Antifungal Activity of Essential Oils from Three Artemisia Species against Colletotrichum gloeosporioides of Mango
Abstract
:1. Introduction
2. Results
2.1. The Antifungal Activities by the Agar Diffusion Method
2.2. Evaluation of the Antifungal Activity of Plant Essential Oils Delivered In Vitro Fumigation
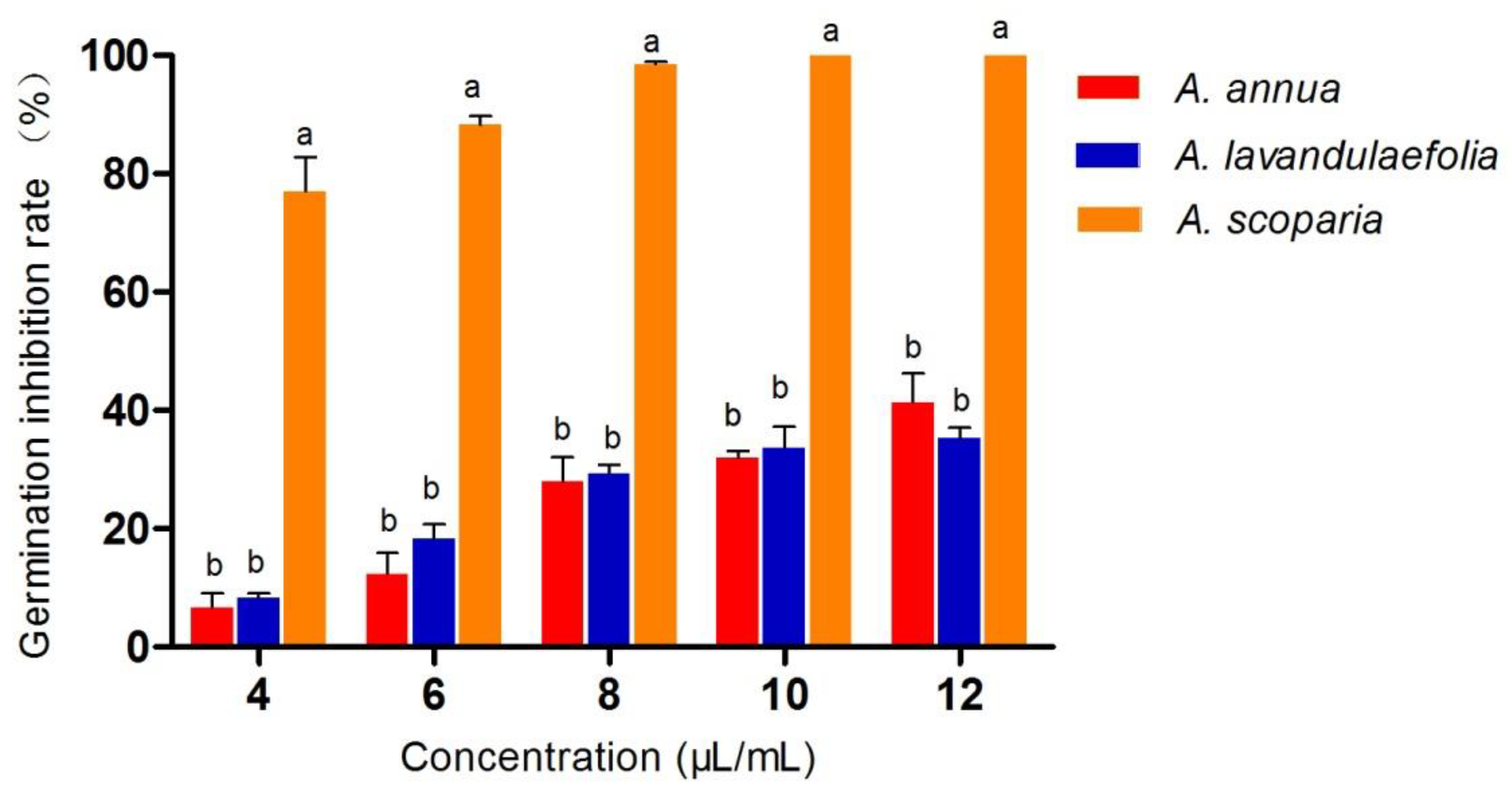
2.3. Effect of Essential Oils on Conidial Germination
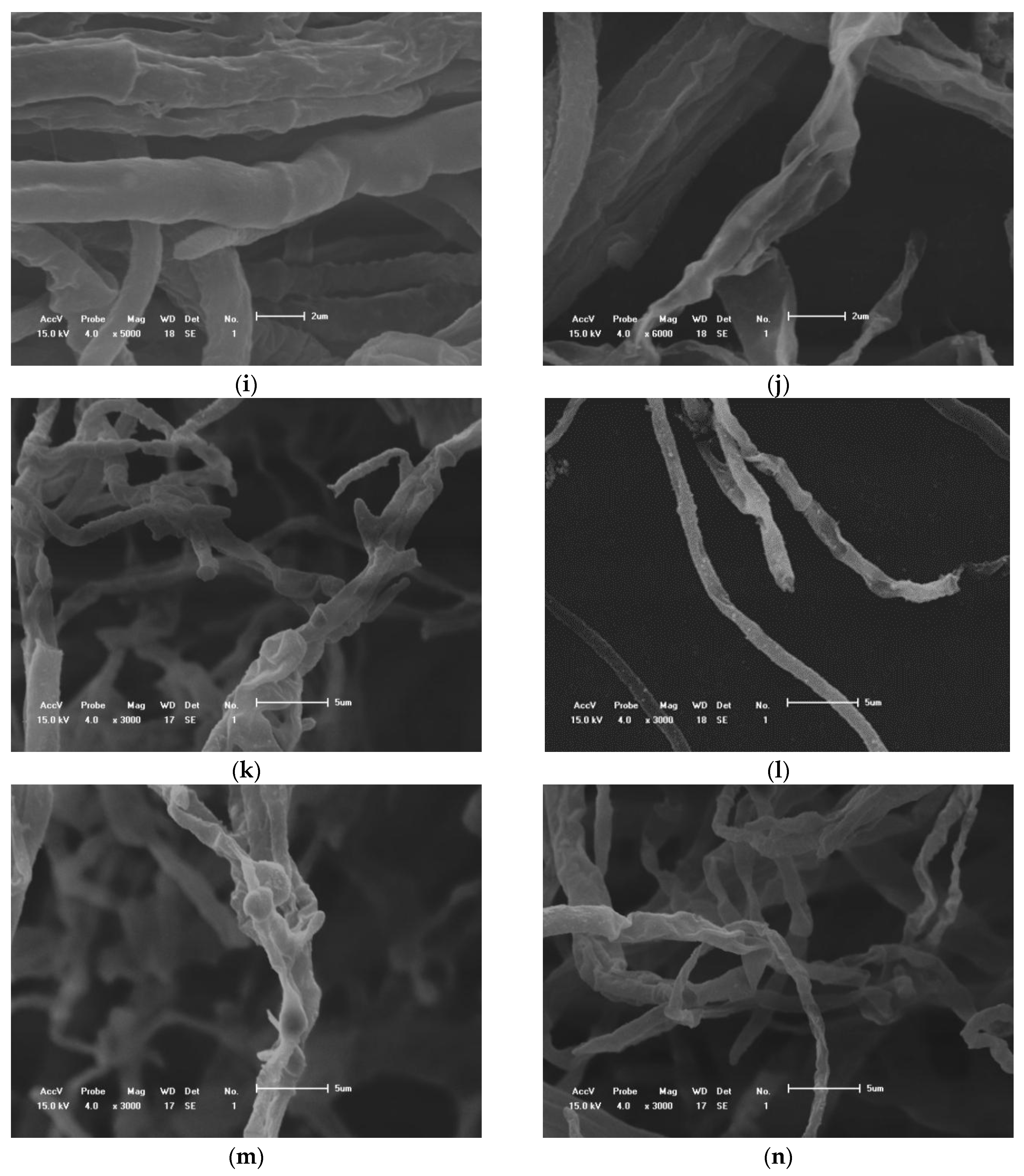
2.4. Determination of the Effect of Essential Oils on Hyphal Morphology
2.5. In Vivo Tests of the Volatile Compounds Produced by A. scoparia Essential Oil
2.6. The Effect of A. scoparia Essential Oil on Natural Morbidity
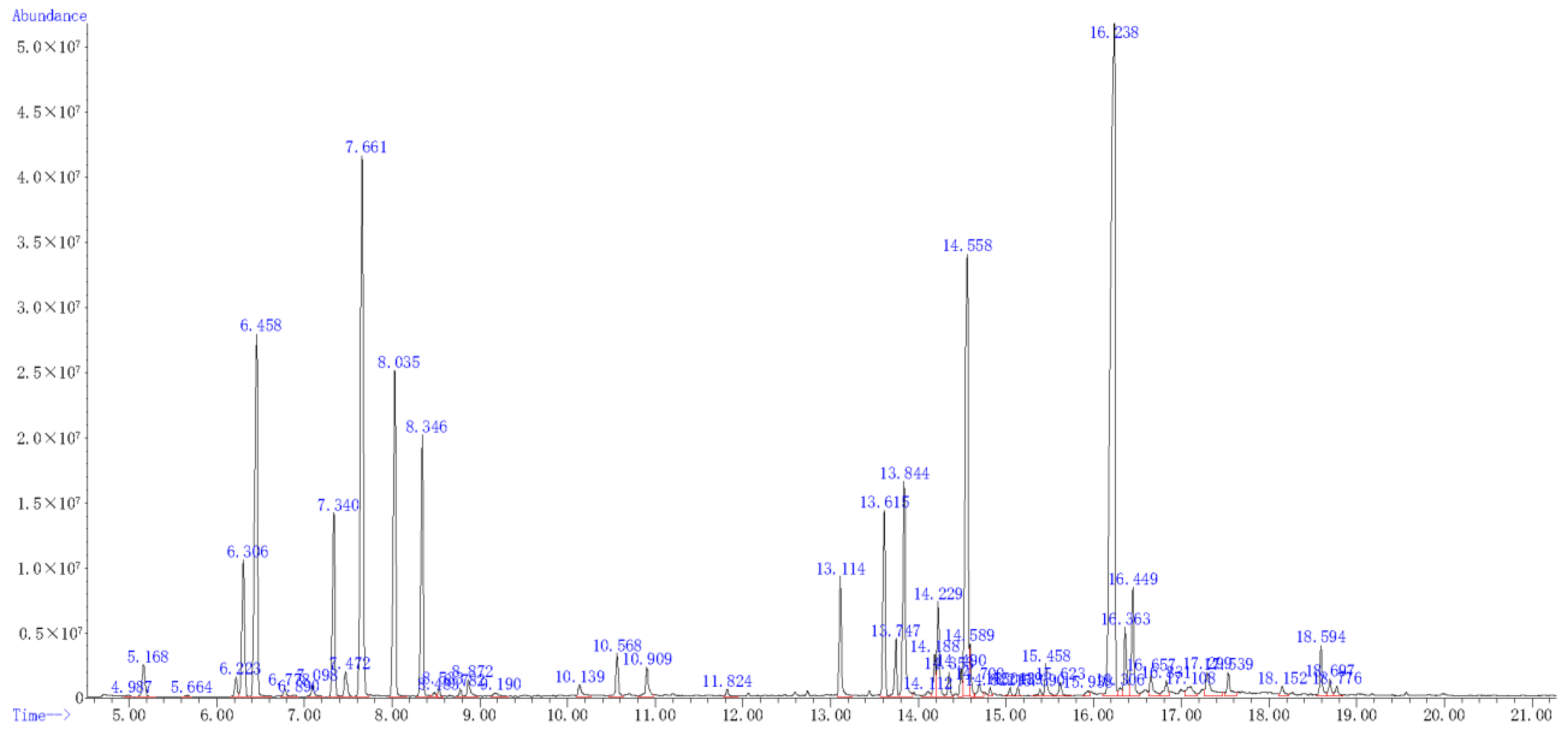
2.7. The Components of A. scoparia Essential Oil
3. Discussion
4. Materials and Methods
4.1. Plant Material and Essential Oil Extraction
4.2. Preparation of Colletotrichum Gloeosporioides
4.3. Investigation of Antifungal Activities by the Agar Diffusion Method
4.4. Evaluation of the Antifungal Activity of Plant Essential Oils Delivered In Vitro Fumigation
4.5. Spore Germination Assays
4.6. The Determination of the Effect of Essential Oils on Hyphal Morphology
4.7. In Vivo Assay
4.8. Effect of Essential Oil Fumigation on Decay Incidence and Mango Fruit Quality
4.9. Determination of Chemical Composition of Essential Oil from A. scoparia
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momeny, E.; Vafaei, N.; Ramli, N. Physicochemical properties and antioxidant activity of a synthetic cocoa butter equivalent obtained through modification of mango seed oil. Int. J. Food Sci. Technol. 2013, 48, 1549–1555. [Google Scholar] [CrossRef]
- Prior, C.; Ryder, K. Effect of low volume copper sprays with polyisobutene sticker on mango blossom blight (Glomerella cingulata) in dominica. Trop. Pest. Manag. 1987, 33, 350–352. [Google Scholar] [CrossRef]
- Arauz, L.F. Mango anthracnose: Economic impact and current options for integrated manage. Plant. Dis. 2000, 84, 600–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latunde-Dada, A.O. Colletotrichum: Tales of forcible entry, stealth, transient confinement and breakout. Mol. Plant. Pathol. 2001, 2, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Yong, H.Y.; Bakar, F.D.A.; Illias, R.M.; Mahadi, N.M.; Murad, A.M.A. Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioides. Braz. J. Microbiol. 2013, 44, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusky, D.; Lichter, A. Activation of quiescent infections by postharvest pathogens during transition from the biotrophic to the necrotrophic stage. FEMS Microbiol. Lett. 2007, 268, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, I. Chemotherapy of latent infection diseases caused by Colletotrichum species in tropical fruits. Australas. Plant. Pathol. 1974, 3, 36. [Google Scholar] [CrossRef]
- Furness, R.W.; Muirhead, S.J.; Woodburn, M. Using bird feathers to measure mercury in the environment: Relationships between mercury content and moult. Mar. Pollut. Bull. 1986, 17, 27–30. [Google Scholar] [CrossRef]
- Dodd, J.C.; Estrada, A.B.; Matcham, J.; Jeffries, P.; Jeger, M.J. The effect of climatic factors on Colletotrichum gloeosporio-ides, causal agent of mango anthracnose, in the Philippines. Plant. Pathol. 1991, 40, 568–575. [Google Scholar] [CrossRef]
- Salte, R.; Bentsen, H.B.; Moen, T.; Tripathy, S.; Bakke, T.A.; Ødegård, J.; Omholt, S.; Hansen, L.P. Prospects for a genetic management strategy to control Gyrodactylus salaris infection in wild Atlantic salmon (Salmo salar) stocks. Can. J. Fish. Aquat. Sci. 2010, 67, 121–129. [Google Scholar] [CrossRef]
- Katan, T. Cross resistance of metalaxyl-resistant Pseudoperonospora cubensis to other acylalanine fungicides. Can. J. Plant. Pathol. 1982, 4, 387–388. [Google Scholar] [CrossRef]
- Elad, Y. Reduced sensitivity of Botrytis cinerea to two sterol biosynthesis-inhibiting fungicides: Fenetrazole and fenethanil. Plant. Pathol. 1992, 41, 47–54. [Google Scholar] [CrossRef]
- Janisiewicz, W.J. Strategies for the use and enhancement of biological control of postharvest fruit decays. Acta Hortic. 2010, 864, 241–247. [Google Scholar] [CrossRef]
- Bowers, J.H.; Locke, J.C. Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of fusarium wilt in the greenhouse. Plant. Dis. 2000, 84, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Momin, R.A.; Nair, M.G. Mosquitocidal, nematicidal, and antifungal compounds from Apium graveolens L. seeds. J. Agric. Food Chem. 2001, 49, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; An, X.N. Plant Essential Oil and Natural Pigment Processing Technology, 2nd ed.; Chemical Industry Press: Beijing, China, 2005; pp. 177–179. [Google Scholar]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Caramiello, R.; Maffei, M.; Chialva, F. Essential oils from some Artemisia species growing spontaneously in North West Italy. Flavour Frag. J. 1995, 10, 25–32. [Google Scholar] [CrossRef]
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.C.; Han, J.X.; Yang, W.Y.; Deng, D.A.; Yue, X.F. Antitumor activities of 4 derivatives of artemisic acid and artemisinin B in vitro. Acta Pharmacol. Sin. 1992, 13, 541–543. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crop. Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Soylu, E.M.; Yigitbas, H.; Tok, F.M.; Soylu, S.; Kurt, S.; Baysal, O.; Kaya, A.D. Chemical composition and antifungal activity of the essential oil of Artemisia annua L. against foliar and soil-borne fungal pathogens. Z. Pflanzenk. Pflanzen. 2005, 112, 229–239. [Google Scholar] [CrossRef]
- Yan, G.; Yuemin, P.; Zhimou, G.; Kun, W.; Mei, P.; Shun, C. Inhibitive ativity of the etracts of plants in Artemisia against Fusarium moniliforme and Fusarium oxysporum F. sp. vasinfectum. Chin. Agric. Sci. Bull. 2009, 25, 206–210. [Google Scholar]
- Jiang, G.B.; Zeng, R.S.; Chert, S.X.; Chen, X.L. Identification and antimicrobial effects of volatiles in traditional Chinese medicine herb Artemisia lavandulaefolia DC. Prodr. J. Shenyang Agri. Univ. 2008, 39, 495–498. [Google Scholar]
- Cha, J.D.; Jeong, M.R.; Choi, H.J.; Jeong, S.I.; Moon, S.E.; Yun, S.I.; Kim, Y.H.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oil of Artemisia lavandulaefolia. Planta Med. 2005, 71, 575–577. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kim, J.Y.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005, 71, 186–190. [Google Scholar] [CrossRef]
- Farzaneh, M.; Ahmadzadeh, M.; Hadian, J.; Tehrani, A.S. Chemical composition and antifungal activity of the essential oils of three species of artemisia on some soil-borne phytopathogens. Commun. Agric. Appl. Biol. Sci. 2006, 71, 1327–1333. [Google Scholar]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from croatia and france. Planta Med. 2003, 69, 158–161. [Google Scholar] [CrossRef]
- Ewais, E.A.; Aly, M.M.; Ismail, M.A.; Shakour, E.; Hassanin, M.F. Antibacterial, antifungal, antitumor and toxicity of essential oils of Salvia officinalis, Thymus vulgaris, Eugenia caryophyllata and Artemisia absinthium. Sci. J. Flowers Ornam. Plants 2014, 1, 265–274. [Google Scholar] [CrossRef]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in western ghats region of North West Karnataka, India. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Batiha, E.S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Rivero-Perez, N. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Montenegro, I.; Said, B.; Godoy, P.; Besoain, X.; Parra, C.; Díaz, K.; Madrid, A. Antifungal activity of essential oil and main components from Mentha pulegium growing wild on the Chilean central coast. Agronomy 2020, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Chuah, T.S.; Tan, Y.Y.; Ismail, B.S. In vitro evaluation of the antifungal activity of some essential oils on post-harvest fungal pathogens of tropical fruits. Plant Prot. Qurat. 2010, 25, 162–164. [Google Scholar]
- Aminifard, M.H.; Mohammadi, S. Efficacy of plant essential oils to control post-harvest decay of sweet cherry (Prunus avium L.) fruit. J. Hortic. Sci. Biotechnol. 2013, 88, 79–84. [Google Scholar] [CrossRef]
- Muazu, S.A.; Channya, F.K.; Chimbekujwo, I.B.; Basiri, B.; Zakari, B.G.; Tukur, K.U.; Fauziya, K.M.; Samuel, K.B. Antifungal activity of garlic (Allium sativum) essential oil and wood ash against post-harvest fruit rot of banana (Musa acuminata L.) in Yola, Adamawa State, Nigeria. Int. J. Plant. Soil Sci. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov. Food Sci. Emerg. Technol. 2009, 10, 97–102. [Google Scholar] [CrossRef]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest. Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Bocate, K.P.; Evangelista, A.G.; Luciano, F.B. Garlic essential oil as an antifungal and anti-mycotoxin agent in stored corn. LWT-Food Sci. Technol. 2021, 147, 111600. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crop Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Bista, U.; Bist, D.B.; Aryal, H.P.; Amgain, L.P.; Shrestha, A. Anti-fungal activities and responses of plant essential oils against post-harvest disease of mango (Mangifera indica L.) Fruit. Int. J. Innov. Stud. Sci. Eng. Technol. 2020, 4863, 5–11. [Google Scholar] [CrossRef]
- Júnior, I.; Sales, N.; Martins, E.R. Fungitoxic effect of concentrations of essential oils on Colletotrichum gloeosporioides, isolated from the passion fruit. Biotemas 2009, 22, 77–83. [Google Scholar]
- Tripathi, A.; Sharma, N.; Sharma, V. In vitro efficacy of Hyptis suaveolens L. (Poit.) essential oil on growth and morphogenesis of Fusarium oxysporum f.sp. gladioli (Massey) Snyder & Hansen. World J. Microbiol. Biotechnol. 2009, 25, 503–512. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Soylu, S.; Yigitbas, H.; Soylu, E.M.; Kurt, Ş. Antifungal effects of essential oils from oregano and fennel on Sclerotinia sclerotiorum. J. Appl. Microbiol. 2007, 103, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Bruni, R.; Andreotti, E.; Rai, M.K.; Vicentini, C.B.; Mares, D. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma 2005, 225, 57–65. [Google Scholar] [CrossRef]
- Sefu, G.; Satheesh, N.; Berecha, G. Antifungal activity of ginger and cinnamon leaf essential oils on mango anthracnose disease causing fungi (C. gloeosporioides). Carpathian J. Food Sci. Technol. 2015, 7, 26–34. [Google Scholar]
- Palhano, F.L.; Vilches, T.T.B.; Santos, R.B.; Orlando, M.T.D.; Ventura, J.A.; Fernandes, P.M.B. Inactivation of Colletotrichum gloeosporioides spores by high hydrostatic pressure combined with citral or lemongrass essential oil. Int. J. Food Microbiol. 2004, 95, 61–66. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Tao, W.; Guo, J.; Yu, T. Effect of citronella essential oil on the inhibition of postharvest Alternaria alternata in cherry tomato. J. Sci. Food Agric. 2014, 94, 2441–2447. [Google Scholar] [CrossRef] [PubMed]
- Bosquez-Molina, E.; Jesús, E.R.; de Bautista-Baños, S.; Verde-Calvo, J.R.; Morales-López, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Chemical composition and insecticidal activity of Artemisia scoparia essential oil against three Coleopteran stored-product insects. J. Asia-Pac. Entomol. 2006, 9, 381–388. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Bamoniri, A.; Sarafraz, M.B.; Batooli, H. Volatile components from Artemisia scoparia Waldst et Kit growing in central Iran. Flavour Frag. J. 2010, 20, 650–652. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Cases, M.A.; Sotomayor, J.A. Watering level effect on Thymus hyemalis Lange essential oil yield and composition. J. Agr. Food Chem. 2003, 51, 5420–5427. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Pirbalouti, A.; Firoznezhad, M.; Craker, L.; Akbarzadeh, M. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages. Rev. Bras. Farmacogn. 2013, 23, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: In vivo ROS detection and alterations in antioxidant enzymes. Biochem. Syst. Ecol. 2012, 44, 390–399. [Google Scholar] [CrossRef]
- Kapoor, R.; Ali, M.; Mir, S.R.; Rafiullah, M.R.M. Essential oil constituents of aerial parts of Artemisia scoparia Waldst. & Kit. Flavour Frag. J. 2010, 19, 109–111. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Islam, M.T.; Da Mata, A.M.O.F.; de Aguiar, R.P.S.; Paz, M.F.C.J.; de Alencar, M.V.O.B.; Ferreira, P.M.P.; de Carvalho Melo-Cavalcante, A.A. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Wu, H.B.; Wu, H.B.; Zhang, J. Wormwood (Artemisia absinthium L.) as a promising nematicidal and antifungal agent: Chemical composition, comparison of extraction techniques and bioassay-guided isolation. Ind. Crop. Prod. 2019, 133, 295–303. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Bogavac, M.; Suvajdzic, L.; Simin, N.; Samojlik, I.; Couladis, M. Chemical composition, antioxidant and antibacterial properties of Achillea collina becker ex heimerl s.l. and A. pannonica scheele essential oils. Molecules 2008, 13, 2058–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Essential Oil | Virulence Regression Equation | Correlation Coefficient (r) | EC50 |
|---|---|---|---|
| A. annua | y = −2.051 + 0.068x | 0.929 | 30.278 |
| A. lavandulaefolia | y = −1.600 + 0.84x | 0.994 | 19.064 |
| A. scoparia | y = −2.106 + 0.226x | 0.954 | 9.320 |
| Essential Oils | Virulence Regression Equation | Correlation Coefficient (r) | EC50 |
|---|---|---|---|
| A. annua | y = −1.161 + 0.72x | 0.978 | 16.194 |
| A. lavandulaefolia | y = −0.689 + 0.73x | 0.886 | 9.485 |
| A. scoparia | y = −0.521 + 0.81x | 0.755 | 6.464 |
| Concentrations (μL) | Rot Area (cm2) ± SD | Control Value (%) |
|---|---|---|
| CK | 12.54 ± 0.56 a | - |
| 50.00 | 8.55 ± 0.33 b | 31.81 |
| 80.00 | 3.99 ± 0.48 d | 38.07 |
| 100.00 | 4.77 ± 0.16 bc | 65.66 |
| 120.00 | 4.93 ± 0.57 cd | 66.23 |
| Concentrations (μL) | Rot Area (cm2) ± SD | Disease Index (%) | Control Value (%) |
|---|---|---|---|
| CK | 47.09 ± 7.10 a | 82.22 | - |
| 50.00 | 8.51 ± 3.72 b | 22.22 | 82.39 |
| 100.00 | 7.31 ± 2.70 b | 17.77 | 85.18 |
| 120.00 | 3.84 ± 3.07 b | 4.44 | 92.06 |
| No. | Compounds | RI | Percent Composition (%) |
|---|---|---|---|
| 1 | 1R-πPinene | 948 | 0.7 |
| 2 | β-Pinene | 943 | 8.0 |
| 3 | 3,3,6-Trimethyl-1,4-heptadien-6-ol | 983 | 0.2 |
| 4 | Limonene | 1018 | 3.3 |
| 5 | (E)-3,7-dimethyl-1,3,6-Octatriene | 976 | 0.6 |
| 6 | 2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-ol | 1206 | 2.0 |
| 7 | 1-methyl-4-(1-methylethyl)-1,4-Cyclohexadiene | 998 | 6.3 |
| 8 | 3,3,6-trimethyl-1,5-Heptadien-4-one | 1042 | 4.6 |
| 9 | 3,3,6-Trimethyl-1,5-heptadien-4-ol | 1068 | 0.1 |
| 10 | 1-methyl-4-(1-methylethylidene)-Cyclohexene | 1052 | 0.2 |
| 11 | 2-ethenyl-1,1-dimethyl-3-methylene-Cyclohexane | 1071 | 0.1 |
| 12 | 3,7-dimethyl-1,6-Octadien-3-ol | 1082 | 0.4 |
| 13 | 2,6-dimethyl-3,7-Octadiene-2,6-diol | 1197 | 0.1 |
| 14 | (R)-5-methyl-2-(1-methylethenyl)-4-Hexen-1-ol | 1146 | 0.3 |
| 15 | (R)-4-methyl-1-(1-methylethyl)-3-Cyclohexen-1-ol | 1137 | 0.8 |
| 16 | ππ-trimethyl-3-Cyclohexene-1-methanol | 1143 | 0.7 |
| 17 | Acetate 5-methyl-2-(1-methylethenyl)-4-Hexen-1-ol | 1270 | 0.1 |
| 18 | 2,4-pentadiynyl-Benzene | 1206 | 11.8 |
| 19 | Caryophyllene | 1424 | 3.2 |
| 20 | (Z)-7,11-dimethyl-3-methylene-1,6,10-Dodecatriene | 1440 | 0.9 |
| 21 | Eugenol | 1392 | 4.0 |
| 22 | πCaryophyllene | 1456 | 0.7 |
| 23 | (R)-2,4a,5,6,7,8-hexahydro-3,5,5,9-tetramethyl-1H-Benzocycloheptene | 1497 | 1.6 |
| 24 | (Z,E)-3,7,11-trimethyl-1,3,6,10-Dodecatetraene | 1486 | 0.4 |
| 25 | [S-(R*,S*)]-5-(1,5-dimethyl-4-hexenyl)-2-methyl-1,3-Cyclohexadiene | 1492 | 0.4 |
| 26 | 1,2-dimethoxy-4-(2-propenyl)-Benzene | 1361 | 10.0 |
| 27 | Octahydro-7-methyl-3-methylene-4-(1-methylethyl)-,3aS,3bR,4S,7R,7aR)-1H-Cyclopenta[1,3]cyclopropa[1,2]benzene | 1339 | 0.6 |
| 28 | [4aR-(4aπ7π8aπ]-decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-Naphthalene | 1469 | 0.3 |
| 29 | 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-Cycloheane | 1431 | 0.2 |
| 30 | (1S-cis)-1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-Naphthalene | 1469 | 0.2 |
| 31 | [1R-(1π3aπ4π8aπ]-decahydro-1,5,5,8a-tetramethyl-1,4-Methanoazulen-9-one | 1576 | 0.2 |
| 32 | 2-methylene-6,8,8-trimethyl-Tricyclo[5.2.2.0(1,6)]undecan-3-ol | 1233 | 0.2 |
| 33 | (E)-3,7,11-trimethyl-1,6,10-Dodecatrien-3-ol | 1564 | 0.6 |
| 34 | Octahydro-3,6,6,7a-tetramethyl-2H-2a,7-Methanoazuleno[5,6-b]oxirene | 1293 | 0.3 |
| 35 | o-Hydroxybiphenyl | 1456 | 0.7 |
| 36 | 2-ethenyl-Naphthalene | 1367 | 23.5 |
| 37 | (−)-Spathulenol | 1536 | 1.3 |
| 38 | Caryophyllene oxide | 1507 | 2.0 |
| 39 | 5-Hydroxy-4,4-dimethyl-1,5-diphenylpent-1-yn-3-one | 2294 | 0.6 |
| 40 | 1,5,5,8-tetramethyl-[1R-(1R,3E,7E,11R)]-12-Oxabicyclo[9.1.0]dodeca-3,7-diene | 1592 | 0.4 |
| 41 | Cubenol | 1580 | 0.5 |
| 42 | [2R-(2π4aπ8aπ]-decahydro-ππ4a-trimethyl-8-methylene-2-Naphthalenemethanol | 1593 | 1.0 |
| 43 | Phenol, 2-methoxy-4-(2-propenyl)-, acetate | 1552 | 0.6 |
| 44 | 1-phenyl-2,4-Hexadiyn-1-one | 1461 | 0.3 |
| 45 | 8a-dimethyl-6-(1-methylethenyl)-2(1H)Naphthalenone,3,5,6,7,8,8a-hexahydro-4 | 1673 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Liu, T.; Zhou, C.; Huang, Y.; Liu, X.; Yuan, H. Antifungal Activity of Essential Oils from Three Artemisia Species against Colletotrichum gloeosporioides of Mango. Antibiotics 2021, 10, 1331. https://doi.org/10.3390/antibiotics10111331
Huang X, Liu T, Zhou C, Huang Y, Liu X, Yuan H. Antifungal Activity of Essential Oils from Three Artemisia Species against Colletotrichum gloeosporioides of Mango. Antibiotics. 2021; 10(11):1331. https://doi.org/10.3390/antibiotics10111331
Chicago/Turabian StyleHuang, Xing, Tiantian Liu, Chunxiang Zhou, Yulin Huang, Xing Liu, and Haibin Yuan. 2021. "Antifungal Activity of Essential Oils from Three Artemisia Species against Colletotrichum gloeosporioides of Mango" Antibiotics 10, no. 11: 1331. https://doi.org/10.3390/antibiotics10111331
APA StyleHuang, X., Liu, T., Zhou, C., Huang, Y., Liu, X., & Yuan, H. (2021). Antifungal Activity of Essential Oils from Three Artemisia Species against Colletotrichum gloeosporioides of Mango. Antibiotics, 10(11), 1331. https://doi.org/10.3390/antibiotics10111331





