ünal, S.; Türkyılmaz, C.; Hirfanoğlu, İ.M.; Atalay, Y. Different Presentations of Cow`s Milk Protein Allergy during Neonatal Period. Turk. J. Pediatr. 2017, 59, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swart, J.F.; Ultee, K. Rectal Bleeding in a Preterm Infant as a Symptom of Allergic Colitis. Eur. J. Pediatr. 2003, 162, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Turner, T.W.S. Allergic Colitis with Pneumatosis Intestinalis in an Infant. Pediatr. Emerg. Care 2018, 34, e14–e15. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef] [Green Version]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic Exposure in Neonates and Early Adverse Outcomes: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmann, K.E.; Lehnick, D.; Buettcher, M.; Schwendener-Scholl, K.; Daetwyler, K.; Fontana, M.; Morgillo, D.; Ganassi, K.; O’Neill, K.; Genet, P.; et al. Impact of Empowering Leadership on Antimicrobial Stewardship: A Single Center Study in a Neonatal and Pediatric Intensive Care Unit and a Literature Review. Front. Pediatr. 2018, 6, 294. [Google Scholar] [CrossRef] [Green Version]
- Blakely, M.L.; Lally, K.P.; McDonald, S.; Brown, R.L.; Barnhart, D.C.; Ricketts, R.R.; Thompson, W.R.; Scherer, L.R.; Klein, M.D.; Letton, R.W.; et al. Postoperative Outcomes of Extremely Low Birth-Weight Infants With Necrotizing Enterocolitis or Isolated Intestinal Perforation: A Prospective Cohort Study by the NICHD Neonatal Research Network. Ann. Surg. 2005, 241, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Maayan-Metzger, A.; Schushan-Eisen, I.; Kuint, J. Management of Isolated Rectal Bleeding in Newborn Infants: Comparison of Two Time Periods. Acta Paediatr. 2009, 99, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Antimicrobial Stewardship in Paediatrics. BMC Infect. Dis. 2016, 16, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, J.; Profit, J.; Lee, H.C.; Dueñas, G.; Bennett, M.V.; Parucha, J.; Jocson, M.A.L.; Gould, J.B. Variations in Neonatal Antibiotic Use. Pediatrics 2018, 142, e20180115. [Google Scholar] [CrossRef] [Green Version]
- Schulfer, A.; Blaser, M.J. Risks of Antibiotic Exposures Early in Life on the Developing Microbiome. PLOS Pathog. 2015, 11, e1004903. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How Colonization by Microbiota in Early Life Shapes the Immune System. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Risnes, K.R.; Belanger, K.; Murk, W.; Bracken, M.B. Antibiotic Exposure by 6 Months and Asthma and Allergy at 6 Years: Findings in a Cohort of 1401 US Children. Am. J. Epidemiol. 2011, 173, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Murk, W.; Risnes, K.R.; Bracken, M.B. Prenatal or Early-Life Exposure to Antibiotics and Risk of Childhood Asthma: A Systematic Review. Pediatrics 2011, 127, 1125–1138. [Google Scholar] [CrossRef]
- Theochari, N.A.; Stefanopoulos, A.; Mylonas, K.S.; Economopoulos, K.P. Antibiotics Exposure and Risk of Inflammatory Bowel Disease: A Systematic Review. Scand. J. Gastroenterol. 2018, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rallis, D.; Balomenou, F.; Maragoudaki, E.; Papastergiou, E.; Machas, P.; Tsabouri, S.; Siomou, E.; Giapros, V. Neonatal Isolated Rectal Bleeding: A Case-control Study. Acta Paediatr. 2021, 110, 2772–2774. [Google Scholar] [CrossRef]
- Ullman, A.J.; Marsh, N.; Mihala, G.; Cooke, M.; Rickard, C.M. Complications of Central Venous Access Devices: A Systematic Review. Pediatrics 2015, 136, e1331–e1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, C.; Álvarez, F.; De La Rua, V.; Medina, A.; Concha, A.; Díaz, J.J.; Menéndez, S.; Arcos, M.L.; Mayordomo-Colunga, J. Mechanical Complications during Central Venous Cannulations in Pediatric Patients. Intensive Care Med. 2009, 35, 1438–1443. [Google Scholar] [CrossRef]
- Rina, P.; Zeng, Y.; Ying, J.; Qu, Y.; Mu, D. Association of Initial Empirical Antibiotic Therapy with Increased Risk of Necrotizing Enterocolitis. Eur. J. Pediatr. 2020, 179, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Knezevic, A.; Shenvi, N.; Hinkes, M.; Keene, S.; Roback, J.D.; Easley, K.A.; Josephson, C.D. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA 2016, 315, 889. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.C.; Klaassen, P.; Niemarkt, H.J.; de Boode, W.P.; Cossey, V.; van Goudoever, J.B.; Hulzebos, C.V.; Andriessen, P.; van Kaam, A.H.; Kramer, B.W.; et al. Risk Factors for Necrotizing Enterocolitis: A Prospective Multicenter Case-Control Study. Neonatology 2018, 114, 277–284. [Google Scholar] [CrossRef]
- Lucas, A.; Cole, T.J. Breast Milk and Neonatal Necrotising Enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
| All Patients (n = 102) | Bell IB (n = 43) | Bell IIA (n = 25) | Bell IIB (n = 25) | Bell III (n = 9) | |
|---|---|---|---|---|---|
| Risk Factors | |||||
| Worsening of general condition (n, %) | 33 (32.4%) | 1 (2.3%) | 6 (24.0%) | 18 (72.0%) | 8 (88.9%) |
| Abdominal X-ray (n = 80) (n, %) | |||||
| —inconspicuous or minor findings | 38 (47.5%) | 21 (100%) | 5 (20.0%) | 12 (48.0%) | 0 (0.0%) |
| — PI | 35 (43.8%) | 0 (0.0%) | 20 (80.0%) | 13 (52.0%) | 2 (22.2%) |
| — PVG or PP | 7 (8.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (77.8%) |
| Abdominal ultrasound (n = 30) (n, %) | |||||
| — inconspicuous or minor findings | 14 (46.7%) | 7 (100%) | 5 (71.4%) | 2 (16.7%) | 0 (0.0%) |
| — PI | 12 (40%) | 0 (0.0%) | 2 (28.6%) | 10 (83.3%) | 0 (0.0%) |
| — PVG or PP | 4 (13.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (100%) |
| Blood count (n = 92) (n, %) | |||||
| — inconspicuous or minor abnormalities | 74 (80.4%) | 33 (100%) | 24 (96.0%) | 13 (52.0%) | 4 (44.4%) |
| — suspicious for NEC | 18 (19.6%) | 0 (0.0%) | 1 (4.0%) | 12 (48.0%) | 5 (55.6%) |
| CRP (n = 89), mg/L median (range) | <5 (<5–150) | <5 (<5–<5) | <5 (<5–56) | 10 (<5–150) | 17 (<5–76) |
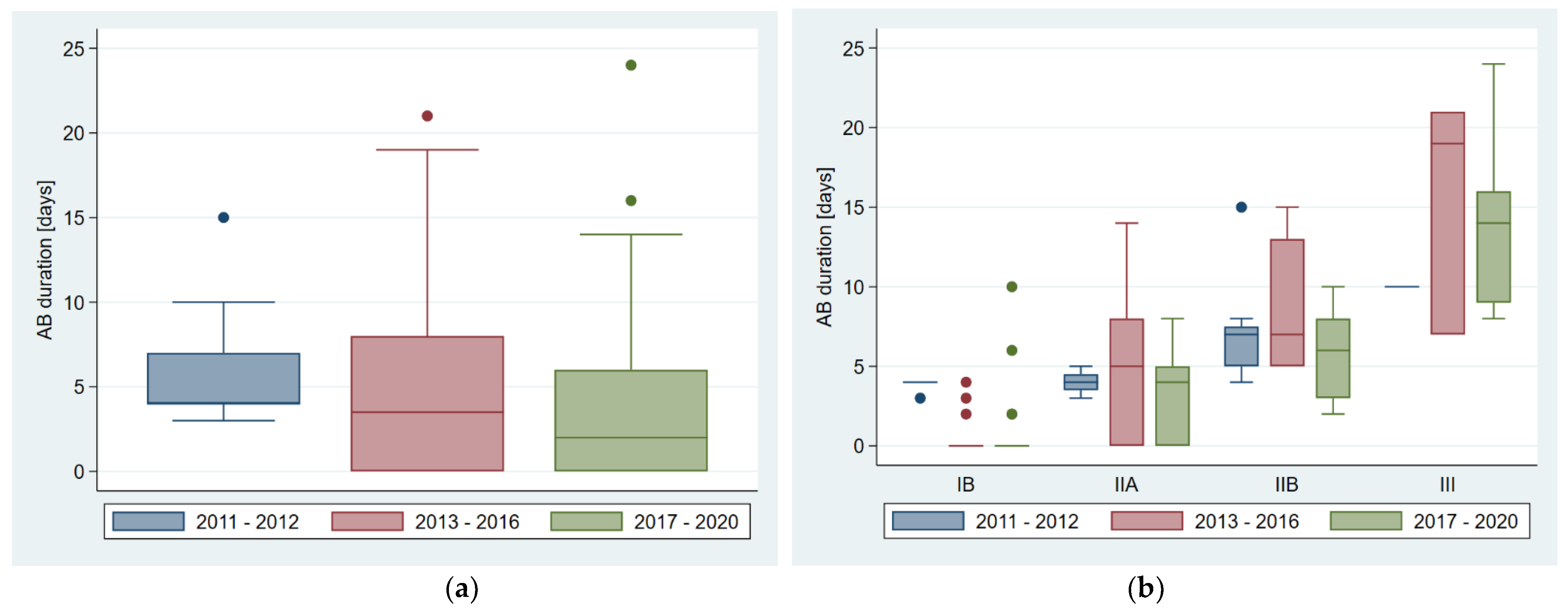
| Time p = Period | Neonates (n), Divided by NEC Stages (n) | Neonates with Antibiotic Therapy (n, %) | Duration of Antibiotic Therapy Days Median (Q1; Q3), Range | Parenteral Nutrition (PN) (n, %) | Change of Feeds (n = 99) (n, %) | Surgery for NEC (n, %) | NEC-Related Death (n, %) |
|---|---|---|---|---|---|---|---|
| 2011–2020 | Total: 102 IB: 43 IIA: 25 IIB: 25 III: 9 | 65 (63.7%) 13 (30.2%) 18 (72.0%) 25 (100%) 9 (100%) | 4 (0; 7), 0–24 0 (0; 2), 0–10 4 (0; 5), 0–14 7 (5; 8), 2–15 14 (9; 19), 7–24 | 60 (58.8%) 10 (23.3%) 17 (68.0%) 24 (96.0%) 9 (100%) | 53 (53.5%) 34 (79.1%) 13 (52.0%) 4 (17.4%) 2 (25.0%) | 6 (5.9%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 6 (66.7%) | 4 (3.9%) 0 (0.0%) 0 (0.0%) 3 (12.0%) 1 (11.1%) |
| p-value Kendall’s tau-b / p-value | <0.001 a 0.58 / <0.001 | N/A 0.65 / <0.001 | <0.001 a 0.60 / <0.001 | <0.001 a −0.46 / <0.001 | <0.001 a 0.39 / <0.001 | 0.023 a 0.23 / 0.014 | |
| 2011–2012 * | Total: 19 IB: 6 IIA: 4 IIB: 8 III: 1 | 19 (100%) 6 (100%) 4 (100%) 8 (100%) 1 (100%) | 4 (4; 7), 3–15 4 (4; 4), 3–4 4 (3.5; 4.5), 3–5 7 (5; 7.5), 4–15 10 (10; 10), 10–10 | 17 (89.5%) 4 (66.7%) 4 (100%) 8 (100%) 1 (100%) | 1 (5.3%) 1 (16.7%) 0 (0.0%) 0 (0.0%) 0 (0.0%) | 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) | 1 (5.3%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (5.3%) |
| 2013–2016 * | Total: 34 IB: 15 IIA: 9 IIB: 7 III: 3 | 19 (55.9%) 3 (20%) 6 (66.7%) 7 (100%) 3 (100%) | 3.5 (0; 8), 0–21 0 (0; 0), 0–4 5 (0; 8), 0–14 7 (5; 13), 5–15 19 (7; 21), 7–21 | 13 (38.2%) 1 (6.7%) 3 (33.3%) 6 (85.7%) 3 (100%) | 16 (47.1%) 13 (86.7%) 3 (33.3%) 0 (0.0%) 0 (0.0%) | 2 (5.9%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 2 (66.7%) | 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
| 2017–2020 * | Total: 49 IB: 22 IIA: 12 IIB: 10 III: 5 | 27 (55.1%) 4 (18.2%) 8 (66.7%) 10 (100%) 5 (100%) | 2 (0; 6), 0–24 0 (0; 0), 0–10 4 (0; 5), 0–8 6 (3; 8), 2–10 14 (9; 16), 8–24 | 30 (61.2%) 5 (22.7%) 10 (83.3%) 10 (100%) 5 (100%) | 36 (73.5%) 20 (90.9%) 10 (83.3%) 4 (40%) 2 (40%) | 4 (8.2%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 4 (80%) | 3 (6.1%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 3 (6.1%) |
| Patient No. | GA Weeks, BW Grams, APGARs’ Score | NEC Stage | Comorbidities | DOL of Symptoms’ Onset | DOL of Start of Antibiotic Therapy for NEC | DOL of NEC Surgery | DOL of Death | Cause of Death |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 1/7, 430 g, 1/4/6 | Bell IIB | IUGR with severe cerebral, cardiac and renal anomalies; chromosomal aberration | 8 | 8 | - | 13 | NEC-related |
| 2 | 27 4/7, 870 g, 2/6/7 | Bell III | PDA with need for surgery (DOL 9) | 5 | 5 | 5, 12 | 13 | NEC-related |
| 3 | 26 1/7, 870 g, 1/1/2 | Bell IIB | Early onset sepsis with multi-organ failure; PFO, PDA, severe PAH, cardiac failure | 7 | 7 | - | 8 | NEC-related, redirection of care |
| 4 | 24 2/7, 700 g, 7/8/8 | Bell IIB | PDA, severe PAH and arterial hypotension; bilateral PIE | 4 | 4 | - | 6 | NEC-related, redirection of care |
| All Patients (n = 102) | Bell IB (n = 43) | Bell IIA (n = 25) | Bell IIB (n = 25) | Bell III (n = 9) | p-Value | Kendall’s tau-b/ p-Value | |
|---|---|---|---|---|---|---|---|
| Risk Factors | |||||||
| Gestational age (GA), weeks median (range) | 33 5/7 (23 4/7–41 5/7) | 34 4/7 (24 5/7–41 5/7) | 34 4/7 (26 0/7–40 5/7) | 30 (23 4/7–40 6/7) | 33 3/7 (27 4/7–36 2/7) | 0.011 a | −0.22/0.003 |
| Birth weight (BW), grams median (range) | 2135 (360–4410) | 2340 (1070–4130) | 2260 (870–3900) | 1300 (360–4410) | 1680 (720–2775) | 0.009 a | −0.24/0.001 |
| Sex (male/female) (n, %) | 55 (53.9%)/ 47 (46.1%) | 23 (53.5%)/ 20 (46.5%) | 11 (44.0%)/ 14 (56.0%) | 17 (68.0%)/ 8 (32.0%) | 4 (44.4%)/ 5 (55.6%) | 0.34 b | −0.04/0.68 |
| DOL at symptoms’ onset median (range) | 13 (2–78) | 18 (3–68) | 13 (2–74) | 8 (3–78) | 12 (4–62) | 0.043 a | −0.22/0.005 |
| Cardiac anomalies (n, %) | 55 (53.9%) | 20 (46.5%) | 9 (36%) | 18 (72%) | 8 (88.9%) | 0.001 b | 0.27/0.002 |
| — without hemodynamic relevance | 34 (33.3%) | 16 (37.2%) | 5 (20%) | 11 (44%) | 2 (22.2%) | ||
| — with hemodynamic relevance | 12 (11.8%) | 3 (7%) | 0 (0.0%) | 5 (20%) | 4 (44.4%) | ||
| — with hemodynamic relevance and need for surgery | 9 (8.8%) | 1 (2.3%) | 4 (16%) | 2 (8%) | 2 (22.2%) | ||
| Asphyxia (n, %) | 5 (4.9%) | 1 (2.3%) | 2 (8.0%) | 2 (8.0%) | 0 (0.0%) | 0.68 b | 0.06/0.53 |
| Sepsis (n, %) | 6 (5.9%) | 1 (2.3%) | 0 (0.0%) | 3 (12.0%) | 2 (22.2%) | 0.031 b | 0.21/0.023 |
| RBC transfusion (n, %) | 15 (14.7%) | 1 (2.3%) | 3 (12.0%) | 8 (32.0%) | 3 (33.3%) | 0.001 b | 0.33/ <0.001 |
| Platelet transfusion (n, %) | 11 (10.8%) | 1 (2.3%) | 0 (0.0%) | 8 (32.0%) | 2 (22.2%) | <0.001 b | 0.32/ <0.001 |
| FFP transfusion (n, %) | 5 (4.9%) | 1 (2.3%) | 0 (0.0%) | 2 (8.0%) | 2 (22.2%) | 0.041 b | 0.19/0.046 |
| Arterial hypotension with need for vasopressors (n, %) | 25 (24.5%) | 6 (14.0%) | 3 (12.0%) | 11 (44.0%) | 5 (55.6%) | 0.003 b | 0.30/0.001 |
| SGA (n, %) | 7 (6.9%) | 1 (2.3%) | 1 (4.0%) | 4 (16.0%) | 1 (11.1%) | 0.11 b | 0.18/0.052 |
| Formula based feeds (n = 95) (n, %) | 26 (27.4%) | 10 (23.3%) | 10 (41.7%) | 6 (27.3%) | 0 (0.0%) | 0.19 b | 0.002/0.99 |
| Antibiotic therapy before symptoms’ onset, days median (range) | 4 (0–25) | 3 (0–14) | 3 (0–17) | 4 (0–25) | 4 (0–13) | 0.69 a | 0.06/0.44 |
| Risk Factors | Odds Ratio | 95%-CI | p-Value |
|---|---|---|---|
| Gestational age (GA) | 0.84 | 0.77–0.92 | <0.001 |
| DOL at symptom onset | 0.97 | 0.94–0.99 | 0.014 |
| FFP transfusion | 13.40 | 2.12–84.52 | 0.006 |
| SGA | 3.65 | 0.87–15.37 | 0.078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyne-Pietschmann, M.; Lehnick, D.; Spalinger, J.; Righini-Grunder, F.; Buettcher, M.; Lehner, M.; Stocker, M. Newborns with Bloody Stools—At the Crossroad between Efficient Management of Necrotizing Enterocolitis and Antibiotic Stewardship. Antibiotics 2021, 10, 1467. https://doi.org/10.3390/antibiotics10121467
Heyne-Pietschmann M, Lehnick D, Spalinger J, Righini-Grunder F, Buettcher M, Lehner M, Stocker M. Newborns with Bloody Stools—At the Crossroad between Efficient Management of Necrotizing Enterocolitis and Antibiotic Stewardship. Antibiotics. 2021; 10(12):1467. https://doi.org/10.3390/antibiotics10121467
Chicago/Turabian StyleHeyne-Pietschmann, Marie, Dirk Lehnick, Johannes Spalinger, Franziska Righini-Grunder, Michael Buettcher, Markus Lehner, and Martin Stocker. 2021. "Newborns with Bloody Stools—At the Crossroad between Efficient Management of Necrotizing Enterocolitis and Antibiotic Stewardship" Antibiotics 10, no. 12: 1467. https://doi.org/10.3390/antibiotics10121467
APA StyleHeyne-Pietschmann, M., Lehnick, D., Spalinger, J., Righini-Grunder, F., Buettcher, M., Lehner, M., & Stocker, M. (2021). Newborns with Bloody Stools—At the Crossroad between Efficient Management of Necrotizing Enterocolitis and Antibiotic Stewardship. Antibiotics, 10(12), 1467. https://doi.org/10.3390/antibiotics10121467






