Abstract
Bacteria of the genus Burkholderia include pathogenic Burkholderia mallei, Burkholderia pseudomallei and the Burkholderia cepacia complex (Bcc). These Gram-negative pathogens have intrinsic drug resistance, which makes treatment of infections difficult. Bcc affects individuals with cystic fibrosis (CF) and the species B. cenocepacia is associated with one of the worst clinical outcomes. Following the repurposing of auranofin as an antibacterial against Gram-positive bacteria, we previously synthetized auranofin analogs with activity against Gram-negatives. In this work, we show that two auranofin analogs, MS-40S and MS-40, have antibiotic activity against Burkholderia clinical isolates. The compounds are bactericidal against B. cenocepacia and kill stationary-phase cells and persisters without selecting for multistep resistance. Caenorhabditis elegans and Galleria mellonella tolerated high concentrations of MS-40S and MS-40, demonstrating that these compounds have low toxicity in these model organisms. In summary, we show that MS-40 and MS-40S have antimicrobial properties that warrant further investigations to determine their therapeutic potential against Burkholderia infections.
1. Introduction
Antibiotics are one of the greatest medical advances of the 20th century, with their widespread discovery starting in the early 1900’s [,]. However, antibiotic resistance is now a global crisis, responsible for approximately 700,000 deaths annually [], with that number projecting to increase each year []. The “golden era” of antibiotic discovery, which lasted approximately 20 years, led to the identification of vancomycin, methicillin, cephalosporins, and many other antibiotics [,]. No new antibiotic from a new class has reached the clinic for many decades []. When a new antibiotic is developed that does not have a novel mechanism of action, resistance mechanisms are already present. Therefore, antimicrobials with novel mechanisms of action are needed to prevent this quickly generated resistance.
Bacteria of the genus Burkholderia [] includes difficult-to-treat human pathogens such as the Burkholderia cepacia complex (Bcc), Burkholderia mallei and Burkholderia pseudomallei []. Bcc is a group of more than 20 species that cause life-threatening bacterial infections in cystic fibrosis (CF) patients []. Burkholderia cenocepacia infections, in particular, have one of the worst clinical outcomes [,], causing decreased lung function and cepacia syndrome, a sepsis with necrotizing pneumonia [,]. Additionally, CF patients infected with B. cenocepacia are often ineligible for lung transplants [], a common life-saving procedure [], because B. cenocepacia infections have a high risk of reoccurrence [].
New antibiotics are urgently needed to combat Burkholderia infections. Promising emerging therapeutics are those with heavy metals [], especially gold [,,,,], which have been used in medicine since 2500 B.C.E. Recently, the gold-containing, anti-arthritis drug auranofin [,] was found to be active against Mycobacterium tuberculosis and Gram-positive bacteria []. Auranofin inhibited the function of the enzyme thioredoxin reductase, interrupting thiol-redox homeostasis [,]. However, auranofin lacked significant activity against Gram-negative bacteria with minimum inhibitory concentrations (MICs) > 16 mg/L [].
We previously found that auranofin is active against Helicobacter pylori and synthesized sugar-modified analogs have improved antibiotic activity and reduced toxicity to mammalian cells []. By varying the structures of the thiol and phosphine ligands on auranofin, we expanded the antibacterial activity of the auranofin analogs to the Gram-negative pathogens including Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Escherichia coli []. In this work, we describe the characterization of two auranofin analogs, WB-19-HL4170 (MS-40S) and WB-19-HL4118 (MS-40), which show potential as antimicrobials against Bcc strains, B. pseudomallei and B. mallei. MS-40S and MS-40 are bactericidal against B. cenocepacia, kill persister cells, and do not select for multistep resistant mutations while maintaining low toxicity to eukaryotic systems.
2. Results
2.1. MIC of Auranofin Derivatives against a Panel of Burkholderia Cepacia Complex Species
We tested auranofin and ten auranofin analogs (Figure 1) against a panel of Bcc bacteria, comprising of clinical isolates from CF patients and strains from environmental sources. Auranofin (Figure 1, top left) and the analogues belonging to group one (Figure 1, top right) were largely inactive against members of the Bcc (Table S1), with most of the MICs being 128 μg/mL or higher. Group one analogs have modifications of the thioglucose ligands, with or without additional replacing of trimethylphosphine (-PMe3) to triethylphosphine (-PEt3) coordinated bonding to the gold atom. Auranofin, however, showed high activity against B. mallei with MICs ranging from 0.25–1 μg/mL. In B. pseudomallei, auranofin had an MIC of 64 μg/mL. Auranofin and the group one derivatives in this study were shown to have diverse activity in other Gram-negative and Gram-positive bacteria, but they were inactive or had low activity in P. aeruginosa, Enterobacter cloacae, and K. pneumoniae [].
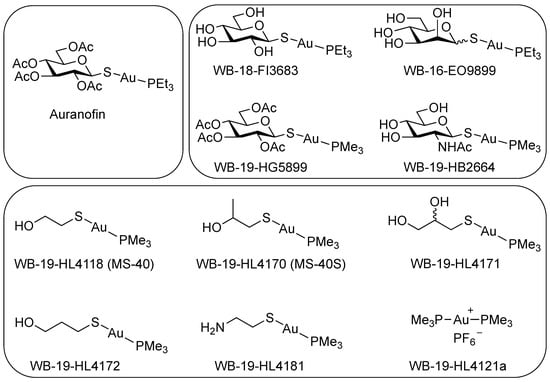
Figure 1.
Chemical structure of auranofin and auranofin analogs. (Top left) Auranofin. (Top right) Group one auranofin analogs containing modifications of the thioglucose and replacement of triethylphosphine (P(CH2CH3)3; PEt3) with trimethylphosphine (P(CH3)3; PMe3). (Bottom) Group two auranofin analogs, contains mercaptoethanol (OHCH2CH2SH) replacing the thioglucose, then further modifications of mercaptoethanol.
Group two (Figure 1, bottom) includes the analog WB-19-HL4118 (MS-40) which had shown a low MIC in Gram-negative and Gram-positive bacteria, such as in A. baumannii, E. cloacae, E. coli, and S. aureus []. Therefore, we synthetized additional analogs with similar structures to MS-40. Group two analogs showed lower MICs against members of the Bcc (Table 1) than group one (Table S1). Remarkably, MS-40 and WB-19-HL4170 (MS-40S) showed the strongest activity (Table 1). These two compounds also have high activity in B. mallei and B. pseudomallei strains as well. The synthesis of MS-40S and MS-40 is shown in Scheme 1. The remaining derivatives from this group have moderate MICs, ranging from 8 to 64 μg/mL, with only a few being 128 μg/mL or higher. The structures of the group two derivatives have substitution of thioglucose ligands with mercaptoethanol (HOCH2CH2SH) or mercaptoethanol modification, suggesting that the thioglucose was unable to permeate into most of the Bcc bacterial cell.

Table 1.
Minimum inhibitory concentrations (MICs) of group two auranofin derivatives against Burkholderia cepacia complex (Bcc) bacteria, B. mallei and B. pseudomallei.
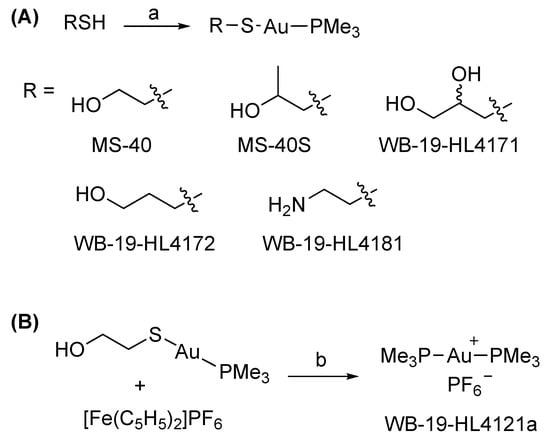
Scheme 1.
(A) Synthesis of MS-40S. (B) Synthesis of MS-40. Reagents and conditions: a: NaOCH3, CH3OH, room temperature, 2 h; b: dichloromethane, 0 °C, 23 h.
Next, we compared the MICs of MS-40S and MS-40 to common antibiotics used to treat CF patients infected with Burkholderia spp. Those include ceftazidime [,], meropenem [,,,], doxycycline [], and tobramycin [,,]. The combination therapy ceftazidime–avibactam is considered the last resort treatment for those infected with Burkholderia species []; therefore, we determined the MIC of ceftazidime–avibactam and these four antibiotics against the Bcc panel (Table 2). The MICs of MS-40 and MS-40S are much lower than the antibiotic tobramycin and are similar to the other antibiotics, including the last resort combination treatment ceftazidime–avibactam. Additionally, doxycycline, against some isolates from the Bcc, had MIC values as low as 1 and 2 μg/mL. Taken together, the initial MIC testing shows MS-40S and MS-40 are comparable to antibiotics used currently in the clinic, having MICs lower than most and even have similar values to the combination therapy ceftazidime–avibactam.

Table 2.
Minimum inhibitory concentrations (MICs) of MS-40S compared to clinical antibiotics used to treat CF patients.
2.2. MS-40S Has Broad Bactericidal Activity
Individuals with CF are commonly infected by multiple bacteria, causing polymicrobial infections [,]. Therefore, for MS-40S and MS-40 to be effective antimicrobials, it is imperative for these compounds to kill additional CF pathogens. Common bacteria that cause CF lung infections, besides Burkholderia spp., are Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans [,]. Other pathogenic Gram-negative bacteria that were shown to, although rarely, cause CF pulmonary infections include Escherichia coli [], Escherichia vulneris [], Klebsiella pneumoniae [], and Acinetobacter species [].
We thus tested MS-40S against CF pathogens to determine their MIC and MBC (minimum bactericidal concentration), and the same was done with MS-40 (Table 3). MS-40S have low MICs for the Gram-positive bacterium S. aureus, one of which is an MRSA strain. MS-40S is also bactericidal against the other Gram-negative bacteria tested with MICs in the range of 1–16 μg/mL and most of the MBCs were between 1- and 4-fold of their respective MICs. P. aeruginosa is a common multi-drug resistant bacterium [], with some strains/clinical isolates being extensively-drug resistant [], and has moderate MIC values between 16 and 64 μg/mL and its MBCs between 2- and 16-fold higher than its MICs. MS-40 shows similar results to MS-40S. Overall, the MICs/MBCs against CF pathogens show MS-40S and MS-40 have broad-spectrum bactericidal activity, indicating their potential as a therapeutic option for CF patients.

Table 3.
MS-40S is bactericidal against other bacteria that infect CF patients.
2.3. MS-40S Does Not Select for Multistep Resistant Mutants
New antimicrobials are urgently needed because resistance to current antibiotics has arisen and spread to many bacteria [,,]. Ideally, resistance will occur slowly for new antimicrobials, or not at all. We therefore characterized the occurrence of resistance to MS-40S due to repeated exposure and continuous growth []. Bacteria grown in the presence of subinhibitory concentrations of each compound (0.5× MIC) were subcultured and grown overnight in Luria–Bertani (LB) broth. These cultures were then used for the next MIC test, and this process was repeated for a total of 24 days (Figure 2, left). We performed this procedure for MS-40S, MS-40 and the antibiotics meropenem and doxycycline. These antibiotics were chosen because they are commonly used to treat cystic fibrosis patients infected with Burkholderia species [,,,,], and they have different mechanisms of action and resistance [,].
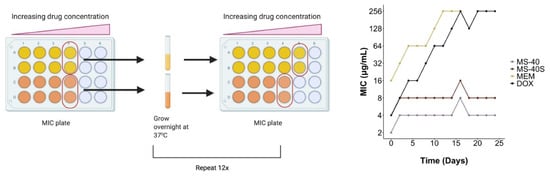
Figure 2.
Resistance is not generated to MS-40S. Repeated exposure of a continuously grown culture to sub-lethal concentrations of the antimicrobials were achieved by determining the MIC of the compound. Then, for each compound, 30 μL of bacteria from the well with growth with the highest concentration of compound was grown overnight and used in the next MIC test. This was repeated over 24 days, with the MIC tested every second day. MEM, meropenem; DOX, doxycycline.
Figure 2 (right) shows that resistance against meropenem and doxycycline arose quickly. While their starting MICs values were 16 and 4 μg/mL, respectively, both MICs reached 256 μg/mL after 12–16 days. Remarkably, no apparent increase in their MICs was observed for MS-40S and MS-40, demonstrating a desired property as a potential therapeutic agent.
2.4. MS-40S Is Bactericidal against Both Replicating and Non-Replicating Cells
Antibiotics are classified either as bactericidal, if they kill cells and reduce the population by 99.99%, or bacteriostatic, if they prevent cell growth/division, but do not kill more than 99.99% of the population [,]. It is common for antibiotics to only target actively dividing cells because their targets are involved in replication or other energy-dependent processes [], rendering them less effective when cells are not replicating or respiring []. In time kill experiments, we found MS-40S to be bactericidal to both exponential (replicating) and stationary (non-replicating) phase cells, and the same was found for MS-40 (Figure 3; top 4 panels). Interestingly, MS-40S is more effective at killing stationary phase (Figure 3; middle right) than exponential phase cells (Figure 3; middle left), reducing the culture by approximately three log10 units and nine log10 units at 4× MIC in the exponential and stationary phase, respectively. For comparison purposes, we show that doxycycline and ceftazidime–avibactam are both unable to kill cells in stationary phase (Figure 3; bottom right) and ceftazidime–avibactam is slow at killing exponential phase cells, regardless of the concentration (Figure 3; bottom left).
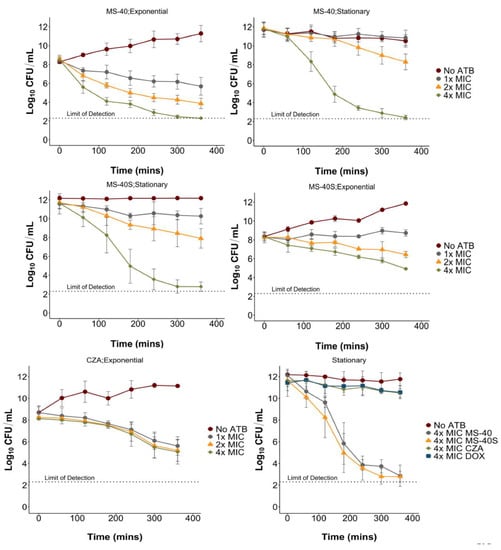
Figure 3.
Exponential and stationary time kills of Burkholderia cenocepacia K56-2. The compounds used were MS-40, MS-40S, and ceftazidime–avibactam (CZA) at 1×, 2×, and 4× MIC, as well as doxycycline (DOX) at 4× MIC. The cultures were grown overnight for stationary phase cells or grown overnight, subcultured, and grown to early exponential phase. Samples were taken every hour for six hours to determine CFU/mL. No ATB; no antibiotic.
The finding that MS-40S and MS-40 are able to kill stationary phase cells highlights their potential as future therapeutics. Stationary phase cells contain a higher amount of persister cells that could be a common cause of relapses in infections [,].
2.5. MS-40S Kill and Inhibit the Formation of Persister Cells
Persister cells, a subpopulation that is not killed by an antimicrobial, are thought to be a common cause of relapses in infections and persistent infections [,]. Persisters are thought to form via randomly overexpressing a resistance factor, decreased growth rate, decreased cellular energy, and/or a slower lag phase [,]. Once the antibiotic is removed, they will begin to grow normally, without inherited resistance, termed “persister awakening” []. A stationary phase population has increased amounts of persisters because it is slower growing and is metabolically dormant []. MS-40S and MS-40 can kill stationary phase cells effectively, so we reasoned that the compounds might kill persister cells.
We exposed an exponentially growing B. cenocepacia K56-2 population to 5× MIC of ciprofloxacin (MIC, 2 μg/mL) for 3 h, to enrich the surviving population in persister cells. After the treatment, surviving cells were washed and collected in phosphate buffered saline (PBS) to prevent persister awakening [], then exposed to the MS-40S and MS-40. Figure 4 show persister cells, in the presence of MS-40S and MS-40 (Figure 4, top panels), are killed to a concentration below/close to the limit of detection, whereas the persister cells re-exposed to ciprofloxacin or those without antibiotics are not killed. This demonstrates that MS-40S and MS-40 can indeed kill persister cells created by other antibiotics.
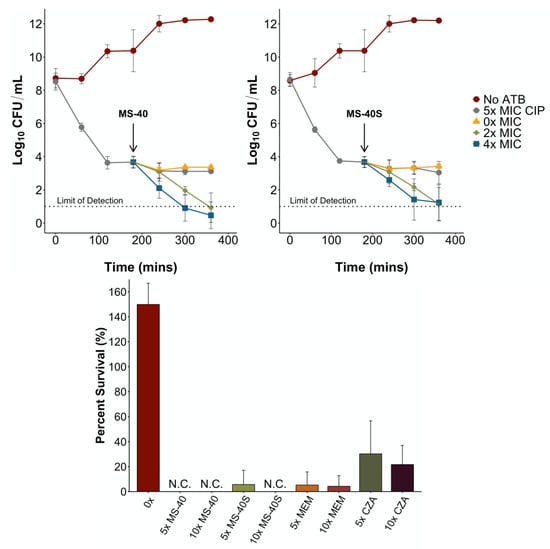
Figure 4.
MS-40S kills and inhibits the formation of persister cells. (Top) An exponential phase culture with approximately 1 × 108 CFU/mL was exposed to 5× MIC ciprofloxacin (CIP) to generate persister cells. At three hours post-exposure, persister cells were washed, resuspended in PBS and exposed to 0×, 2×, or 4× MIC of MS-40 (top left) and MS-40S (top right) for an additional three hours. No ATB; no antibiotic. (Bottom) An overnight culture of B. cenocepacia K56-2 was incubated with the corresponding antimicrobial, MS-40, MS-40S, meropenem (MEM), or ceftazidime–avibactam (CZA). Percent survival was calculated by the log10 CFU/mL of the surviving population/log10 CFU/mL of the initial population. N.C., no colonies.
To determine the amount of persisters remaining after 24 h of exposure, we performed the persister frequency assay []. We exposed a culture with a CFU/mL of 1 × 108, to one of the antimicrobials for 24 h. Compounds tested included MS-40S, meropenem, ceftazidime–avibactam, and MS-40. The remaining cells, enriched in persisters, were plated on LB to determine CFU/mL and percent survival was calculated by the log10 CFU/mL values. After 24 h, no persisters were formed after exposure to 10× MIC MS-40S, and a very low persister frequency with 5× MIC MS-40S (Figure 4, bottom). No persisters were formed exposed to 5× and 10× MIC of MS-40 and meropenem also produced a low amount of persisters at both concentrations tested. The last resort combination therapy ceftazidime–avibactam (CZA) produced the most persisters in this assay, with approximately 30% of the culture enriched in persisters surviving after treatment.
Taken together, these results show MS-40S, as well as MS-40, can kill persister cells created by other antibiotics and can inhibit persister cell formation. This suggests that MS-40S, as well as MS-40, have the potential to effectively eradicate an infection, reducing the risk of a relapse in infection after the treatment regime.
2.6. C. elegans and G. mellonella Toxicity
In preliminary cytotoxicity tests, MS-40 was shown to have lower toxicity in human A549 cells than auranofin [,]; however, the novel MS-40S has not been tested. To show that these compounds are safe for eukaryotic cells, we first used C. elegans as a model organism.
We performed a survival assay with Caenorhabditis elegans exposed to MS-40S, and three clinical antibiotics: the combination ceftazidime–avibactam, meropenem, and doxycycline, as well as MS-40 (Table 4). We calculated the Survival100/MIC value, which is a ratio of the highest concentration with 100% survival to the compound’s MIC []. This is a preliminary view to a compound’s therapeutic index. MS-40S has similar Survival100/MIC values as clinical antibiotics, with MS-40S, doxycycline, and ceftazidime–avibactam having values of 8, 16, and 32, respectively, and MS-40 and meropenem having a value of four. Similar to C. elegans, Galleria larvae were also well tolerated to MS-40S (Table 5). This was compared to MS-40 and a clinical antibiotic, doxycycline, which has similar MIC values to MS-40S and MS-40. Concentrations used ranged from 10 to 1 mg/kg. MS-40S and MS-40 were safe for the larvae at concentrations of 10 mg/kg with a percent survival of approximately 80% and higher. This was similar as the clinical antibiotic, doxycycline. Taken together, C. elegans and Galleria toxicity models show that MS-40S, as well as MS-40, have low toxicity in these eukaryotic organisms.

Table 4.
Percent survival of C. elegans exposed to MS-40, MS-40S, and clinical antibiotics.

Table 5.
Percent survival of Galleria exposed to doxycycline (DOX), MS-40, and MS-40S.
3. Discussion
Here, we show initial antibiotic properties of two auranofin analogs, MS-40 and the novel compound MS-40S, against the cystic fibrosis pathogen B. cenocepacia K56-2. The antibiotic properties were explored in parallel with commonly used antibiotics, namely doxycycline, meropenem, and ceftazidime–avibactam. This comparison shows MS-40S and MS-40 to have potential to be developed as antibiotics. One difference between MS-40S and MS-40 and the antibiotics used in this study is the ability of MS-40S and MS-40 to eliminate non-replicating cells. It is common for antibiotics to act on essential targets, such as those involved in cell wall synthesis, DNA replication, and translation []. In stationary phase, most of these processes are decreased, preventing the antibiotics from acting upon the cell. We confirmed this with two antibiotics with different mechanisms of action (MOA): doxycycline, a tetracycline that binds to the 30 s subunit of the ribosome, preventing translation elongation [], and ceftazidime–avibactam, a cephalosporin–β-lactamase inhibitor combination that inhibits cell wall synthesis []. These two antibiotics did not kill cells in stationary phase. Our data show that MS-40S and MS-40 are bactericidal against both replicating and non-replicating cells. Interestingly, MS-40S was shown to kill a greater amount of stationary phase cells than exponential phase, unlike MS-40. This suggests that the MOA of these two compounds may be slightly different.
B. cenocepacia strains are inherently resistant to many available antibiotics [,,], leaving only a few available for treatment. As shown in the resistance studies, resistance is not easy to achieve for MS-40S and MS-40. Mutational resistance can be achieved by altering the antibiotic gene targets, decreasing the binding affinity of the antimicrobial to the gene product, decreasing the uptake/increase in efflux, or, lastly, by changing global responses such as changing a metabolic pathway []. Meropenem and doxycycline quickly generated multistep resistance, possibly by one of the mechanisms listed above; however, resistance did not emerge for MS-40S and MS-40. This might be due to MS-40S and MS-40 not being affected by the change of porins/efflux pumps that can cause resistance to other antimicrobials [], especially in Burkholderia species [,,]. Alternately, mutations in the gene targets of MS-40S and MS-40 could have resulted in a reduced fitness of the resistant mutant cell, preventing the mutant from outcompeting the sensitive cells [,].
Additionally, MS-40S and MS-40 can clear difficult-to-eradicate persister cells which commonly cause relapses in infections []. This suggests these compounds could eradicate the difficult-to-treat persistent infections in the CF lung, helping CF patients infected with Burkholderia species become eligible for lung transplants by []. MS-40S and MS-40 were shown to eliminate persister cells in two ways. The first way was by killing an enriched population of ciprofloxacin-generated persisters, reducing the population by a further 1–2 log10 CFU/mL. The second way was from a stationary-phase population of bacteria exposed solely to MS-40S and MS-40, with MS-40S only having a small amount of persisters at 5× MIC and MS-40 producing no persisters. Alternatively, the persister frequency was low for meropenem (5–7%), and approximately 30% for ceftazidime–avibactam, similar to the amount produced by Burkholderia pseudomallei exposed to ceftazidime []. Therefore, MS-40S and MS-40 could be used on their own to eliminate infections, or in tandem with current antibiotics to help eradicate infections [].
We have also showed MS-40S and MS-40 have low toxicity in C. elegans and G. mellonella. One limitation of these compounds is that we did not observe in vivo antibiotic activity in C. elegans and G. mellonella infected with B. cenocepacia K56-2 (data not shown). To help explain why we were not seeing a protective effect, we tested the MIC of MS-40S and MS-40 in 50% human serum. In the presence of 50% human serum, the MICs of both compounds increased to 128 μg/mL, suggesting these compounds bind non-specifically to proteins, which could decrease their antimicrobial activity. To increase the efficacy of these potent antimicrobials, a drug delivery system must be developed. One possible route is the creation of an MS-40S/MS-40-loaded liposome, as has been shown with rifampicin in the treatment of pulmonary Mycobacterium abscessus infections []. Creating an aerosolized antimicrobial therapy would also allow us to achieve higher concentrations of the drug, and increase lung penetration, which is especially important for CF pulmonary infections [].
Auranofin, and previously published auranofin analogues, inhibits thioredoxin reductase, an enzyme that plays a role in thiol-homeostasis in the cell [,]. However, it is unclear whether MS-40S and MS-40 share the same target with auranofin. It is assumed that the active component of auranofin is the gold atom, which binds the sulfur in the active site of thioredoxin reductase, inhibiting the formation of the critical disulfide bond, disrupting the function of the enzyme [,]. Auranofin was shown to have other effects on the bacterial cell, such as inhibiting DNA, protein, and cell wall synthesis. Thus, thioredoxin reductase may not be the sole antimicrobial target []. It is assumed that MS-40 and MS-40S will have similar effects inside the cell as auranofin, possibly having a multi-target mechanism of action. The lack of multi-step resistance may support this multi-target mechanism of action because multiple mutations would be needed to generate resistance. The other potential targets of auranofin and auranofin derivatives are not known. Other factors in the mechanism of action of the antimicrobials, which can explain differences in activity among compounds with slightly different structures, can be associated efflux pumps and transporters. Future research avenues could include determining the mechanism of action of MS-40S and MS-40, including the targets of the compounds, permeability factors, such as transporters and efflux pumps, and how these compounds kill persister cells and stationary phase cells.
4. Materials and Methods
4.1. Materials
All reagents and solvents were used as received from Sigma-Aldrich or Fisher Scientific unless noted. Reactions were monitored by thin layer chromatography (TLC) using TLC plates pre-coated with silica gel 60 F254 (SiliCycle, Québec, QC, Canada), visualized with a handheld ultraviolet device either directly or after staining with 5% H2SO4 in ethanol. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance Spectrospin DRX500 spectrometer, referenced either to the non-deuterated residual solvent peaks or tetramethyl silane peak (TMS, δ 0.00 ppm). 31P NMR spectra were recorded on a Bruker Avance Spectrospin DPX200 spectrometer, using freshly prepared triphenylphosphine solution (0.1 M in CDCl3, δ −6.00 ppm) as the external standard.
4.2. Bacterial Strains and Growth Conditions
Strains used are shown in Table S2. All strains were grown in LB at 37 °C with shaking at 230 rpm. B. ubonensis was grown at 30 °C with shaking at 230 rpm. New Brunswick Innova40 shaking incubator was used for liquid cultures. A Barnstead Lab-Line Standing Incubator was used for LB-agar plates and 96-well plates.
4.3. Synthesis of Auranofin and Auranofin Derivatives
Auranofin, WB-19-HL4118 (MS-40), WB-18-FI3683, WB-16-EO9899, WB-19-HG5899 and WB-19-HB2664 were synthesized following our previously published protocol (22).
WB-19-HL4170 (MS-40S). To a solution of Me3PAuCl (150 mg, 0.486 mmol) and 1-mercapto-2-propanol (43 μL, 0.486 mmol) in MeOH (10 mL), NaOCH3 was added (25 wt% in methanol, 125 µL). The solution was stirred at room temperature for 2 h. The reaction mixture was then concentrated on a rotary evaporator, diluted with dichloromethane, and poured into water followed by 3 times extraction by dichloromethane. The combined organic phase was dried over Na2SO4, concentrated, passed through a PTFE syringe filter (0.2 μm), and dried under vacuum to afford the product as light beige crystals (172 mg, 97%). 1H NMR (500 MHz, CDCl3) δ 3.70 (dqd, J = 9.4, 6.1, 3.4 Hz, 1H), 3.47 (s, 1H), 3.09 (dd, J = 12.7, 3.3 Hz, 1H), 2.82 (dd, J = 12.7, 9.0 Hz, 1H), 1.60 (d, J = 10.4 Hz, 5H), 1.24 (d, J = 6.1 Hz, 2H), (Figure S1). 13C NMR (126 MHz, CDCl3) δ 70.41, 38.47, 21.57, 16.03 (d, J = 35.7 Hz), (Figure S2). 31P NMR (162 MHz, CDCl3) δ −0.31 (Figure S3).
WB-19-HL4171, WB-19-HL4172 and WB-19-HL4181 were synthesized following the same procedure as MS-40 above.
WB-19-HL4171. Light yellow crystals (183 mg, 99%) from Me3PAuCl (150 mg, 0.486 mmol) and 1-thioglycerol (36 μL, 0.486 mmol). 1H NMR (500 MHz, CDCl3) δ 3.83–3.60 (m, 4H), 3.10 (dd, J = 12.8, 4.4 Hz, 1H), 3.00 (dd, J = 12.8, 7.7 Hz, 1H), 2.93 (s, 1H, OHα), 2.32 (s, 1H, OHβ), 1.60 (d, J = 10.4 Hz, 9H), (Figure S4). 13C NMR (126 MHz, CDCl3) δ 74.49, 65.58, 32.65, 16.13 (d, J = 35.8 Hz), (Figure S5). 31P NMR (162 MHz, CDCl3) δ −0.36, (Figure S6).
WB-19-HL4172. Light grey semi-solids (175 mg, 99%) from Me3PAuCl (150 mg, 0.486 mmol) and 3-mercapto-1-propanol (42 μL, 0.486 mmol). 1H NMR (500 MHz, CDCl3) δ 3.83 (t, J = 5.9 Hz, 2H), 3.19 (s, 1H), 3.04 (t, J = 6.8 Hz, 2H), 1.92 (p, J = 6.6 Hz, 2H), 1.60 (d, J = 10.4 Hz, 9H), (Figure S7). 13C NMR (126 MHz, CDCl3) δ 62.30, 39.20, 25.82, 16.07 (d, J = 35.5 Hz), (Figure S8). 31P NMR (162 MHz, CDCl3) δ −0.41, (Figure S9).
WB-19-HL4181. Colorless viscous solids (113 mg, quantitative) from Me3PAuCl (100 mg, 0.324 mmol) and cysteamine hydrochloride (37 mg, 0.324 mmol). 1H NMR (500 MHz, CDCl3) δ 3.01 (t, J = 6.3 Hz, 2H), 2.88 (t, J = 6.3 Hz, 2H), 1.99 (s, 2H), 1.59 (d, J = 10.4 Hz, 9H), (Figure S10). 13C NMR (126 MHz, CDCl3) δ 47.89, 32.94, 16.02 (d, J = 35.6 Hz), (Figure S11). 31P NMR (162 MHz, CDCl3) δ −0.15, (Figure S12).
WB-19-HL4121a. To a solution of 2-mercaptoethanolatotrimethylphosphine gold(I) (MS-40, 200 mg, 0.570 mmol) in 100 mL of dichloromethane, ferrocenium hexafluorophosphate (95 mg, 0.286 mmol) was added. The solution was stirred at 0 °C for 23 h. After filtration, the filtrate was concentrated and was then transferred to a 20-mL scintillation vial with a total volume of ~5 mL. The solution was placed in an ether vapor environment at 4 °C overnight. The yellow crystals formed were washed by diethyl ether and were further purified by preparative silica gel TLC (10:1 v/v dichloromethane/methanol) to give the product as a white solid (22 mg, 7.5%). 1H NMR (400 MHz, CD2Cl2) δ 1.60 (t, J = 4.1 Hz, 1H), (Figure S13). 31P NMR (162 MHz, CD2Cl2) δ 8.2, 143.8 (septet, JPF = 710 Hz), (Figure S14).
4.4. Auranofin Derivatives and Antibiotic Stock Solutions
Stock solutions were prepared by dissolving the compounds in dimethyl sulfoxide (DMSO) at a concentration of 20 mg/mL. Antibiotics were suspended at the following concentrations: tobramycin (Alfa Aesar, Haverhill, MA, USA), 10 mg/mL in H2O; chloramphenicol (Sigma, St. Louis, MO, USA), 20 mg/mL in ethanol; ceftazidime (Sigma), 10 mg/mL in 0.1 M NaOH; meropenem (Sigma), 10 mg/mL in DMSO; doxycycline (Sigma), 25 mg/mL in H2O; ciprofloxacin (Sigma), 10 mg/mL in 0.1 M HCl; and avibactam (MedKoo Biosciences, Morrisville, NC, USA), 10 mg/mL in DMSO.
4.5. Antimicrobial Susceptibility Testing and Multistep Resistance to Active Derivatives
The compounds were diluted from their stock solutions to 256 μg/mL in Cation-Adjusted Mueller Hinton Broth (CAMHB) for use in the experiment. Determination of the MIC was followed by standards set by the Clinical Laboratory Standards Institute (CLSI) []. The 96-well plates were filled with 50 μL of CAMHB, combined with a concentration gradient of compound to be tested. Bacterial culture was diluted to a turbidity equal to MacFarland Standard 0.5, then diluted 100-fold in CAMHB. A total of 50 μL of culture was transferred into each well. After incubation at 37 °C with no shaking for 18 h, MIC was read visually as the lowest concentration of antibiotic that prevented growth.
To determine the rate of multistep resistance mutations from serial passaging, the assay was performed as described previously [,]. From the MIC plate, 30 µL from the well that had bacterial growth at the highest concentration of the antimicrobial (0.5× MIC) for each of the compounds tested, was inoculated into 2 mL of LB without compound and incubated overnight at 37 °C with shaking. These overnight cultures were then used as the culture for a second MIC test, and this was repeated 12 times for a total of 24 days of continuous growth.
4.6. Time Kill Assays
Bacterial cultures were grown overnight and either subcultured to an OD600 of 0.025 or left in stationary phase. If subcultured, the bacteria were grown to early exponential phase (OD600 of 0.13–0.18). The bacteria were exposed to the antibiotics at 1×, 2×, and 4× the MIC, as well as no antibiotic for a negative control. Each hour from time zero to six hours, a sample of each condition was serially diluted to a dilution factor of 10−8, and 5 μL of each dilution was spotted onto LB agar. Plates were incubated for 24 h at 37 °C to determine CFU/mL.
4.7. Time Kill of Persister Cells
The generation and collection of persister cells was adapted from Bahar et al. []. Briefly, persister cells were generated by subculturing an overnight culture of B. cenocepacia K56-2 in LB and grown until it reached early exponential phase (OD600 of 0.13–0.18). The culture was then exposed to 5× MIC of ciprofloxacin (CIP; MIC = 2 μg/mL) with 0× MIC as a control for three hours. For the initial time zero count, a sample was taken and diluted to a factor of 10−8 and 5 μL was spotted onto LB. After the initial count, ciprofloxacin was added to the corresponding culture. A sample was taken every hour for three hours for CFU/mL counts, as mentioned above. After the third hour, the remaining population, enriched in persister cells, was collected, washed, and resuspended in phosphate buffered saline (PBS), divided into five tubes, and again exposed to 5× MIC ciprofloxacin or different concentrations of MS-40S or MS-40 (2× and 4× the MIC), along with a no antibiotic condition as a control. Samples were taken every hour for an additional three hours. Plates were incubated at 37 °C for 24 h and counted for CFU/mL.
4.8. Persister Frequency Assay
The persister frequency assay was performed as described in Ross et al. []. An overnight culture of B. cenocepacia K56-2 was subcultured to a concentration of 1 × 108 CFU/mL in 2 mL LB. Antimicrobials tested, meropenem, ceftazidime–avibactam, MS-40S, and MS-40 were added to a final concentration of 5× and 10× MIC. The cultures were exposed to the antibiotics for 24 h at 37 °C with shaking. After 24 h the culture was plated on LB to determine CFU/mL. Plates were incubated at 37 °C for 24 h.
4.9. C. elegans Survival
Caenorhabditis elegans was used as a model organism to test the toxicity of the compounds. The survival was performed as described in Selin et al. []. C. elegans DH26 eggs were incubated at 26 °C until they reached the L4 stage, at approximately 48 h. L4 stage worms were collected and washed with M9 media. Worms were suspended in 100 μL of M9 and transferred to the NGMII plates containing E. coli OP50. Approximately ten non-infected C. elegans OP50-fed worms, in triplicate, were exposed to a serial dilution of antibiotics to be tested in liquid killing media (LKM; 80% M9 buffer 20% liquid NGMII) in a 96-well plate. The range of concentrations used was 4–128 μg/mL for the following antimicrobials: MS-40S, MS-40, meropenem, doxycycline, and ceftazidime–avibactam, along with a no antibiotic control. Worms were counted at day 0 and incubated at 25 °C. After 24 h, worms were counted for percent survival and the Survival100/MIC ratio was calculated. Worms that appeared straight were considered dead, and those were moving and S-shaped were counted as alive. Three experimental replicates were performed.
4.10. Galleria Toxicity
Galleria mellonella was also used as a model organism to study the toxicity of MS-40S and MS-40. The experiments were performed as done in Naguib and Valvano 2018 [] and Cruz et al., 2018 []. Galleria larvae were stored at 16 °C in wood shavings and used within 2 weeks of receiving them. Larvae, with an approximate weight of 250 mg, were injected with 10 μL in the last, left proleg using a Hamilton micro-syringe (Hamilton, Nevada, USA). For each compound, MS-40S, MS-40, and doxycycline were diluted in PBS, and 10, 5, 2, and 1 mg/kg were injected in 10 worms for each condition. A total of 10 worms were also injected with 10 μL of PBS for a negative control, and 10 worms were not injected. Survival was measured every 24 h for 72 h. Larvae were considered dead if non-motile and unresponsive to touch. Three experimental replicates were performed. Survival curves were made on GraphPad Prism 6.
5. Conclusions
To conclude, we have shown MS-40 and the novel compound MS-40S have potent bactericidal activity towards pathogenic Burkholderia, including the cystic fibrosis multi-drug resistant pathogens from the Bcc, B. pseudomallei and B. mallei. MS-40S and MS-40 kill both B. cenocepacia replicating and non-replicating cells, including persister cells, with little occurrence of resistance. MS-40S and MS-40 also have bactericidal activity against other pathogens involved in the CF lung microbiome. We also demonstrate in C. elegans and Galleria models, MS-40S and MS-40 were non-toxic. The novel compounds are comparable to current clinical antibiotics used to help those infected with B. cenocepacia and other Burkholderia species. We propose that MS-40S and MS-40 have unique properties as antimicrobials and studying the mechanism of action of these will help in the development of novel antibiotics to treat multi-drug resistant CF lung infections.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10121443/s1, Table S1. Minimum inhibitory concentrations (MICs) of group one auranofin derivatives against a panel of Burkholderia cepacia complex (Bcc) bacteria. Table S2. Bacterial species and strains used in this study. Figure S1. 1H NMR spectrum of compound WB-19-HL4170 (MS-40S) in CDCl3. Figure S2. 13C NMR spectrum of compound WB-19-HL4170 (MS-40S) in CDCl3. Figure S3. 31P NMR spectrum of compound WB-19-HL4170 (MS-40S) in CDCl3. Figure S4. 1H NMR spectrum of compound WB-19-HL4171 in CDCl3. Figure S5. 13C NMR spectrum of compound WB-19-HL4171 in CDCl3. Figure S6. 31P NMR spectrum of compound WB-19-HL4171 in CDCl3. Figure S7. 1H NMR spectrum of compound WB-19-HL4172 in CDCl3. Figure S8. 13C NMR spectrum of compound WB-19-HL4172 in CDCl3. Figure S9. 31P NMR spectrum of compound WB-19-HL4172 in CDCl3. Figure S10. 1H NMR spectrum of compound WB-19-HL4181 in CDCl3. Figure S11. 13C NMR spectrum of compound WB-19-HL4181 in CDCl3. Figure S12. 31P NMR spectrum of compound WB-19-HL4181 in CDCl3. Figure S13. 1H NMR spectrum of compound WB-19-HL4121a in CD2Cl2. Figure S14. 31P NMR spectrum of compound WB-19-HL4121a in CD2Cl2.
Author Contributions
Conceptualization, D.M. and S.T.C.; Formal analysis, D.M.; Funding acquisition, M.Y. and S.T.C.; Investigation, D.M. and Z.L.Y.; Methodology, D.M., B.W., D.T., S.H.L., A.M.H., Z.L.Y., M.Y. and S.T.C.; Project administration, S.T.C.; Resources, S.T.C. and M.Y.; Supervision, M.Y. and S.T.C.; Writing—original draft, D.M., B.W. and A.M.H.; Writing—review and editing, D.M., D.T., M.Y. and S.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
Work was supported by a research grant from Cystic Fibrosis Canada and a project grant from CIHR to S.T.C., and in part by a grant from the National Institute of Allergy and Infectious Diseases (R21AI140418 to M.Y.). D.M. was supported by a scholarship from the Canadian Institutes of Health Research (CIHR). A.M.H. was supported by the Vanier Canada Graduate Scholarship. Z.L.Y. was supported by scholarship from Research Manitoba.
Data Availability Statement
Not required.
Acknowledgments
We would like to thank Catherine Deschênes (Laboratoire Régional de Microbiologie) for some of the Burkholderia isolates used in this study, to Ayush Kumar (University of Manitoba) for providing P. aeruginosa PA01 and PA7 strains and A. baumannii ATCC 17978. We would also like to thank the NMR facility in the Department of Chemistry at the University of Manitoba. This work utilized NIAID’s suite of preclinical services for MIC assessments against B. pseudomallei and B. mallei (Contract No. 75N93019D00001/75N93020F00001).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gould, K. Antibiotics: From prehistory to the present day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 4-2. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 5, 1007. [Google Scholar] [CrossRef]
- Rhodes, K.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updates 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Sfeir, M.M. Burkholderia cepacia complex infections: More complex than the bacterium name suggest. J. Infect. 2018, 77, 166–170. [Google Scholar] [CrossRef]
- Lobo, L.J.; Noone, P.G. Respiratory infections in patients with cystic fibrosis undergoing lung transplantation. Lancet Respir. Med. 2014, 2, 73–82. [Google Scholar] [CrossRef]
- Los-Arcos, I.; Len, O.; Gomez, M.T.M.; González-López, J.J.; Saéz-Giménez, B.; Deu, M.; Nuvials, X.; Ferrer, R.; Román, A.; Gavaldà, J. Lung transplantation in two cystic fibrosis patients infected with previously pandrug-resistant Burkholderia cepacia complex treated with ceftazidime–avibactam. Infection 2019, 47, 289–292. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia Infections in Cystic Fibrosis Patients: Drug Resistance and Therapeutic Approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Dupont, L. Lung transplantation in cystic fibrosis patients with difficult to treat lung infections. Curr. Opin. Pulm. Med. 2017, 23, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.V.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Oosterhuis, D.; Bonsignore, R.; Casini, A.; Olinga, P.; Scheffers, D. An Organogold Compound as Potential Antimicrobial Agent against Drug-Resistant Bacteria: Initial Mechanistic Insights. ChemMedChem 2021, 16, 3060–3070. [Google Scholar] [CrossRef] [PubMed]
- May, H.C.; Yu, J.-J.; Guentzel, M.N.; Chambers, J.P.; Cap, A.P.; Arulanandam, B.P. Repurposing Auranofin, Ebselen, and PX-12 as Antimicrobial Agents Targeting the Thioredoxin System. Front. Microbiol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Novelli, F.; Recine, M.; Sparatore, F.; Juliano, C. Gold(I) complexes as antimicrobial agents. Il Farm. 1999, 54, 232–236. [Google Scholar] [CrossRef]
- Owings, J.P.; McNair, N.N.; Mui, Y.F.; Gustafsson, T.N.; Holmgren, A.; Contel, M.; Goldberg, J.B.; Mead, J.R. Auranofin andN-heterocyclic carbene gold-analogs are potent inhibitors of the bacteriaHelicobacter pylori. FEMS Microbiol. Lett. 2016, 363, 148. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571. [Google Scholar] [CrossRef]
- Kean, W.F.; Hart, L.; Buchanan, W.W. Auranofin. Br. J. Rheumatol. 1997, 36, 560–572. [Google Scholar] [CrossRef]
- Finkelstein, A.E.; Walz, D.T.; Batista, V.; Mizraji, M.; Roisman, F.; Misher, A. Auranofin. New oral gold compound for treatment of rheumatoid arthritis. Ann. Rheum. Dis. 1976, 35, 251–257. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef]
- Epstein, T.D.; Wu, B.; Moulton, K.D.; Yan, M.; Dube, D.H. Sugar-Modified Analogs of Auranofin Are Potent Inhibitors of the Gastric Pathogen Helicobacter pylori. ACS Infect. Dis. 2019, 5, 1682–1687. [Google Scholar] [CrossRef]
- Wu, B.; Yang, X.; Yan, M. Synthesis and Structure–Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens. J. Med. Chem. 2019, 62, 7751–7768. [Google Scholar] [CrossRef] [PubMed]
- Balwan, A.; Nicolau, D.P.; Wungwattana, M.; Zuckerman, J.B.; Waters, V. Clinafloxacin for Treatment of Burkholderia cenocepacia Infection in a Cystic Fibrosis Patient. Antimicrob. Agents Chemother. 2016, 60, 1–5. [Google Scholar] [CrossRef]
- Kitt, H.; Lenney, W.; Gilchrist, F.J. Two case reports of the successful eradication of new isolates of Burkholderia cepacia complex in children with cystic fibrosis. BMC Pharmacol. Toxicol. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Laboudi, A.; Etherington, C.; Whitaker, P.; Clifton, I.; Conway, S.; Denton, M.; Peckham, D. Acute Burkholderia cenocepacia pyomyositis in a patient with cystic fibrosis. J. Cyst. Fibros. 2009, 8, 273–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilchrist, F.J.; Webb, A.K.; Bright-Thomas, R.J.; Jones, A. Successful treatment of cepacia syndrome with a combination of intravenous cyclosporin, antibiotics and oral corticosteroids. J. Cyst. Fibros. 2012, 11, 458–460. [Google Scholar] [CrossRef]
- Salizzoni, S.; Pilewski, J.; Toyoda, Y. Lung transplant for a patient with cystic fibrosis and active Burkholderia Cenocepacia pneumonia. Exp. Clin. Transplant. 2014, 12, 487–489. [Google Scholar]
- Huang, Y.J.; LiPuma, J.J. The Microbiome in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 59–67. [Google Scholar] [CrossRef]
- Cribbs, S.K.; Beck, J.M. Microbiome in the pathogenesis of cystic fibrosis and lung transplant-related disease. Transl. Res. 2017, 179, 84–96. [Google Scholar] [CrossRef]
- Vandeplassche, E.; Tavernier, S.; Coenye, T.; Crabbé, A. Influence of the lung microbiome on antibiotic susceptibility of cystic fibrosis pathogens. Eur. Respir. Rev. 2019, 28, 190041. [Google Scholar] [CrossRef]
- Edwards, B.D.; Somayaji, R.; Greysson-Wong, J.; Izydorczyk, C.; Waddell, B.; Storey, D.G.; Rabin, H.R.; Surette, M.G.; Parkins, M.D. Clinical Outcomes Associated with Escherichia coli Infections in Adults with Cystic Fibrosis: A Cohort Study. In Open Forum Infectious Diseases; Oxford University Press: Oxford, MS, USA, 2019; Volume 7, p. ofz476. [Google Scholar] [CrossRef]
- Burns, J.L.; Emerson, J.; Stapp, J.R.; Yim, D.L.; Krzewinski, J.; Louden, L.; Ramsey, B.W.; Clausen, C.R. Microbiology of Sputum from Patients at Cystic Fibrosis Centers in the United States. Clin. Infect. Dis. 1998, 27, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Sabtu, N.; Enoch, D.A.; Brown, N.M. Antibiotic resistance: What, why, where, when and how? Br. Med. Bull. 2015, 116, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Waglechner, N.; Wright, G.D. Antibiotic resistance: It’s bad, but why isn’t it worse? BMC Biol. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Abutaleb, N.; Elmagarmid, K.A.; Seleem, M. Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant enterococci. Sci. Rep. 2018, 8, 8353. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Macrobiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Corona, F.; Martinez, J.L. Phenotypic Resistance to Antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef]
- Wood, T.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Wilmaerts, D.; Windels, E.; Verstraeten, N.; Michiels, J. General Mechanisms Leading to Persister Formation and Awakening. Trends Genet. 2019, 35, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Liu, Z.; Totsingan, F.; Buitrago, C.; Kallenbach, N.R.; Ren, D. Synthetic dendrimeric peptide active against biofilm and persister cells of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 8125–8135. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Myers, J.N.; Muruato, L.A.; Tapia, D.; Torres, A.G. Evaluating New Compounds to Treat Burkholderia pseudomallei Infections. Front. Cell. Infect. Microbiol. 2018, 8, 210. [Google Scholar] [CrossRef]
- Selin, C.; Stietz, M.S.; Blanchard, J.E.; Gehrke, S.S.; Bernard, S.; Hall, D.; Brown, E.D.; Cardona, S.T. A Pipeline for Screening Small Molecules with Growth Inhibitory Activity against Burkholderia cenocepacia. PLoS ONE 2015, 10, e0128587. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.F.; Neubauer, H.; Frickmann, H.; Hagen, R.M. Correlation of RpsU Gene Sequence Clusters and Biochemical Properties, GC—MS Spectra and Resistance Profiles of Clinical Burkholderia Spp. Isolates. Eur. J. Microbiol. Immunol. 2016, 6, 25–39. [Google Scholar] [CrossRef]
- Guglierame, P.; Pasca, M.R.; De Rossi, E.; Buroni, S.; Arrigo, P.; Manina, G.; Riccardi, G. Efflux pump genes of the resistance-nodulation-division family in Burkholderia cenocepacia genome. BMC Microbiol. 2006, 6, 66. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Evolutionary Trajectories to Antibiotic Resistance. Annu. Rev. Microbiol. 2017, 71, 579–596. [Google Scholar] [CrossRef]
- Durao, P.; Balbontín, R.; Gordo, I. Evolutionary Mechanisms Shaping the Maintenance of Antibiotic Resistance. Trends Microbiol. 2018, 26, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.J.; Stokes, J.M.; Collins, J.J. Eradicating Bacterial Persisters with Combinations of Strongly and Weakly Metabolism-Dependent Antibiotics. Cell Chem. Biol. 2020, 27, 1544–1552.e3. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.; Sennato, S.; De Santis, F.; Forte, J.; Fraziano, M.; Casciardi, S.; Marianecci, C.; Bordi, F.; Carafa, M. Rifampicin–Liposomes for Mycobacterium abscessus Infection Treatment: Intracellular Uptake and Antibacterial Activity Evaluation. Pharmaceutics 2021, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; van Ingen, J.; van der Laan, R.; Herrmann, J.-L. Liposomal drug delivery to manage nontuberculous mycobacterial pulmonary disease and other chronic lung infections. Eur. Respir. Rev. 2021, 30, 210010. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (Ed.) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1. [Google Scholar]
- Yarlagadda, V.; Akkapeddi, P.; Manjunath, G.B.; Haldar, J. Membrane Active Vancomycin Analogues: A Strategy to Combat Bacterial Resistance. J. Med. Chem. 2014, 57, 4558–4568. [Google Scholar] [CrossRef]
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18. [Google Scholar] [CrossRef]
- Cruz, L.I.B.; Lopes, L.F.F.; Ribeiro, F.D.C.; de Sa, N.P.; Lino, C.I.; Tharmalingam, N.; De Oliveira, R.B.; Rosa, C.A.; Mylonakis, E.; Fuchs, B.B.; et al. Anti-Candida albicans Activity of Thiazolylhydrazone Derivatives in Invertebrate and Murine Models. J. Fungi 2018, 4, 134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).