Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Period
2.2. Data Analysis and Data Provenance
2.3. Population
2.4. Calculation of Estimated Antibiotic Consumption Rate
2.5. Antibiotic Inclusion Criteria
2.6. Ethics Approval
3. Results
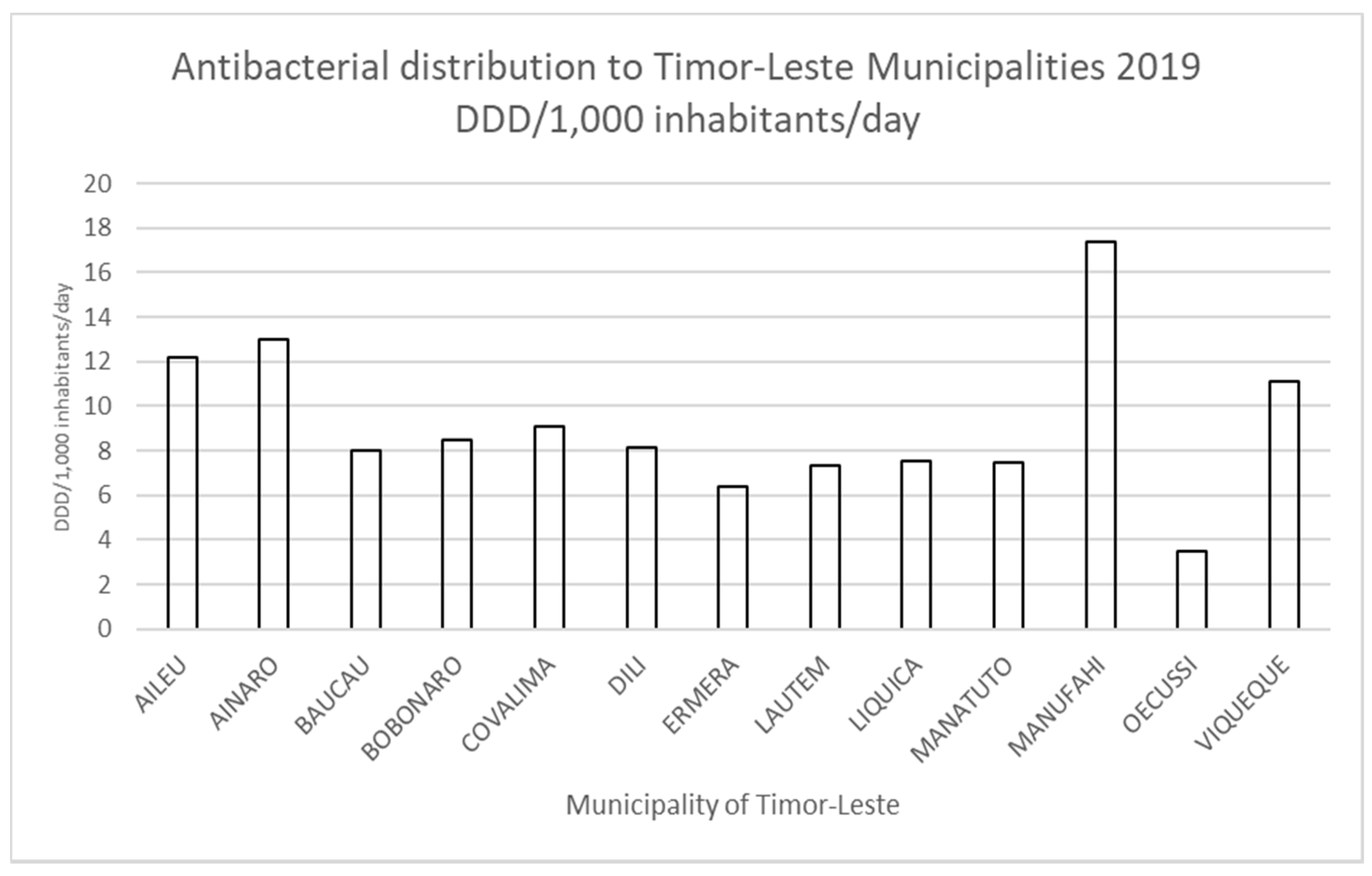
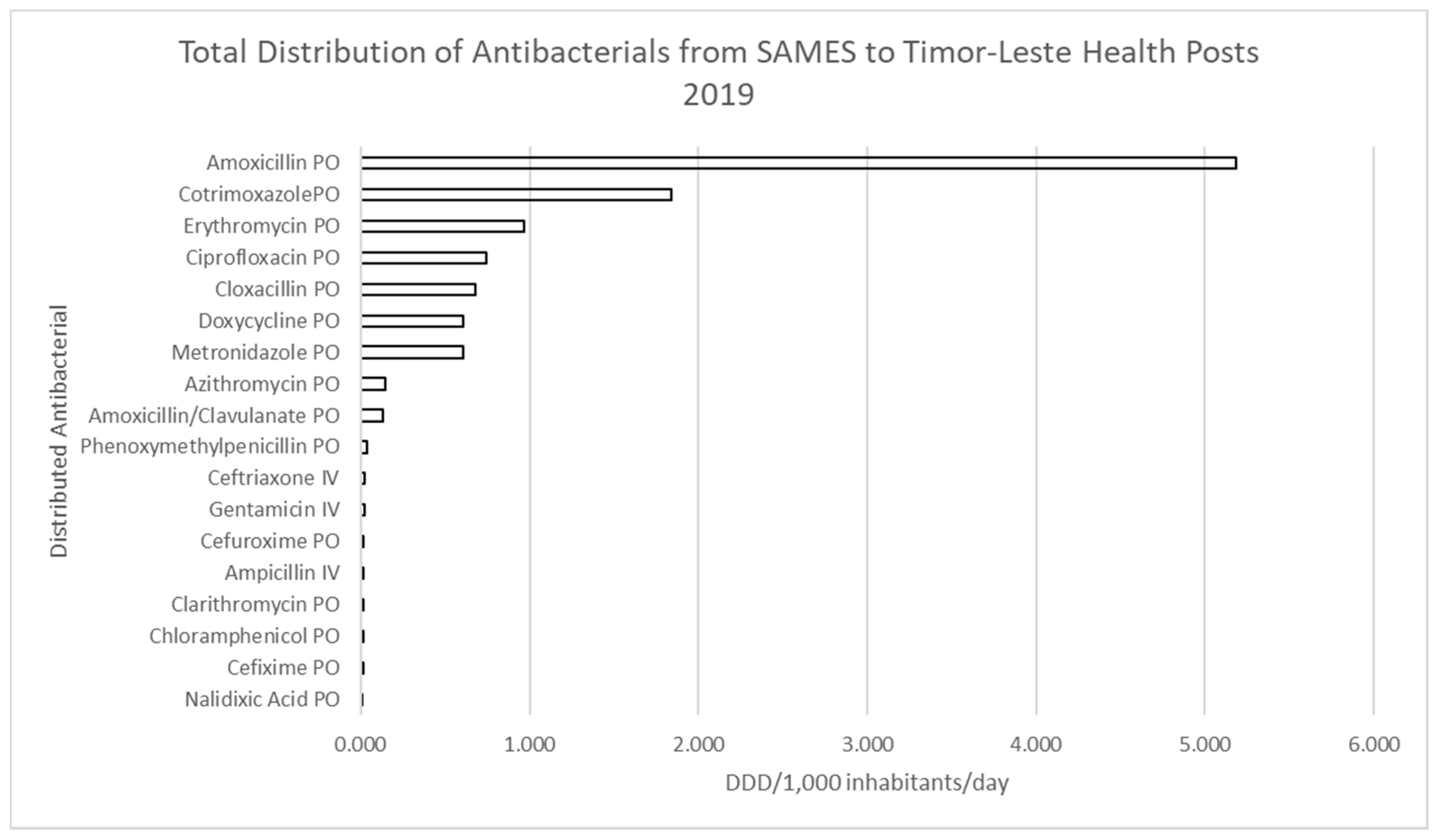
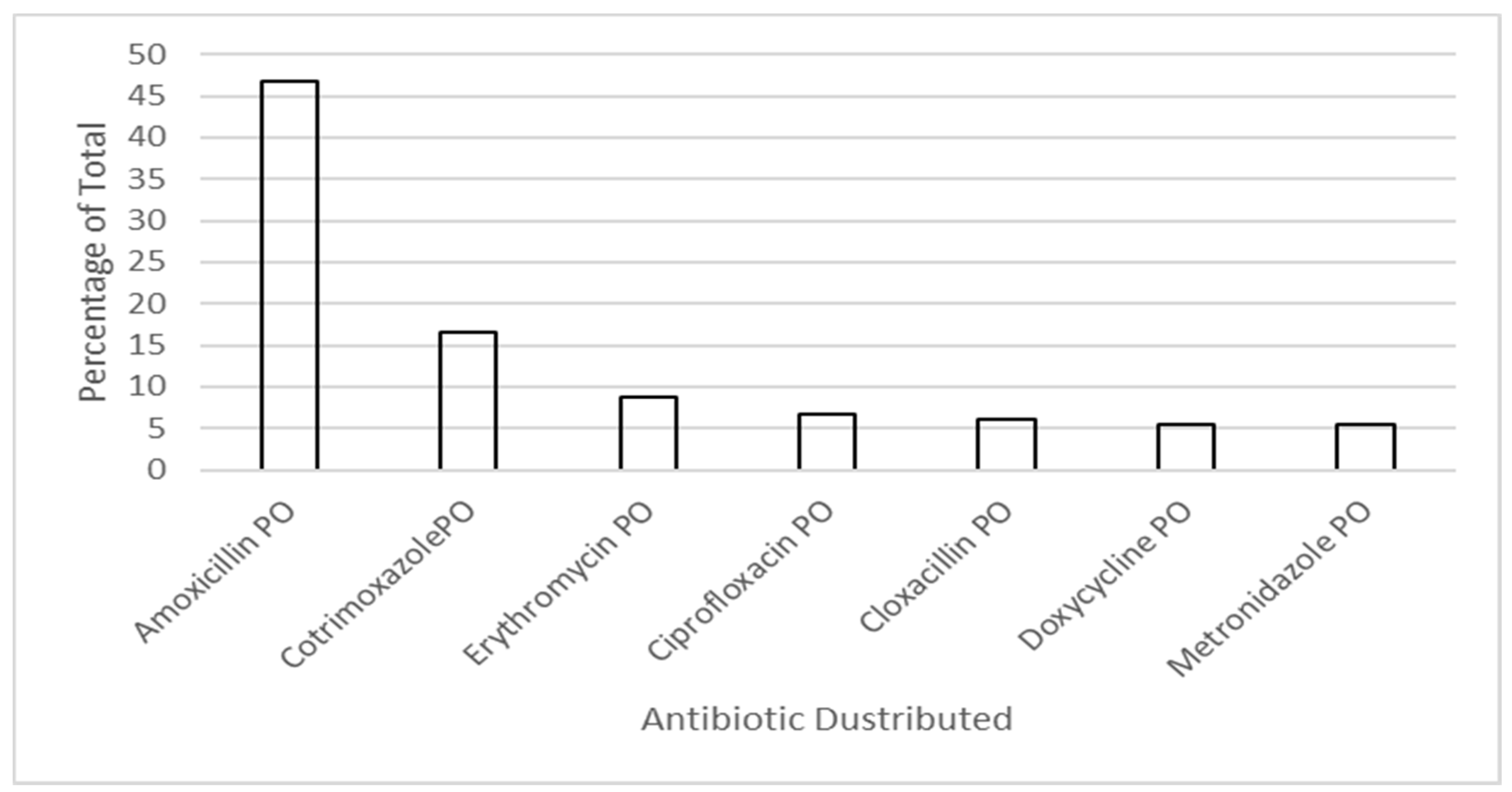
3.1. Antimicrobial Distribution in Timor-Leste
3.1.1. Consumption of Restricted Items
3.1.2. Comparison of Distribution and Dispensing Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective anti microbials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Hassali, M.A. Travellers take heed: Outbreak of extensively drug resistant (XDR) typhoid fever in Pakistan and a warning from the US CDC. Travel Med. Infect. Dis. 2018, 27, 127. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antimicrob. Resist. 2016, 84. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London: Review on Antimicrobial Resistance. 2014. Available online: https://amrreview.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 20 May 2021).
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef] [PubMed]
- Guinness, L.; Paul, R.C.; Martins, J.S.; Asante, A.; A Price, J.; Hayen, A.; Jan, S.; Soares, A.; Wiseman, V. Determinants of health care utilisation: The case of Timor-Leste. Int. Health 2018, 10, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Donà, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Al Maani, A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries: A position statement for the international society for infectious diseases. J. Infect. Dis. 2019, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. 2018. Available online: http://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf?ua=1 (accessed on 18 May 2021).
- World Health Organization. WHO Methodology for a Global Programme on Surveillance of Antimicrobial Consumption. 2017. Available online: www.who.int/medicines/areas/rational_use/WHO_AMCsurveillance_1.0.pdf (accessed on 18 May 2021).
- World Health Organisation. Timor-Leste Essential Medicines List (TLEML), 3rd ed.; World Health Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef]
- Martins, N.; Trevena, L.J. Implementing what works: A case study of integrated primary health care revitalisation in Timor-Leste. Asia Pac. Fam. Med. 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Timor-Leste Ministeriu Finansas. Population and Housing Census 2015. Preliminary Results. 2015. 1 Jan 2019. Available online: http://www.statistics.gov.tl/wp-content/uploads/2015/10/1-Preliminary-Results-4-Printing-Company-19102015.pdf (accessed on 18 May 2021).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Global Essental Medicines; World Health Organization. EMLs around the World. 2021. Available online: https//global.essentialmeds.org/dashboard/countries/122 (accessed on 18 May 2021).
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Marr, I.; Sarmento, N.; O’Brien, M.; Lee, K.; Gusmao, C.; de Castro, G.; Janson, S.; Tong, S.Y.; Baird, R.W.; Francis, J.R. Antimicrobial resistance in urine and skin isolates in Timor-Leste. J. Glob. Antimicrob. Resist. 2018, 13, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Nji, E.; Kazibwe, J.; Hambridge, T. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 3372. [Google Scholar] [CrossRef] [PubMed]
- Smale, E.M.; Egberts, T.C.G.; Heerdink, E.R.; Van den Bemt, B.J.F.; Bekker, C.L. Waste-minimising measures to achieve sustainable supply and use of medication. Sustain. Chem. Pharm. 2021, 2, 100400. [Google Scholar] [CrossRef]
- Bekker, C.L.; Gardarsdottir, H.; Egberts, A.C.G.; Bouvy, M.L.; Van den Bemt, B.J.F. Pharmacists’ Activities to Reduce Medication Waste: An International Survey. Pharmacy 2018, 6, 94. [Google Scholar] [CrossRef]
| Antimicrobial | ATC Code | WHO ATC DDD | Route | Restriction |
|---|---|---|---|---|
| Amoxicillin | J01CA04 | 1.5 g | Oral | HP1 |
| Amoxicillin/Clavulanate | J01CR02 | 1.5 g (amoxicillin) | Oral | DHC |
| Ampicillin | J01CA01 | 6 g | Injection | DHC |
| Azithromycin | J01FA10 | 0.3 g | Oral | DHC |
| Benzathine Benzylpenicillin | J01CE08 | 3.6 g (benzyl penicillin) | Injection | HP1 |
| Benzyl Penicillin | J01CE01 | 3.6 g | Injection | HP1 |
| Cefixime | J01DD08 | 0.4 g | Oral | DHC |
| Ceftriaxone | J01DD04 | 2 g | Injection | Spec |
| Cefuroxime | J01DC02 | 0.5 g | Oral | Spec |
| Chloramphenicol | J01BA01 | 0.3 g | Oral | HP1 |
| Chloramphenicol | J01BA01 | 0.3 g | Injection | HP1 |
| Ciprofloxacin | J01MA02 | 1 g | Oral | DHC |
| Clindamycin | J01FF01 | 1.8 g | Injection | Spec |
| Clindamycin | J01FF01 | 1.2 g | Oral | Spec |
| Cloxacillin | J01CF02 | 2 g | Injection | HP1 |
| Cloxacillin | J01CF02 | 2 g | Oral | HP1 |
| Cotrimoxazole 400 mg + 80 mg | J01EE01 | 4 tablets/40 mL | Oral | HP1 |
| Doxycycline | J01AA02 | 0.1 g | Oral | HP1 |
| Erythromycin | J01FA01 | 1 g | Oral | HP1 |
| Gentamicin | J01GB03 | 0.24 g | Injection | SDHC |
| Meropenem | J01DH02 | 3 g | Injection | Spec |
| Metronidazole | P01AB01 | 2 g | Oral | HP1 |
| Metronidazole | J01XD01 | 1.5 g | Injection | HP1 |
| Nalidixic Acid | J01MB02 | 4 g | Oral | DHC |
| Phenoxymethylpenicillin | J01CE02 | 2 g | Oral | HP1 |
| Vancomycin | J01XA01 | 2 g | Injection | Spec |
| Cefazolin | J01DB04 | 3 g | Injection | Hosp |
| Cefotaxime | J01DD01 | 4 g | Injection | Spec |
| Ceftazidime * | J01DD02 | 4 g | Injection | Spec |
| Clarithromycin | J01FA09 | 0.5 g | Oral | Hosp |
| Fusidic Acid | J01XC01 | 1.5 g | Oral | HNGV Spec |
| Municipality | Total DDD/1000 Inhabitants/Day | DDD/1000 Inhabitants/Day Amoxicillin | % Amoxicillin (of Total Distributed) |
|---|---|---|---|
| Aileu | 12.2 | 4.6 | 37.7 |
| Ainaro | 13 | 4.9 | 37.7 |
| Baucau | 8 | 4.1 | 51.2 |
| Bobonaro | 8.5 | 3.8 | 44.7 |
| Cova Lima | 9.1 | 5.3 | 58.2 |
| Dili | 8.1 | 4.5 | 55.5 |
| Ermera | 6.4 | 2.5 | 39.1 |
| Lautem | 7.3 | 4.5 | 61.6 |
| Liquica | 7.5 | 3.5 | 46.6 |
| Manatuto | 7.5 | 5.1 | 68 |
| Manufahi | 17.4 | 6.8 | 39.1 |
| Oecusse-Ambeno | 3.5 | 0 | 0 |
| Viqueque | 11.1 | 4.9 | 44.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, L.; Bongers, A.; Yan, J.; Francis, J.R.; Marr, I.; Lake, S.; Martins, S. Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns. Antibiotics 2021, 10, 1468. https://doi.org/10.3390/antibiotics10121468
Harris L, Bongers A, Yan J, Francis JR, Marr I, Lake S, Martins S. Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns. Antibiotics. 2021; 10(12):1468. https://doi.org/10.3390/antibiotics10121468
Chicago/Turabian StyleHarris, Lisa, Alexander Bongers, Jennifer Yan, Joshua R Francis, Ian Marr, Susanna Lake, and Santana Martins. 2021. "Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns" Antibiotics 10, no. 12: 1468. https://doi.org/10.3390/antibiotics10121468
APA StyleHarris, L., Bongers, A., Yan, J., Francis, J. R., Marr, I., Lake, S., & Martins, S. (2021). Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns. Antibiotics, 10(12), 1468. https://doi.org/10.3390/antibiotics10121468






