Antibiotic Use and Misuse in Dentistry in India—A Systematic Review
Abstract
1. Introduction
- The prevalence of prescribing antibiotics for dental problems;
- Clinical (therapeutic/prophylactic) and non-clinical indications where antibiotics are prescribed in dentistry;
- The types and regimen of antibiotics used;
- The difference, if any, between rural and urban populations, adults and children, males and females, and socioeconomic classes;
- Differences in antibiotic prescription based on provider characteristics;
- The factors influencing practitioners’ prescription patterns; and
- The reasons for self-medication with antibiotics and their sources.
2. Methods
2.1. Protocol Registration
2.2. Information Sources and Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
- Case reports and case series;
- Studies involving dental students;
- Studies performed in vitro; and
2.5. Research Question
- were seeking treatment from dental practitioners, dental specialists or other healthcare providers (including general medical practitioners, informal healthcare providers, etc.) for oral/dental problems; or
- had taken at least one course of antibiotics to help with dental/oral problems (irrespective of whether they completed the course or not), without consulting a dentist or other health practitioner (self-medication).
- Indications for dental antibiotic prescription: clinical (therapeutic/prophylactic) and non-clinical.
- The types and regimens of antibiotics used.
- The difference, if any, between rural and urban populations, adults and children, male and female, and socioeconomic classes.
- The difference, if any, between prescriber (provider) characteristics (general dental practitioner/specialist dental practitioner/general medical practitioner/Informal healthcare provider; male/female; urban/rural).
- The factors influencing practitioners’ prescription patterns, e.g., source of knowledge, such as monographs, textbooks and journals, colleagues, continuing professional development programmes, etc.
- The sources of antibiotics, if self-prescribed, and the reasons for self-medication.
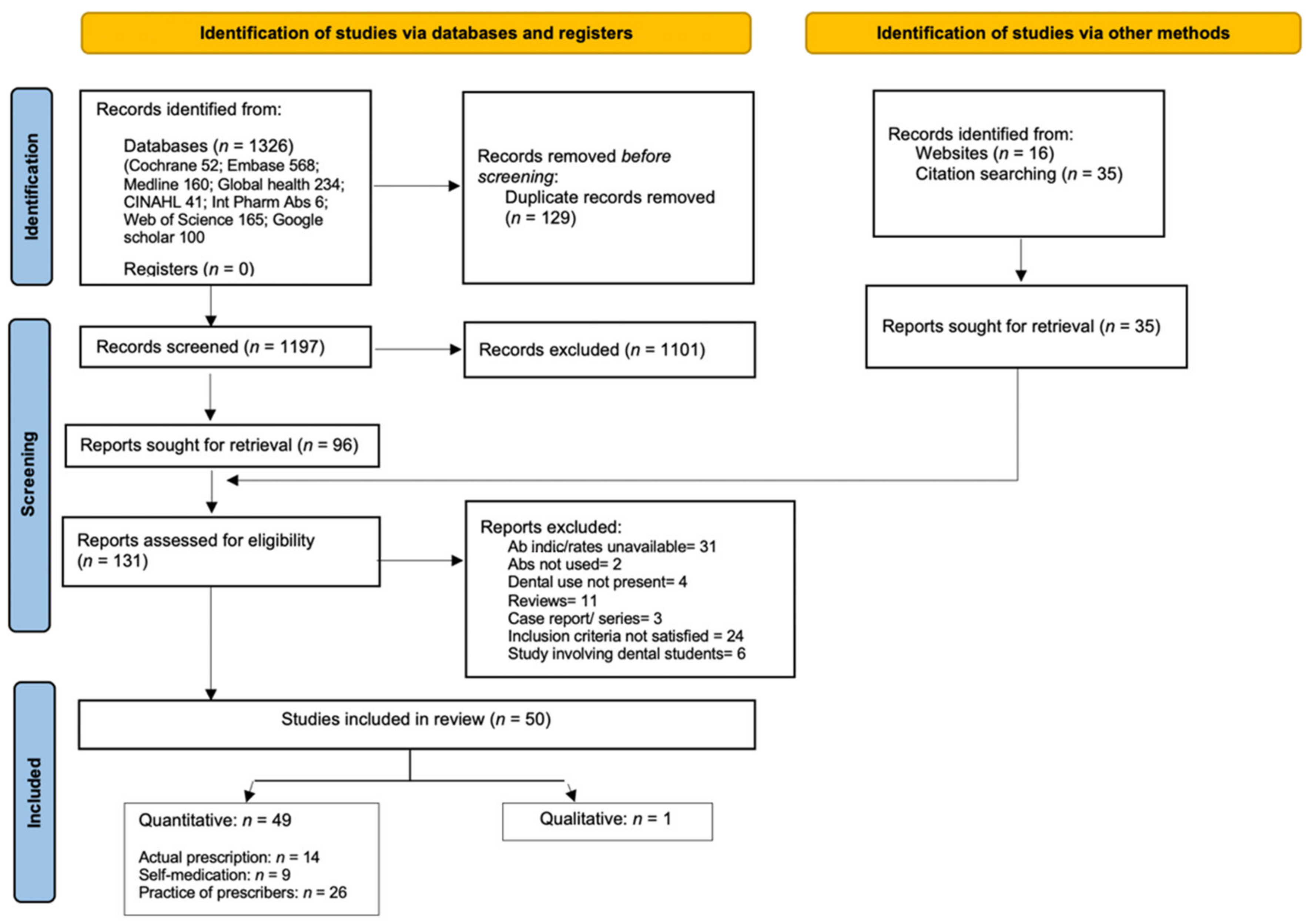
2.6. Study Screening and Selection
2.7. Data Extraction and Synthesis
- Studies exploring antibiotic prescription rates by dentists and other healthcare providers for dental problems;
- Studies that investigated self-medication practices by the general population for dental/oral problems; and
- Studies that explored indications, and the knowledge and practice of dentists in prescribing antibiotics for dental conditions.
2.8. Data Analysis
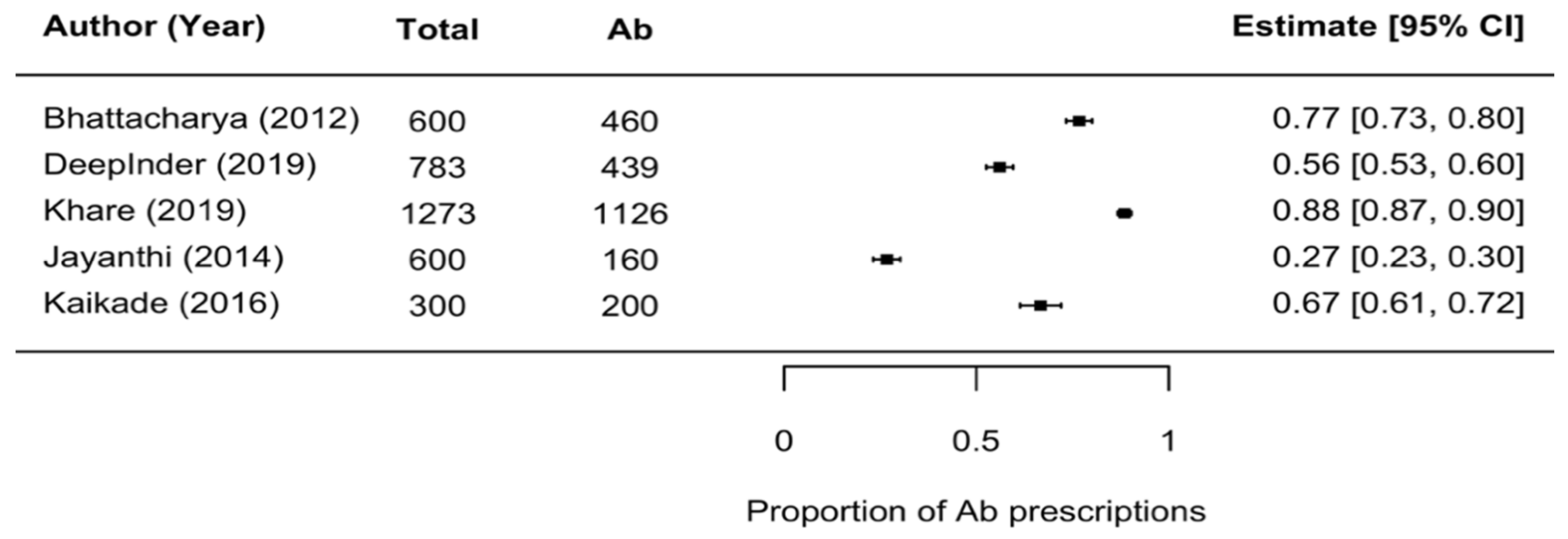
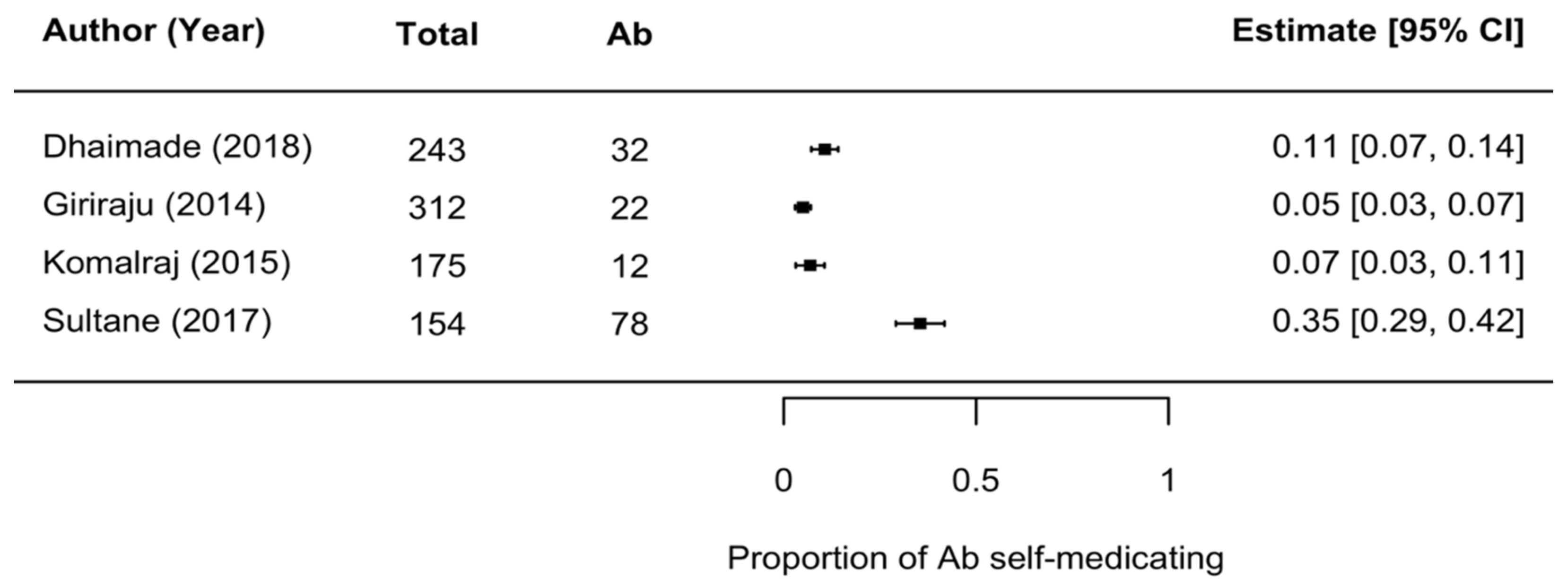
2.9. Rate of Antibiotic Use
2.10. Clinical and Non-Clinical Indications for Prescribing Antibiotics
2.11. Types and Regimen of Antibiotics Used
2.12. Quality Assessment
3. Results
3.1. Study Characteristics
3.1.1. Study Design
3.1.2. Participants
| Actual Antibiotic Prescription | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Location; Urban/Rural | Setting | Type of Population (Adult/Child) and Age Range | Male and Female % | Healthcare Provider/Prescriber | Number Eligible/Retrieved | Number of Prescriptions with Antibiotics | Outcome Evaluated | Outcome Evaluation Method | |
| Bhattacharya, 2012 | Bilaspur, Chattisgarh. Urban | 3 primary care + 2 tertiary care hospitals | Adult (age ≥ 18 years) | n/r | Dentist | 600/ 600 | 463 | Drugs prescribed for toothache | Prescriptions | |
| Chandy, 2016 | Vellore, TN. Urban/Rural | Small hospitals, GP clinics, pharmacy shops | Adult/Child | n/r | GPs, pharmacists/dentists? | 353/ 353 | 353 (all were Ab orescriptions | Pattern of antibiotic use in community—Ab use for various health problems (including dental) were assessed. | Prescriptions | |
| Datta-Datta, 2015 | Chennai, TN. Urban | Tertiary care teaching | Adult (age ≥ 18 years) | n/r | Oral Medicine specialist | 300/ 300 | Not available | Drug utilisation pattern of oral medicine department | Prescriptions | |
| Deep Inder-Pawan Kumar, 2019 | South Delhi. Urban | Tertiary care teaching | Adult/Child (>10 years) | 68.5%, 31.5% | Dentist | 783/ 1000 | 439 | Drug utilisation pattern at dental outpatients | Prescriptions | |
| Fayisa, 2019 | Malappuram, Kerala. Rural | Tertiary care teaching | Adult/Child (5–63 years) | 42.4%, 57.6% | Dentist | 2802/ 2802 | Not available | Drug utilisation and prescribing trends of antibiotics | Prescriptions | |
| Jayanthi- Naidu, 2014 | Mysore, Karnataka. Urban | Tertiary care teaching | Child (specific age range not reported) | n/r | Paediatric dentist/ | 600/ 600 | 160 | Drug utilisation and cost analysis in paediatric outpatients | Prescriptions | |
| Kaikade, 2016 | Dhule, Maharashtra. Urban | Tertiary care teaching | Child (specific age range not reported) | n/r | Paediatric dentist | 300/ 300 | 200 | Antibiotic prescription pattern in paediatric dentistry outpatients | Prescriptions | |
| Khare, 2019 | Ujjain, MP. Rural | Primary care | Adult/Child (not reported) | n/r | Informal Healthcare Providers | 1273/ 1273 | 1126 | Practices and seasonal changes in antibiotic prescription for common illness | Prescriptions | |
| Patel NN, 2014 | Piparia, Vadodara, Gujarat. Rural | Tertiary care teaching | Adult/Child (not reported) | 61.5%, 38.5% | Dentist | 200/ 200 | Not available | Utilisation pattern of antimicrobial agents | Patient interview and hospital case record | |
| Patel PS, 2016 | Vadodara, Gujarat. Urban | Tertiary care hospital | Adult/Child (not reported) | 53.6%, 46.4% | Dentist | 934/ 934 | Not available | Drug utilisation pattern at dental outpatients department | Patient case records | |
| Salman, 2009 | Aligarh, UP. Urban | Tertiary care teaching | Adult/Child (not reported) | n/r | Dentist | Not reported | Not available | Drug prescribing pattern in the outpatients department | Prescriptions | |
| Sharma M, 2014 | Jaipur, Rajastan. Urban | Tertiary care teaching | Child (2–16 years) | n/r | Paediatric dentist | 619/ 619 | Not available | Drug prescribing pattern in paediatric dentistry outpatients | Prescriptions | |
| Suhaib, 2017 | Aligarh, UP. Urban | Tertiary care teaching | Adult/Child (11–70 years) | 54%, 46% | Dentist | 100/ 115 | Not available | Antimicrobial prescription pattern in dental outpatients | Prescriptions | |
| Self-Medication | ||||||||||
| Study | Location | Setting | Type of Population (Adult/Child) and Age Range | Male, Female % | Prescriber | Number Reported/Chosen (Response Rate) | Num using Abs/Total Self-Medicating(Antibiotic Self-Medication Rate) | Outcomes Evaluated | Outcome Evaluation Method | Other Outcomes Evaluated |
| Dhaimade-Banga 2018 | Tertiary care teaching hospital, Mumbai, Maharashtra. | Urban | Adults 25–70 years mean age 36.22 | 45.3%, 54.7% | Self | 300/ 300 | 32/ 243 | Prevalence of self-medication for dental problems | Questionnaire | Source of medication, reasons for self-medicating. |
| Giriraju, 2014 | Tertiary care teaching hospital, Davangere, Karnataka. | Urban | Adults 18–65 years Mean age 38.8 | 75.6%, 24.4% | Self | 410/ 410 | 22/ 312 | Prevalence and perception about self-medication for oral health problems | Questionnaire | Source of medication, triggering factors, reasons for self-medicating, level of education and SES |
| Komalraj, 2015 | Tertiary care teaching hospital, Bengaluru, Karnataka. | Urban | Adults ≥ 18 years Mean age 38.8 ± 12.76 | 61.7%, 38.3% | Self | 175/ 175 | 12/ 175 | Prevalence of self-medication for dental problems | Questionnaire | Source of medication, triggering factors, reasons for self-medicating, level of education and SES |
| Shamsudeen, 2018 | Tertiary care teaching hospital, Chennai, TN. | Urban | Adults 18–65 years 36 ± 15.62 | 48.7%, 51.3% | Self | 610/ 610 | Not available | Prevalence, knowledge, practice of antibiotic self-medication | Interview- based on questionnaire | Source of medication, reasons for self-medicating. |
| Simon, 2015 | Tertiary care teaching hospital, Manipal, Karnataka. | Rural | Adults 18–66 years 33.51 ± 12.98 | 34%, 66% | Self | 400/ 400 | 10/ 120 | Prevalence, pattern and awareness about self-medic for oral health problems | Interview based on questionnaire | Source of medication, triggers, reasons for self-medicating, level of education |
| Sultane, 2017 | Tertiary care teaching hospital, Udaipur, Rajasthan. | Urban | Adults 18–65 years | 56.8%, 43.2% | Self | 220/ 220 | 78/154 | Prevalence of self-medication for dental problems | Questionnaire | Source of medication, triggering factors, reasons for self-med, level of education |
| Gandhi | Tertiary care teaching hospital, Gujarat | Rural | Adults 21–60 years | 51.3%, 48.7% | Self | 230/ 230 | Not available | Prevalence of self-medication for oral/dental problems | Questionnaire | Awareness about self-medication, and the risk factors among rural population |
| Rawlani | Tertiary care teaching hospital, Wardha | Rural | Adults 7–70 years | 54.3%, 45.7% | Self | 175/ 175 | Not available | Prevalence of self-medication for dental problems | Questionnaire | Factors associated with self-medication for dental problems. |
| Mahmoud, M.A. | Hyderabad, Telangana state | Urban | Adults > 18 years | 62.3%, 37.7% | Self | 175/ 175 | Not available | Prevalence of antibiotic self-medication in the community | Questionnaire | Reasons for antibiotic use, criteria for antibiotic selection and source of information, knowledge on impact of self-medication. |
| Indications for Antibiotic Prescription | ||||||||||
| Study | Location | Setting | Population Evaluated | Mean Age/Age Stratification | Male % | Number Reported/Chosen (Response Rate) | Outcome Evaluated | Type of Antibiotic | Outcome Evaluation Method | |
| Datta, 2014 | Tertiary care teaching hospital, Mohali, Punjab. | Urban/Rural Primary and tertiary care; India-various | Dentists performing implant surgery | n/r | n/r | 332/ 350 | Antibiotics for routine implant placement | Prophylactic | Questionnaire | |
| Garg, 2013 | Tertiary care teaching hospital, Indore. | Urban/Rural Primary and tertiary care; India-various | Dental practitioners | 31.58 ± 7.2 years | 55.3%, 44.7% | 552/ 1600 | Pulp and periapical diseases | Therapeutic | Questionnaire | |
| Goud, 2012 | Tertiary care teaching hospital, Bhopal. | Urban/Rural Primary and tertiary care. | Dental practitioners | n/r | n/r | 80/ 120 | Various dental diseases and minor surgical procedures | Prophylactic + therapeutic | Questionnaire | |
| Gowri, 2015 | Tertiary care teaching hospital, Meerut, UP. | Urban Tertiary care | Interns, junior residents and specialist dentists. | n/r | n/r | 120/ 120 | Various dental diseases and minor surgical procedures | Prophylactic + therapeutic | Questionnaire | |
| Jayadev, 2014 | Tertiary care teaching hospital, Hyderabad. | Urban Primary and tertiary care. | Dentists | 21–30 years 70.5%; 31–40 years 23.2%; 41–60 years 6.3% | 51.4%, 48.6% | 344/ 400 | Pulp and periapical pathologies | Therapeutic + Prophylactic | Questionnaire | |
| Karibasappa, 2014 | Tertiary care teaching hospital, Dhule. | Urban Primary and tertiary care. | BDS and MDS qualified dentists | n/r | 54%, 46% | 82/ 82 | Various oral conditions and routine dental treatment | Prophylactic + therapeutic | Questionnaire | |
| Kaul, 2018 | Tertiary care teaching hospital, Kolkata. | Urban Primary and tertiary care. | BDS and MDS qualified dentists | 71% respondents were <30 years | 62%, 38% | 115/ 300 | Various. Not clearly stated | Prophylactic + therapeutic | Questionnaire | |
| Konde, 2017 | Tertiary care teaching hospital, Bangalore. | Urban Primary and tertiary care. | Dental practitioners and paediatric dentists. | n/r | n/r | 200/ 200 | Various paediatric oral conditions | Prophylactic + therapeutic | Questionnaire | |
| Kumar, 2013 | Tertiary care teaching hospital, Secunderabad. | Urban Primary and tertiary care. | Dentists | 28.6 ± 6.5 years (21–25 years 42.1%; 26–30 years 29.2%; 31–35 years 12.5%; 36–40 years 9.3%; 41+ years 6.9% | 50%, 50% | 216/ 246 | Pulp and periapical pathologies | Therapeutic | Questionnaire | |
| Peedikayil, 2012 | Tertiary care teaching hospital, Kannur. | Urban/Rural Primary and tertiary care. | Dentists | 36.7 ± 10.7 years (<25 years 19.35%; 26–40 years 47.58%; 41–55 years 29.03%; >55 years 4.03% | 56.4%, 43.6% | 248/ 300 | Various dental infections and routine dental procedures | Prophylactic and therapeutic | Questionnaire | |
| Saini, 2014 | Tertiary care teaching hospital, Jaipur. | Urban/Rural Primary and tertiary care. | Dental practitioners | Mean age 41 years | n/r | 500/ 525 | Dental infection and routine dental procedures | Prophylactic and therapeutic | Questionnaire | |
| Sam Prasad, 2017 | Tertiary care teaching hospital, Chennai. | Urban. Primary care. | Dental practitioners | Mean 41.88 years. Age range 24–67 years. | 57%, 43% | 100/ 100 | Unclear | Unclear | Questionnaire | |
| Shafia, 2019 | Tertiary care teaching hospital, Srinagar. | Urban. Primary and tertiary care. | GDPs and specialist dental practitioners | n/r | n/r | 247/ 300 | Various dental infections and routine dental procedures | Prophylactic and therapeutic | Questionnaire | |
| Wasan, 2017 | Tertiary care teaching hospital, New Delhi. | Urban Primary and tertiary care. | GDPs, specialist trainees and specialist practitioners | 27.9 ± 7 years | 41%, 59% | 539/ 667 | Various dental conditions | Prophylactic and therapeutic | Questionnaire | |
| Gour, 2013 | Tertiary care teaching hospital, Jaipur. | Urban Primary and tertiary care. | Dentists | n/r | 56%, 44% | 150/ 175 | Various dental infections and prophylaxis | Prophylactic and therapeutic | Questionnaire | |
| Harsh Vardhan, 2017 | Tertiary care teaching hospital, Mallaram, Talangana. | Urban/Rural Primary and tertiary care. | Dentists and specialist dental practitioners | n/r | 70%, 30% | 450/ 700 | Non-clinical reasons | N/a | Questionnaire | |
| Nandkeoliar, 2016 | Tertiary care teaching hospital, Imphal, Manipur. | Urban/Rural Primary and tertiary care. | Dentists | 21–25 years 28%; 26–30 years 36%; 31–35 years 23%; 36–40 years 4%; >41 years 9% | n/r | 100/ 122 | Various acute and chronic dental conditions and routine dental procedures | Prophylactic and therapeutic | Questionnaire | |
| Naveen, 2015 | Tertiary care teaching hospital, Bangalore. | Urban Tertiary care | Dentists and specialist dentists | n/r | 47% | 202/ 245 | Various dental infections and prophylaxis for medically compromised patients | Prophylactic and therapeutic | Questionnaire | |
| Padda, 2016 | Tertiary care teaching hospital, Ferozepur, Punjab. | Urban/Rural Primary care. | Dentists | n/r | 60% | 200/ 200 | Antibiotics prescription for various clinical signs and dental conditions | Therapeutic | Questionnaire | |
| Patait, 2015 | Tertiary care teaching hospital, Sangamner, Maharashtra. | Urban Tertiary care | Dentists and specialist dentists. | n/r | n/r | 41/ 42 | Various dental conditions | Therapeutic | Questionnaire | |
| Punj, 2018 | Tertiary care teaching hospital, Mangalore. | Urban Primary care. | Dentists | n/r | 57.8% | 173/ Not known | Unclear | Prophylactic and therapeutic | Questionnaire | |
| Puranik, 2018 | Tertiary care teaching hospital, Bengaluru. | Urban Primary care. | Dentists | 56% ≤ 35 years; 44% >35 years | 54.3% | 400/ 400 | Various oral conditions and dental procedures | Prophylactic and therapeutic | Questionnaire | |
| Srinivasan, 2017 | Tertiary care teaching hospital, Vellore. | Urban Primary care. | Dentists | 25–35 years 70%; ≥36 years 30% | 54% | 117/ 150 | Various dental conditions and procedures and non-clinical reasons | Prophylactic and therapeutic | Questionnaire | |
| Tripathi 2020 | Tertiary care teaching hospital, Secunderabad, | Urban Primary and tertiary care | Dentists | 25–34 years 77.9%, 35–44 y 16%, 45–54 years 3.1%, 55–64 y 1.5%, >65 y 1.5% | 52.7%, 47.3% | 363/568 | Implant therapy and management of peri-implantitis | Therapeutic | Questionnaire (online) | |
| Kaul. R. 2021 | Tertiary care hospital, Manipur | Urban/ rural Primary and tertiary care | Dentists | 20–30 years 63.4%, 31–40 y 30.8%, 41–50 years 4%, >51 years 1.8% | 40.6%, 59.4% | 276/400 | Pain and infection control in children | Prophylactic | Questionnaire (online) | |
| Savithra Prakash | Pharmacies | Urban, Primary care | Pharmacists | n/r | n/r | 61/68 | Dispensing for toothache/toothache with fever | Therapeutic | Simulated patients | |
| Shoeb Ahmed | Hyderabad | Urban, variable | Dentists, pharmacists | 23–60 years | 60% | 25/25 | Perception about reasons for AMR | n/a | Interviews (qualitative research) | |
3.1.3. Study Setting
3.2. Quality Assessment
3.3. Primary Outcomes
Rate of Antibiotic Use for Dental/Oral Problems
- Rate of antibiotic prescriptions in clinical dental settings
- b.
- Rate of over-the-counter antibiotic use (self-medication) for dental problems
- c.
- Rate of antibiotics prescribed in dentistry compared to other healthcare fields
| Author | Total Antibiotic Prescriptions in All Fields of (Human) Healthcare | Number of Prescriptions in Dentistry Alone | Proportion of Antibiotic Prescriptions Accounted for by Dentistry |
|---|---|---|---|
| Khare [38] (rural) | 11,336 | 1126 | 9.93% |
| Chandy [47] (urban and rural) | 10,800 | 353 | 3.3% |
3.4. Secondary Outcomes
3.4.1. Indications for Antibiotics
Therapeutic Indications for Antibiotic Prescription
| Indication Identified | Proportion of Dentists Prescribing % | Mean Dentists’ Proportion Prescribing % (SD) |
|---|---|---|
| Acute pulpitis | 30 [89], 13 [32], 71 [74], 43.6 [34], 76.5 [75], 49.1 [77], 63.8 [83], 60.8 [85] | 50.98 (20.17) |
| Irreversible pulpitis | 37.6 [68], 53 [89], 7.8 [71], 35 [32], 60.6 [33], 75 [74], 85.5 [77] | 50.64 (26.34) |
| Pulpitis (non-specific) | 72 [72], 54.8 [76], 23 [35] | 50.26 (20.66) |
| Acute apical periodontitis | 71.6 [68], 10 [71], 65.2 [33] | 48.93 (27.65) |
| Chronic apical periodontitis | 38.2 [68], 3.4 [71], 44.9 [33] | 28.83 (18.19) |
| Apical periodontitis (non-specific) | 87.8 [72], 85.5 [77], 39 [35] | 70.7 (22.48) |
| Necrotic pulp/periapical abscess with sinus tract/discharge | 46.9 [68], 15 [71], 57 [32], 69.4 [33], 55 [77] | 48.66 (20.46) |
| Periapical/dentoalveolar abscess | 98.8 [72], 50 [32], 95 [74], 98.7 [77], 88 [69] | 86.1 (20.6) |
| Periapical abscess with extra oral swelling (includes space infection, cellulitis, spreading infection, systemic involvement) | 90.2 [68], 56.4 [71], 97.6 [72], 70 [32], 92.1 [34], 93 [33], 91.6 [74], 82.5 [75], 76.2 [76], 98.5 [77], 88.8 [83], 91.9 [85] | 85.7 (12.46) |
| Periodontal abscess | 84 [69], 94 [74], 88.1 [34], 77 [75], 68.3 [83], 88.1 [85] | 83.25 (8.42) |
| Pericoronitis | 77 [69], 75.6 [72], 92 [74], 76.7 [34], 80 [75], 76.2 [76], 81.1 [83], 28.7 [35] | 73.4 (18.83) |
| Soft tissue infections | 90 [89] | 90 |
| Chronic periodontitis | 33 [89], 65 [74], 47.5 [34], 51 [75] | 48.63 (10.7) |
| Acute periodontitis | 26 [89] | 26 |
| Acute gingivitis | 23 [89], 74 [74] | 48.5 (25.5) |
| Chronic gingivitis | 3 [89], 50 [75], 28.2 [83] | 27.07 (19.2) |
| Acute necrotising gingivitis | 90 [74], 82 [75], 69 [76] | 80.3 (8.65) |
| Dry socket | 58 [74], 57.9 [34], 35 [75], 45.2 [76], 41.8 [83], 53.2 [85] | 48.5 (9.36) |
| Dental caries | 18.3 [72], 53 [35] | 36.5 (17.35) |
| Viral infections | 37.5 [70], 24.2 [35] | 30.85 (6.6) |
| Other therapeutic indications identified: Sinusitis [83], trismus [56], tooth sensitivity [72], periodontal pocket [72], halitosis [72], peri-implantitis [87], and peri-implant mucositis [87]. | ||
Prophylactic Indications for Prescribing Antibiotics
| Prophylactic Indications for Prescribing Antibiotics | ||
|---|---|---|
| Indication Identified | Proportion of Dentists Prescribing % | Mean Dentists’ Proportion Prescribing % (SD) |
| Tooth fracture/trauma | 28.7 [34], 56.7 [35], 46.3 [81], 52.5 [86] | 46.05 (10.7) |
| Scaling | 2.5 [83], 42 [74], 18 [81] | 20.8 (16.23) |
| Restoration | 6 [86] | 6 |
| Periapical surgery | 96.3 [72],22.5 [35], 34 [83] | 50.93(32.42) |
| Extraction | 67 [69], 72 [72], 91 [74], 84.7 [34], 72.6 [77], 76 [81], 13.6 [83], 54.5 [35], 39 [86], 26 [73] | 59.64 (24.4) |
| Surgical extractions | 90.2 [72], 14 [35] | 52.1 (38.1) |
| Removal of impacted teeth | 76.7 [69], 96.3 [72], 72.8 [34], 96 [81], 69.2 [83], 89.6 [85], 10 [35] | 72.94 (27.63) |
| Periodontal/flap surgery | 77 [69], 96.3 [72], 86 [81], 25.7 [35] | 71.25 (31.37) |
| Minor oral surgeries | 60 [70], 27.1 [77] | 43.5 (16.45) |
| Soft tissue surgery | 88 [71] | 88 |
| Routine Implants | 85.5 [67], 92.7 [72], 86 [81], 25 [35] | 72.3 (27.4) |
| Root canal treatment | 84.1 [72], 78.7 [34], 71.4 [76], 20.9 [77], 60 [81], 76.6 [82], 88.8 [83], 27.7 [35], 44.8 [71] | 61.4 (24.85) |
| Replantation of avulsed tooth | 89 [32], 32.4 [83] | 60.7 (28.3) |
| Other prophylactic indications identified: asymptomatic impacted tooth [72]; trauma to primary tooth [86], restoration of primary teeth [86], and extraction of primary teeth [86] | ||
Antibiotic Prescription (Prophylaxis) in Medically Compromised Patients
| Antibiotic Prescription for Medically Compromised Patients | ||
|---|---|---|
| Indication Identified | Proportion of Dentists Prescribing % | Mean Dentists’ Proportion Prescribing % (SD) |
| Medically compromised (unspecified) | 3.3 [70] | 3.3 |
| Diabetes (Type 1) | 45 [73], 19.5 [35], 45.2 [83] | 36.57 (12.07) |
| Diabetes (Type 2) | 78 [81], 81 [82], 58.4 [85] | 72.47 (10.02) |
| Blood dyscrasias/bleeding disorders | 76 [73], 13.5 [35], 91.4 [83] | 60.3 (33.68) |
| Pregnancy | 32 [81], 54.2 [82], 25 [74] | 37.07 (12.45) |
| Other indications identified for medically compromised patients (prophylactic): RCT in medically compromised patients [34,69], hypertension [34,83], kidney transplant [34], liver failure [34], respiratory disorders [35,73], epilepsy [83], hyper- and hypothyroidism [83], immunocompromised [74,83], carcinoma of the large intestine [74], and infectious diseases [81] | ||
Non-Clinical Reasons for Prescribing Antibiotics
| Non-Clinical Indication (Reasons) for Antibiotic Prescription | ||
|---|---|---|
| Indication Identified | Proportion of Dentists Prescribing% | Mean Dentists’ Proportion Prescribing % (SD) |
| Patient expectation | 5.6 [71], 4 [32], 35 [73], 57.32 [84], 45 [74], 8.4 [34], 5 [80], 7.5 [35], 55 [83], PNS [88] | 24.76 (21.71) |
| Pressure of time and workload | 7.8 [71], 5 [34], 3 [80], 39 [35] | 13.7 (14.71) |
| Fear of loss of patient | 38 [84], 53 [83], proportion not available [88] | 45.5 (7.5) |
| Unsure diagnosis | 36.1 [71], 14.5, 42 [74], 19.8 [34], 6 [80], 77.7 [83] | 32.68 (23.57) |
| Delaying/incomplete treatment | 34.79 [71], 9 [32], 51 [73], 49.5 [34], 9 [80] | 30.66 (18.57) |
| Patient’s SES | 9.9 [34], 48.7 [83] | 29.3 (19.4) |
| Poor oral hygiene and patients’ habits (gutka chewing) | Proportion not available [88] | n/av |
| Market pressure from pharmaceutical companies and Mutual commercial interests. | 5.6 [71], 4 [32], 35 [73], 57.32 [84], 45 [74], 8.4 [34], 5 [80], 7.5 [35], 55 [83], PNS [88] | 24.76 (21.71) |
| Maintain dentist’s reputation | 7.8 [71], 5 [34], 3 [80], 39 [35] | 13.7 (14.71) |
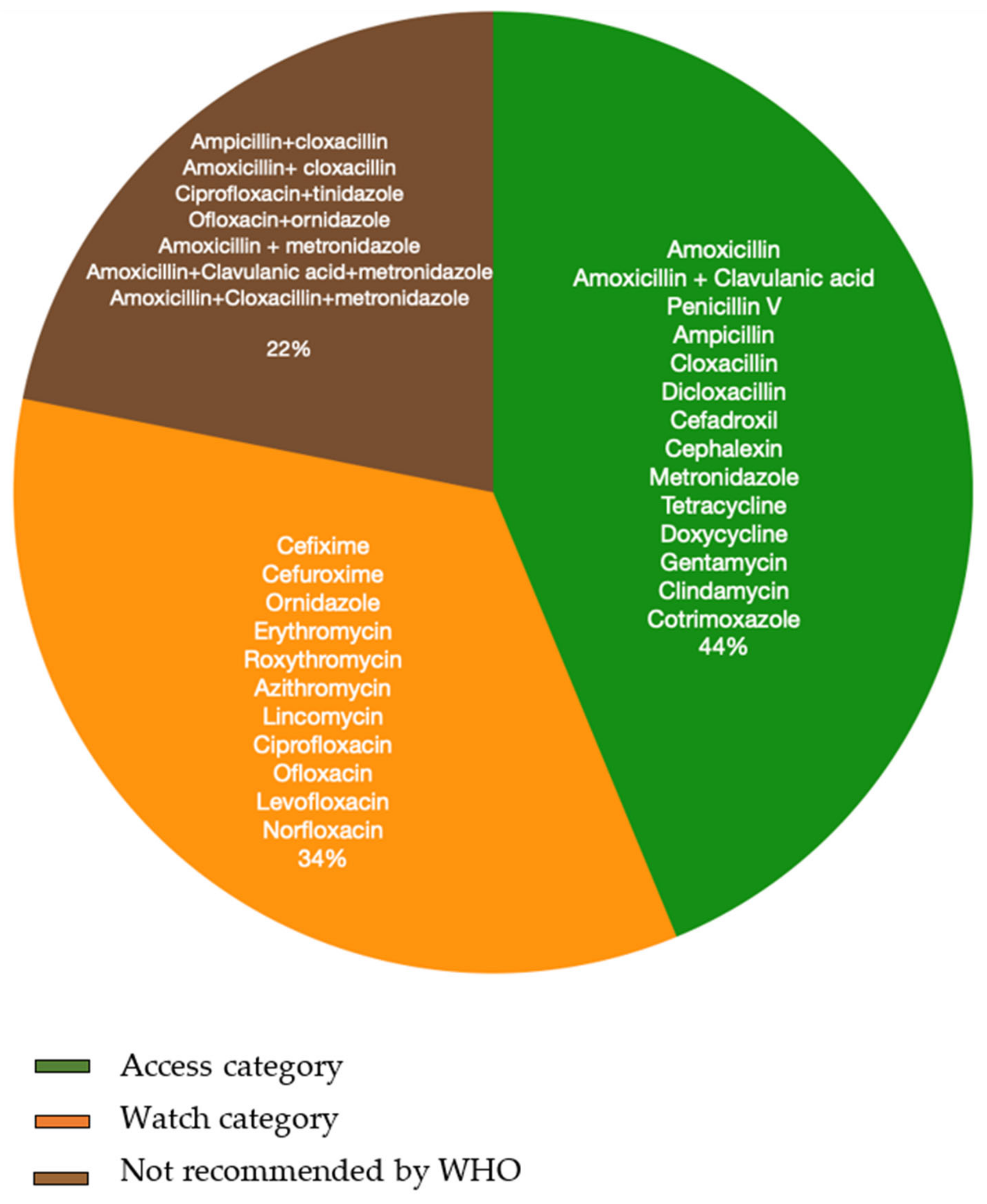
3.4.2. Antibiotics Used
Types and Regimen of Antibiotics
Combination Antibiotics and Fixed Dose Drug Combinations (FDC)
- Combinations Identified from Questionnaire Surveys
- b.
- Combinations Identified from Prescription Audits (Actual Prescriptions)
3.4.3. Antibiotic Use in Different Settings and Populations
- Difference in Antibiotic Prescription Rate between the Urban and Rural Population
- b.
- Difference in Antibiotic Self-Medication Rates between Urban and Rural Population
- c.
- Difference in Antibiotic Prescription Rate between Adults and Children
- d.
- Difference in Prescription Rate Based on Prescriber Characteristics
3.4.4. Factors Influencing Practitioners’ Prescription Pattern and/or Choice of Antibiotics
3.4.5. Reasons for Self-Medication for Dental Problems
- avoidance of the dentist;
- easy accessibility to antibiotics without prescription and the ability to use these repeatedly as and when there is dental pain;
- time constraints and cost of dental treatment;
- immediate relief from dental pain,
- mutual trust between the pharmacist and customers (dental patients), in the form of credits given by pharmacies to buy antibiotics, the ability of patients to return or replace antibiotics when they do not work.
4. Discussion
4.1. Antibiotic Prescription Rate
4.2. Antibiotic Prescription in Dentistry versus Medicine
4.3. Self-Medication Rate
4.4. Indications for Antibiotic Prescription
4.5. Types of Antibiotics
4.6. Providers
4.7. Role of Pharmacists
5. Limitations
6. Future Research and Clinical Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resitant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2014. Available online: http://amr-review.org/ (accessed on 20 June 2020).
- Viens, A.M.; Littmann, J. Is Antimicrobial Resistance a Slowly Emerging Disaster? Public Health Ethics 2015, 8, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Littmann, J.; Viens, A.M. The Ethical Significance of Antimicrobial Resistance. Public Health Ethics 2015, 8, 209–224. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.; Piddock, L. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; Group, E.P. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic/Antimicrobial Resistance (AR/AMR). Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 15 August 2020).
- Rosenblatt-Farrell, N. The landscape of antibiotic resistance. Environ. Health Perspect. 2009, 117, A244–A250. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Review on Antimicrobial Resistance. Antimicrobial Resitance: Tackling a Crisis for the Health and Wealth of Nations; Thermo Fisher Scientific, Inc.: London, UK, 2014. [Google Scholar]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 26 November 2020).
- Frost, J.C.I.; Joshi, J.; Faure, K.; Laxminarayan, R. Access Barriers Antibiot; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2019. [Google Scholar]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Basu, S.; Garg, S. Antibiotic prescribing behavior among physicians: Ethical challenges in resource-poor settings. J. Med. Ethics Hist. Med. 2018, 11, 5. [Google Scholar] [PubMed]
- Ranjalkar, J.; Chandy, S.J. India’s National Action Plan for antimicrobial resistance—An overview of the context, status, and way ahead. J. Family Med. Prim. Care 2019, 8, 1828–1834. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Chaudhury, R.R. Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med. 2016, 13, e1001974. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.J.; Hsueh, K.; Sallah, Y.H.; Feng, Q.; Jafarzadeh, S.R.; Munshi, K.D.; Lockhart, P.B.; Thornhill, M.H.; Henderson, R.R.; Fraser, V.J. An evaluation of dental antibiotic prescribing practices in the United States. J. Am. Dent. Assoc. 2017, 148, 878–886.e1. [Google Scholar] [CrossRef]
- Hicks, L.A.; Chien, Y.W.; Taylor, T.H.; Haber, M., Jr.; Klugman, K.P. Active Bacterial Core Surveillance T. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin. Infect. Dis. 2011, 53, 631–639. [Google Scholar] [CrossRef]
- Marra, F.; George, D.; Chong, M.; Sutherland, S.; Patrick, D.M. Antibiotic prescribing by dentists has increased: Why? J. Am. Dent. Assoc. 2016, 147, 320–327. [Google Scholar] [CrossRef]
- Poveda Roda, R.; Bagan, J.V.; Sanchis Bielsa, J.M.; Carbonell Pastor, E. Antibiotic use in dental practice. A review. Med. Oral. Patol. Oral Cir. Bucal. 2007, 12, E186–E192. [Google Scholar] [PubMed]
- Teoh, L.; Stewart, K.; Marino, R.; McCullough, M. Antibiotic resistance and relevance to general dental practice in Australia. Aust. Dent. J. 2018, 63, 414–421. [Google Scholar] [CrossRef]
- Suda, K.J.; Calip, G.S.; Zhou, J.; Rowan, S.; Gross, A.E.; Hershow, R.C.; Perez, R.I.; McGregor, J.; Evans, C.T. Assessment of the Appropriateness of Antibiotic Prescriptions for Infection Prophylaxis Before Dental Procedures, 2011 to 2015. JAMA Netw. Open 2019, 2, e193909. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Tonkin-Crine, S.; Pavitt, S.H.; McEachan, R.; Douglas, G.V.A.; Aggarwal, V.; Sandoe, J.A.T. Factors associated with antibiotic prescribing for adults with acute conditions: An umbrella review across primary care and a systematic review focusing on primary dental care. J. Antimicrob. Chemother. 2019, 74, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Agnihotry, A.; Gill, K.S.; Stevenson, R.G.; Fedorowicz, Z.; Kumar, V.; Sprakel, J.; Cohen, S.; Thompson, W. Irreversible Pulpitis—A Source of Antibiotic Over-Prescription? Braz. Dent. J. 2019, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Agnihotry, A.; Thompson, W.; Fedorowicz, Z.; van Zuuren, E.J.; Sprakel, J. Antibiotic use for irreversible pulpitis. Cochr. Database Syst Rev. 2019, 5, CD004969. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.E.; Hanna, D.; Rowan, S.A.; Bleasdale, S.C.; Suda, K.J. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Infect. Dis. 2019, 6, ofz067. [Google Scholar] [CrossRef] [PubMed]
- Loffler, C.; Bohmer, F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care—A systematic review. PLoS ONE 2017, 12, e0188061. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J. Am. Dent. Assoc. 2019, 150, 906–921.e12. [Google Scholar] [CrossRef]
- NICE. Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. Available online: https://www.nice.org.uk/guidance/cg64/chapter/Recommendations (accessed on 10 August 2020).
- Thornhill, M.H.; Dayer, M.J.; Durkin, M.; Lockhart, P.B.; Baddour, L.M. Oral antibiotic prescribing by NHS dentists in England 2010–2017. Br. Dent. J. 2019, 227, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- George, J. Antibiotics in India. Br. Dent. J. 2020, 228, 60. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Angrish, P.; Jain, P.; Saha, S.; Sengupta, A.V.; Mukherjee, S. A Survey on the Use of Antibiotics among the Dentists of Kolkata, West Bengal, India. Int. J. Clin. Pediatr. Dent. 2018, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.P.; Kaushik, M.; Kumar, P.U.; Reddy, M.S.; Prashar, N. Antibiotic prescribing habits of dental surgeons in hyderabad city, India, for pulpal and periapical pathologies: A survey. Adv. Pharmacol. Sci. 2013, 2013, 537385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naveen, N.G.S.P.; Vanishree, N.; Patnaik, S.; Bharath, C.; Keerthi Prasad, K.S.; Bullappa, D. Current Trends in Prescription of Antibiotics among Dentists Working in Various Dental Colleges of Bangalore City, India—A Cross Sectional Study. Int. J. Oral Health Med. Res. 2015, 2, 8–14. [Google Scholar]
- Puranik, M.P.; Sabbarwal, B.; Bose, S. Dental practitioner’s knowledge and practices regarding antibiotic prescription and development of resistance: A cross-sectional study. J. Indian Assoc. Pub. Health Dent. 2018, 16, 144–148. [Google Scholar]
- Mythri Halappa, N.B.; Kumar, S.; Sreenivasa, H. SWOT Analysis of Dental Health Workforce in India: A Dental alarm. J. Clin. Diagn. Res. 2014, 8, ZE03–ZE05. [Google Scholar]
- Gambhir, R.S.; Brar, P.; Singh, G.; Sofat, A.; Kakar, H. Utilization of dental care: An Indian outlook. J. Nat. Sci. Biol. Med. 2013, 4, 292–297. [Google Scholar] [CrossRef]
- Khare, S.; Purohit, M.; Sharma, M.; Tamhankar, A.J.; Lundborg, C.S.; Diwan, V.; Pathak, A. Antibiotic Prescribing by Informal Healthcare Providers for Common Illnesses: A Repeated Cross-Sectional Study in Rural India. Antibiotics 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf;jsessionid=D7CD72EA869FE7B496DFC8EF647D7B94?sequence=1 (accessed on 31 May 2020).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Tetzlaff, J.M.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- WHO. AWaRe. 2019. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 19 March 2020).
- NHP. National List of Essential Medicines. Available online: https://www.nhp.gov.in/NHPfiles/NLEM%2C%202015.pdf (accessed on 9 October 2020).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- JBI. Critical Appraisal Checklist for Qualitative Research. 2017. Available online: https://joannabriggs.org/critical-appraisal-tools (accessed on 19 March 2020).
- Bhattacharya, A.; Nigam, A.; Satpathy, A.; Sahu, P. Study of drug used in treatment of toothache in various hospitals and private clinics of bilaspur region of Chhattisgarh (India). Asian J. Pharm. Clin. Res. 2012, 5, 215–221. [Google Scholar]
- Borole Priyanka, M.; Chaudhari Yogesh, S.; Amit, M.; Patil Ishwardas, D.; Shinde Sagar, K.; Manisha, B. Current clinical practices of antimicrobials in periodontal disease: A survey. Int. J. Pharm. Phytopharm. Res. 2013, 2, 415–416. [Google Scholar]
- Chandy, S.J.; Thomas, K.; Mathai, E.; Antonisamy, B.; Holloway, K.A.; Stalsby Lundborg, C. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: A repeated cross-sectional study in Vellore, South India. J. Antimicrob. Chemother. 2013, 68, 229–236. [Google Scholar] [CrossRef]
- Fayisa, K.A.P.; Sudhakar, A. Drug utilization patterns of antibiotics in outpatient department of educare dental hospital at Malappuram, Kerala, India. Int. J. Basic Clin. Pharmacol. 2019, 8, 930–933. [Google Scholar]
- Jayanthi, M.K.; Sushma, N.V. Drug utilization pattern and pharmacoeconomic study in paediatric dentistry at a tertiary hospital. Int. J. Pharm. Pharm. Sci. 2014, 6, 70–72. [Google Scholar]
- Swapnil, B.; Kaikade, N.P. Prescribing pattern of antibiotics in pedodontics OPD of tertiary care dental hospital in Dhule district. Int. J. Basic Clin. Pharmacol. 2016, 5, 1462–1465. [Google Scholar]
- Pawan, K.; Deep Inder. A Study of Drug Utilization Pattern (DUP), at Dental Outpatient Department of a Teaching Institution, in South Delhi, India. Int. J. Pharm. Chin. Med. 2019, 3, 1–5. [Google Scholar]
- Patel, N.N.; Desai, H.; Modi, N.; Shah, N. Drug Utilization Pattern Of Antimicrobial Agents In Dental Outpatients Of A Tertiary Care Teaching Rural Hospital. Natl. J. Integr. Res. Med. 2014, 5. [Google Scholar]
- Patel, P.S.; Patel, S.N.; Bhave, A. Evaluation of prescribing pattern at dental outpatient department at a hospital, Gujarat. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Pratiti, D.; Datta, P.P. Drug utilization pattern in oral medicine department of Saveetha Dental College, Tamil Nadu, India. Natl. J. Med Res. 2015, 5, 272–274. [Google Scholar]
- Salman, M.T.; Khan, F.A.; Rahman, S.Z.; Makhdoom, M. Drug prescribing pattern in dental teaching hospital. JK Sci. 2009, 11, 107. [Google Scholar]
- Sharma, M.; Tandon, S.; Chugh, T.; Sharma, S.; Parmod, P.S.; Aggarwal, V.; Kashyap, N. Drug abuse in paediatric dentistry: A cross-sectional study. J. Clin. Diagn. Res. JCDR 2014, 8, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Suhaib, M.; Ahmad, F.; Ahmad, M. Prescription pattern of antimicrobial agents among dental practitioners in a tertiary care center in North India. Asian J. Pharm. Clin. Res. 2017, 10, 192–195. [Google Scholar] [CrossRef][Green Version]
- Giriraju, A. Perception about self-medication practices for oral health problems among the general population of Davangere city, Karnataka, India. J. Indian Assoc. Public Health Dent. 2014, 12, 219–225. [Google Scholar] [CrossRef]
- Dhaimade, P.A.; Banga, K.S. Evaluation of chief complaints of patients and prevalence of self-medication for dental problems: An institutional study. Int. J. Community Med. Public Health 2018, 5, 674–681. [Google Scholar] [CrossRef]
- Gandhi, S.G.R.; Nayyar ASGandhi, S.; Gandhi, R.A.; Nayyar, A.S. Assessment of abuse of self-medication for oral and dental problems among 21–60 years aged populace residing in the rural areas of Belgaum Taluk, Karnataka, India. A questionnaire study. Arch. Med. Health Sci. 2016, 4, 180–184. [Google Scholar]
- Rawlani, S.M.R.S.; Bhowte, R.; Degwekar, S.; Rawlani, S.; Chandak, R. Prevalence of self-medication among dental patients in rural area of Maharashtra, India: A cross-sectional study. Indian J. Oral Sci. 2015, 6, 51–54. [Google Scholar] [CrossRef]
- Shamsudeen, S.M.; Priya, R.S.; Sujatha, G.; Muruganandhan, J.; Manikandan, K. Self-medication with antibiotics: A knowledge, attitude, and practice appraisal of 610 dental patients in Chennai, India, from 2016 to 2017. J. Educ. Health Promot. 2018, 7, 66. [Google Scholar] [PubMed]
- Simon, A.K.; Rao, A.; Rajesh, G.; Shenoy, R.; Pai, M.B.H. Trends in self-medication for dental conditions among patients attending oral health outreach programs in coastal Karnataka, India. Indian J. Pharmacol. 2015, 47, 524–529. [Google Scholar] [PubMed]
- Sultane, P.C.S.; Bhat, N.; Choudhary, S.; Todkar, M.; Singh, P.; Patel, S.A.; Patel, S.D. Perception about Self-medication Practices for Oral Health Problems among Patients attending Dental Hospital, Udaipur, India. Int. J. Oral Care Res. 2017, 5, 54735551. [Google Scholar] [CrossRef]
- KomalRaj, M.R.; Bhat, K.B.; Aruna, C.N. Self medication practices for ORAL health problems among DENTAL patients in Bangalore: A cross sectional study. IOSR J. Pharm. 2015, 10, 68–75. [Google Scholar]
- Mahmoud, M.A.; Wajid, S.; Naqvi, A.A.; Samreen, S.; Althagfan, S.S.; Al-Worafi, Y. Self-Medication with Antibiotics: A Cross-Sectional Community-Based Study. Lat. Am. J. Pharm. 2020, 39, 348–353. [Google Scholar]
- Datta, R.; Grewal, Y.; Batth, J.S.; Singh, A. Current Trend of Antimicrobial Prescription for Oral Implant Surgery Among Dentists in India. J. Maxillofac. Oral Surg. 2014, 13, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Agrawal, N.; Tewari, R.K.; Kumar, A.; Chandra, A. Antibiotic prescription pattern among Indian oral healthcare providers: A cross-sectional survey. J. Antimicrob. Chemother. 2014, 69, 526–528. [Google Scholar] [CrossRef]
- Goud, S.R.; Nagesh, L.; Fernandes, S. Are we eliminating cures with antibiotic abuse? A study among dentists. Niger. J. Clin. Pract. 2012, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.; Mehta, D.; Kannan, S. Antibiotic use in dentistry: A crosssectional survey from developing country. J. Orofac. Sci. 2015, 7, 90–94. [Google Scholar]
- Jayadev Karunkar, P.; Viswanath, B.; Siddhartha Chinmayi, S.; Chaitanya, B. Knowledge and pattern of antibiotic and non narcotic analgesic prescription for pulpal and periapical pathologies—A survey among dentists. J. Clin. Diagn. Res. 2014, 8, 10–14. [Google Scholar]
- Karibasappa, G.N.; Sujatha, A. Antibiotic resistance-a concern for dentists. IOSR J. Dent. Med. Sci. 2014, 13, 112–118. [Google Scholar]
- Konde, S.; Jairam, L.S.; Peethambar, P.; Noojady, S.R.; Kumar, N.C. Antibiotic overusage and resistance: A cross-sectional survey among pediatric dentists. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nandkeoliar, T.P.P.; Bhushan, P.; Kour, A.; Basnett, P. Antibiotics prescribing habits: An evaluation of dental practitioners in Manipur, North east India. Int. J. Basic Clin. Pharmacol. 2016, 5, 623–627. [Google Scholar] [CrossRef]
- Padda, S.G.G.; Kaur, B. Prescription pattern of drugs among general dentists in north India. Int. J. Curr. Res. 2016, 8. [Google Scholar]
- Patait, M.U.N.; Rajderkar, M.; Kedar, S.; Shah, K.; Patait, R. Antibiotic prescription: An oral physician’s point of view. J. Pharm. Bioall. Sci. 2015, 7, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Peedikayil, F.C.; Narayan, A. A survey of antibiotics prescribing practices of dentists in Kerala state. SRM J. Res. Dent. Sci. 2012, 3, 109. [Google Scholar]
- Prakash, S.P.S.; Lakshminarayan, N. Assessment of pharmacists’ oral health advice to clients without prescription using “By Proxy” method. J. Indian Assoc. Public Health Dent. 2016, 14, 323–326. [Google Scholar] [CrossRef]
- Prasad, S.; Rajesvari, R. Antibiotic prescribing practice among general dental practitioners. J. Oral Med. Oral Surg. Oral Pathol. Oral Radiol. 2017, 3, 14–16. [Google Scholar]
- Punj, A.S.S.; Thomas, B.; Ramesh, A. Knowledge awareness and prescription practice of antibiotics among private dental practitioners in Mangalore. J. Educ. Ethics. Dent. 2016, 6, 72–77. [Google Scholar] [CrossRef]
- Saini, N.; Saini, V.; Mehta, P.W. Misuse of antibiotics: A potential threat. IOSR J. Dent. Med. Sci. 2014, 13, 68–72. [Google Scholar] [CrossRef]
- Shafia Karan, K.; Sudangha, B.; Neerja, S. Trends of antibiotics prescription amongst general dental practitioners and specialist dental practitioners in Delhi NCR: A survey. Ann. Int. Med Dent. Res. 2019, 5, DE4. [Google Scholar]
- Srinivasan, K.C. Current trends in antibiotic prescribning practices by dentists- cross sectional study. Int. J. Sci. Res. 2017, 6, 58–63. [Google Scholar]
- Vardhan, T.H.L.N.; Haritha, B. Exploring the pattern of antibiotic prescription by dentists: A questionnaire-based study. J. NTR Univ. Health Sci. 2017, 149–153. [Google Scholar] [CrossRef]
- Wasan, H.G.P.; Mathur, A.; Mutneja, E.; Mathur, V.P.; Gupta, Y.K. Influence of qualification and practice settings of dental practitioners on antimicrobial prescribing in Delhi and National Capital Region, India. J. Nat. Sci. Biol. Med. 2017, 8, 29–34. [Google Scholar]
- Kaul, R.; Sandhu, H.S.; Talwar, B.S.; Chengappa, D.; Bali, A.; Koul, R. Oral pain and infection control strategies for treating children and adolescents in India. J. Fam. Med. Prim. Care 2021, 10, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Vasudevan, S.; Palle, A.R.; Gedela, R.K.; Punj, A.; Vaishnavi, V. Awareness and management of peri-implantitis and peri-mucositis among private dental Practitioners in Hyderabad—A cross-sectional study. J. Indian Soc. Periodontol. 2020, 24, 461–466. [Google Scholar] [PubMed]
- Ahmed, S. The Socio-cultural Dynamics of Antibiotic Misuse in Hyderabad City, India: A Qualitative Study of Dentist and Pharmacist. J. Karnali Acad. Health Sci. 2019, 2, 197–202. [Google Scholar] [CrossRef]
- Gour, P.R.; Kohli, S.; Advani, U.; Kulshreshtha, S.; Jain, A.; Parakh, R. Prescription pattern of antimicrobial agents by dental practitioners: A questionnaire based study. Int. J. Basic Clin. Pharmacol. 2013, 2, 311–314. [Google Scholar] [CrossRef][Green Version]
- Kaur, A.; Bhagat, R.; Kaur, N.; Shafiq, N.; Gautam, V.; Malhotra, S.; Suri, V.; Bhalla, A. A study of antibiotic prescription pattern in patients referred to tertiary care center in Northern India. Ther. Adv. Infect. Dis. 2018, 5, 63–68. [Google Scholar] [CrossRef] [PubMed]
- WHO. Quality Assurance of Medicines terminology Database. Available online: https://www.who.int/medicines/areas/quality_safety/quality_assurance/QASterminology-updated-october2018.pdf (accessed on 15 March 2020).
- WHO. Using Indicators to Measure Country Pharmaceutical Situations. Available online: https://www.who.int/medicines/publications/WHOTCM2006.2A.pdf (accessed on 12 August 2020).
- Cope, A.L.; Chestnutt, I.G.; Wood, F.; Francis, N.A. Dental consultations in UK general practice and antibiotic prescribing rates: A retrospective cohort study. Br. J. Gen. Pract. 2016, 66, e329–e336. [Google Scholar] [CrossRef] [PubMed]
- Halling, F.; Neff, A.; Heymann, P.; Ziebart, T. Trends in antibiotic prescribing by dental practitioners in Germany. J. Craniomaxillofac. Surg. 2017, 45, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- ICMR. Treatment Guidelines for Antimicrobial Use in Common Syndromes, 2nd ed.; ICMR: New Delhi, India, 2019.
- Panda, A.; Pradhan, S.; Mohapatro, G.; Kshatri, J.S. Predictors of over-the-counter medication: A cross-sectional Indian study. Perspect. Clin. Res. 2017, 8, 79–84. [Google Scholar] [CrossRef]
- Nepal, G.; Bhatta, S. Self-medication with Antibiotics in WHO Southeast Asian Region: A Systematic Review. Cureus 2018, 10, e2428. [Google Scholar] [CrossRef]
- Ali, A.S.; Ahmed, J.; Sonekhi, G.B.; Fayyaz, N.; Zainulabdin, Z.; Jindani, R. Practices of self-medication with antibiotics among nursing students of Institute of Nursing, Dow University of Health Sciences, Karachi, Pakistan. J. Pak. Med. Assoc. 2016, 66, 235–237. [Google Scholar] [PubMed]
- Mohannad Naser, M.E.T. Self-medication for Oral and Non-oral Conditions among Visitors of Outpatient Clinics—Alexandria-Egypt. Asian J. Dent. Sci. 2018, 1, 1–8. [Google Scholar]
- Cherry, W.R.; Lee, J.Y.; Shugars, D.A.; White, R.P., Jr.; Vann, W.F., Jr. Antibiotic use for treating dental infections in children: A survey of dentists’ prescribing practices. J. Am. Dent. Assoc. 2012, 143, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.L.; Francis, N.A.; Wood, F.; Chestnutt, I.G. Antibiotic prescribing in UK general dental practice: A cross-sectional study. Commun. Dent. Oral Epidemiol. 2016, 44, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.J.; Feng, Q.; Warren, K.; Lockhart, P.B.; Thornhill, M.H.; Munshi, K.D.; Henderson, R.R.; Hsueh, K.; Fraser, V.J. Assessment of inappropriate antibiotic prescribing among a large cohort of general dentists in the United States. J. Am. Dent. Assoc. 2018, 149, 372–381.e1. [Google Scholar] [CrossRef] [PubMed]
- Vessal, G.; Khabiri, A.; Mirkhani, H.; Cookson, B.D.; Askarian, M. Study of antibiotic prescribing among dental practitioners in Shiraz, Islamic Republic of Iran. East Mediterr. Health J. 2011, 17, 763–769. [Google Scholar] [CrossRef] [PubMed]
- McGettigan, P.; Roderick, P.; Kadam, A.; Pollock, A.M. Access, Watch, and Reserve antibiotics in India: Challenges for WHO stewardship. Lancet Glob. Health 2017, 5, e1075–e1076. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Ramachandran, S.S. Fixed dose drug combinations: Issues and challenges in India. Indian J. Pharmacol. 2016, 48, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quinonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e865. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Kotwani, A. Need to improve availability of "access" group antibiotics and reduce the use of “watch” group antibiotics in India for optimum use of antibiotics to contain antimicrobial resistance. J. Pharm. Policy Pract. 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Sudhinaraset, M.; Ingram, M.; Lofthouse, H.K.; Montagu, D. What is the role of informal healthcare providers in developing countries? A systematic review. PLoS ONE 2013, 8, e54978. [Google Scholar]
- Bloom, G.; Merrett, G.B.; Wilkinson, A.; Lin, V.; Paulin, S. Antimicrobial resistance and universal health coverage. BMJ Glob. Health 2017, 2, e000518. [Google Scholar] [CrossRef]
- Shet, A.; Sundaresan, S.; Forsberg, B.C. Pharmacy-based dispensing of antimicrobial agents without prescription in India: Appropriateness and cost burden in the private sector. Antimicrob. Resist. Infect. Control. 2015, 4, 55. [Google Scholar] [CrossRef]
- Priya, S.; Madan Kumar, P.D.; Ramachandran, S. Knowledge and attitudes of pharmacists regarding oral health care and oral hygiene products in Chennai city. Indian J. Dent. Res. 2008, 19, 104–108. [Google Scholar] [PubMed]
- Bawazir, O.A. Knowledge and attitudes of pharmacists regarding oral healthcare and oral hygiene products in Riyadh, Saudi Arabia. J. Int. Oral Health 2014, 6, 10–13. [Google Scholar] [PubMed]

| Study ID | Treatment | Prescription Rate among General Dentist (BDS) | Prescription Rate among Specialists (MDS) |
|---|---|---|---|
| Goud [69] | RCT | 50 | 40 |
| Surgical removal of impacted teeth | 76 | 80 | |
| Karibasappa [72] | Periodontal pocket | 74.5 | 48.1 |
| Tooth fracture | 54.5 | 29.6 | |
| Pulpitis | 89 | 37 | |
| Apical periodontitis | 96.4 | 70.4 | |
| Periapical abscess | 98.2 | 85.2 | |
| Konde [73] | Reversible pulpitis | 28 | 2 |
| Irreversible pulpitis | 84 | 36 | |
| Apical periodontitis | 96 | 71 | |
| Simple extraction | 45 | 7 | |
| Periapical abscess | 94 | 78 | |
| Dry socket | 96 | 45 | |
| Shafia [82] | RCT | 83.6 | 69.6 |
| Wasan [85] | Acute pulpitis | 65.6 | 50.4 |
| Dry socket | 54.9 | 50 | |
| Periodontal abscess | 88.6 | 87.1 |
| Groups | Number of Clinical Indications | Mean | Standard Deviation | Mean Difference | 95% Confidence Interval of Mean Difference | p Value | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Dentist | 17 | 74.9 | 21.53 | 22.81 | −39.36 | 6.7 | 0.009 |
| Specialist | 17 | 52.1 | 25.6 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuvaraghan, A.; King, R.; Larvin, H.; Aggarwal, V.R. Antibiotic Use and Misuse in Dentistry in India—A Systematic Review. Antibiotics 2021, 10, 1459. https://doi.org/10.3390/antibiotics10121459
Bhuvaraghan A, King R, Larvin H, Aggarwal VR. Antibiotic Use and Misuse in Dentistry in India—A Systematic Review. Antibiotics. 2021; 10(12):1459. https://doi.org/10.3390/antibiotics10121459
Chicago/Turabian StyleBhuvaraghan, Aarthi, Rebecca King, Harriet Larvin, and Vishal R. Aggarwal. 2021. "Antibiotic Use and Misuse in Dentistry in India—A Systematic Review" Antibiotics 10, no. 12: 1459. https://doi.org/10.3390/antibiotics10121459
APA StyleBhuvaraghan, A., King, R., Larvin, H., & Aggarwal, V. R. (2021). Antibiotic Use and Misuse in Dentistry in India—A Systematic Review. Antibiotics, 10(12), 1459. https://doi.org/10.3390/antibiotics10121459








