Beneficial Effects of Anticoagulants on the Clinical Outcomes of COVID-19 Patients
Abstract
:1. Introduction
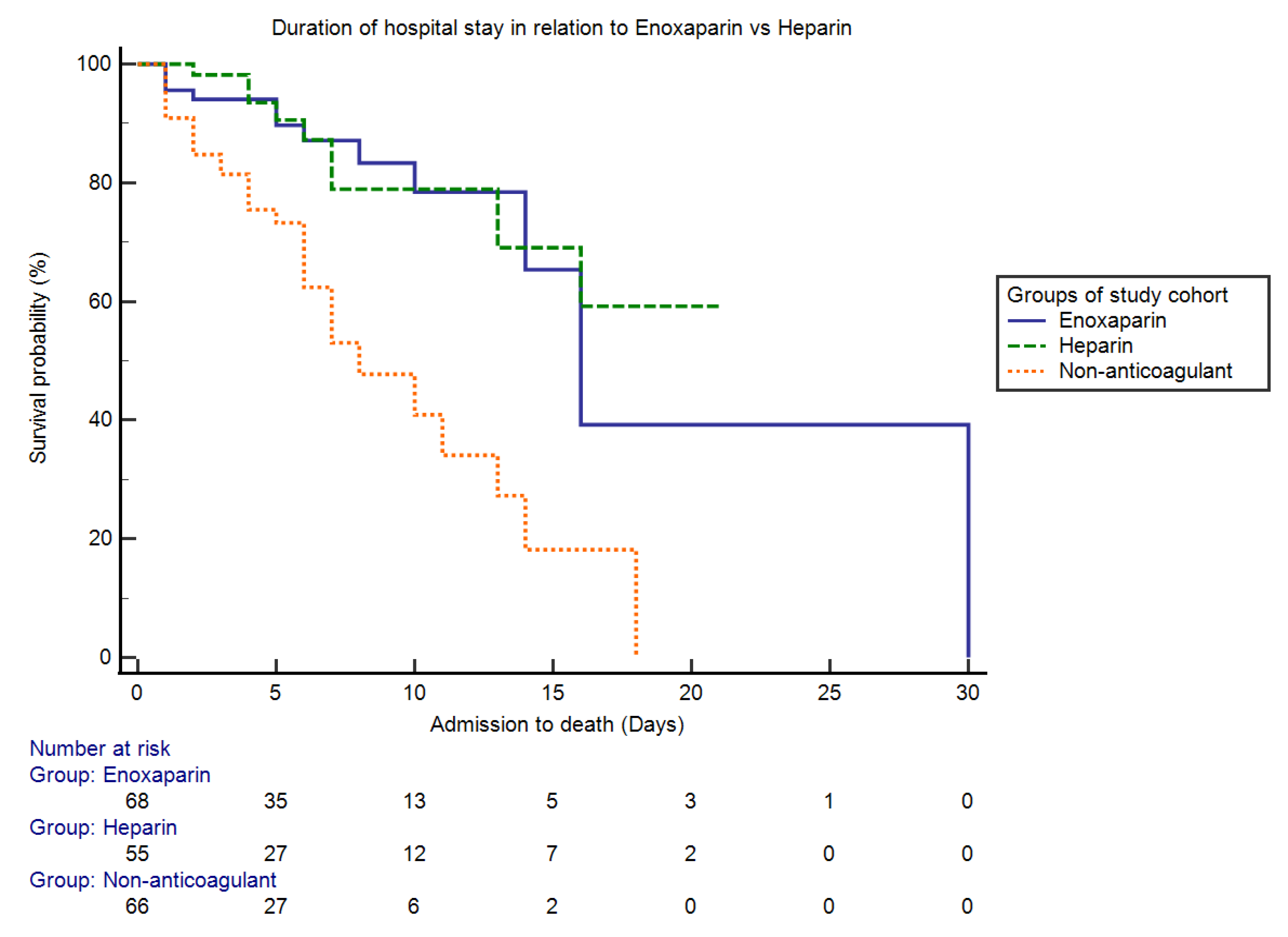
2. Results
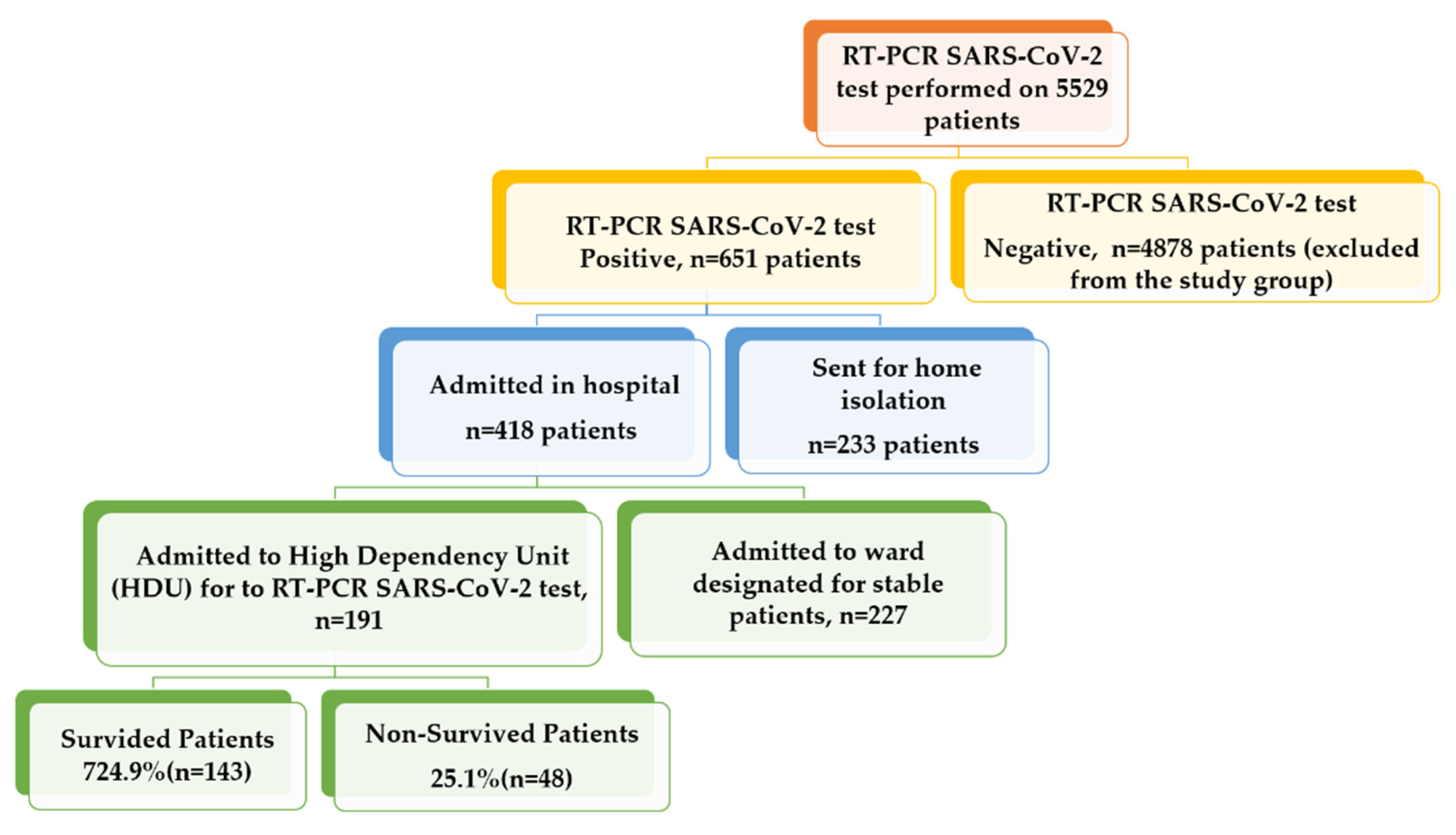
2.1. Patients’ Characteristics
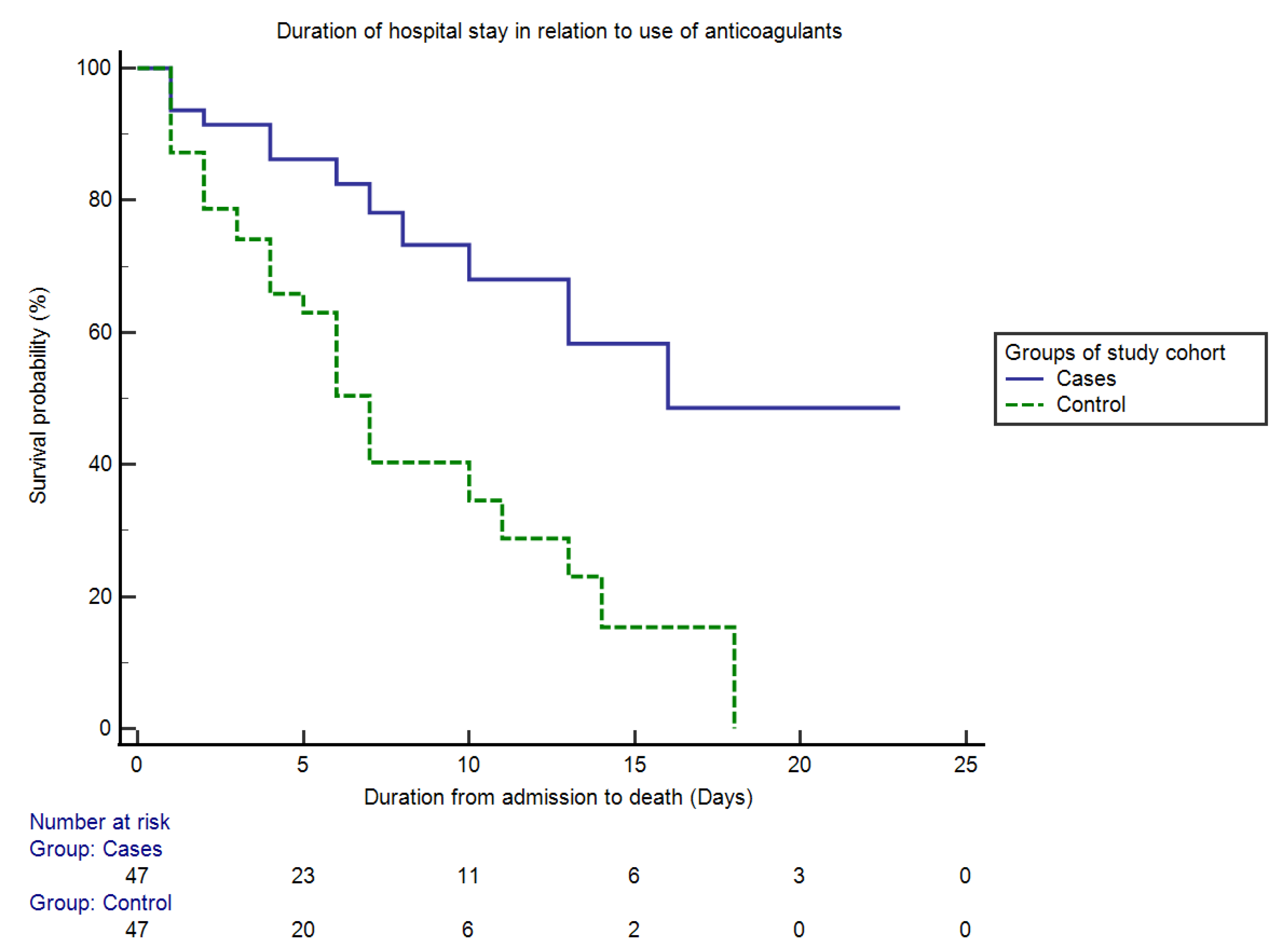
2.2. Propensity-Score-Based Case–Control Matching
3. Discussion
4. Materials and Methods
4.1. Study Cohort and Inclusion Criteria
- Patients with moderate disease (SpO2 > 90%, but <94%) whose chest X-ray shows infiltrates in <50% of total lung fields or whose high-resolution chest CT shows peripheral ground glass opacities.
- Patients with severe disease (SpO2 < 90% + stable vital signs, respiratory rate > 30/min and chest X-ray showing infiltrates >50% of total lungs) or high-resolution chest CT showing extensive peripheral ground glass opacities.
- Lung involvement alone, evident by chest X-ray or HRCT;
- Cytokine release syndrome (CRS), which is a systemic inflammatory response that can be triggered by a variety of factors, such as infections and certain drugs. CRS was diagnosed by a documented rise in inflammatory markers (CRP > 70 mg/L, LDH > 300 U/L, ferritin > 700 ng/mL and D-dimers > 1000 ng/mL (or > 1 mcg/mL)), with a rising tendency of these markers in the last 24 h;
- Evidence of myocarditis (electrocardiogram changes, positive troponin T, elevated proBNP or echo findings);
- Sepsis with or without septic shock;
- Combination of above phases.
4.2. Exclusion Criteria
- Those COVID-19 patients who had mild symptoms and were advised to isolate at home;
- Those patients who were admitted to the stable COVID-19 ward.
- Group I: enoxaparin in a therapeutic dose (1 mg/kg × 12 hourly) subcutaneously;
- Group II: enoxaparin in a prophylactic dose (40 mg × once a day) subcutaneously;
- Group III: heparin in a therapeutic dose (80 units/kg loading dose, then 18 units/kg/hour);
- Group IV: heparin in a prophylactic dose (5000 units × 12 hourly) subcutaneously;
- Group V: oral anticoagulants (rivaroxaban, 15–20 mg, once a day).
4.3. Therapeutic Dose of Enoxaparin or Heparin (Group I and Group III)
- D-dimer levels more than 3 times the upper limit;
- Documented presence of thromboembolic disease (Doppler ultrasound for deep vein thrombosis or CT scan findings for pulmonary embolism);
- Strong clinical suspicion of thromboembolic disease, even without investigation.
4.4. Prophylactic Dose of Enoxaparin or Heparin (Group II and Group IV)
4.5. Oral Anticoagulants (Group V)
4.6. Study Method
4.7. Propensity-Based Case–Control Matching
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 2 November 2021).
- Guidelines Clinical Management Guidelines for COVID-19 Infections. 2020. Available online: https://www.nih.org.pk/wp-content/uploads/2020/06/20200106-Clinical-Management-Guidelines-for-COVID-19-infection-v2.pdf (accessed on 10 November 2021).
- Kollias, A.; Kyriakoulis, K.G.; Dimakakos, E.; Poulakou, G.; Stergiou, G.S.; Syrigos, K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020, 189, 846–847. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [CrossRef] [Green Version]
- Turshudzhyan, A. Anticoagulation Options for Coronavirus Disease 2019 (COVID-19)-Induced Coagulopathy. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Gavioli, E.M.; Sikorska, G.; Man, A.; Rana, J.; Vider, E. Current Perspectives of Anticoagulation in Patients With COVID-19. J. Cardiovasc. Pharmacol. 2020, 76, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 117, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kipshidze, N.; Dangas, G.; White, C.J.; Kipshidze, N.; Siddiqui, F.; Lattimer, C.R.; Carter, C.A.; Fareed, J. Viral Coagulopathy in Patients With COVID-19: Treatment and Care. Clin. Appl. Thromb. Hemost. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.S.; Manolis, T.A.; Manolis, A.A.; Papatheou, D.; Melita, H. COVID-19 Infection: Viral Macro-and Micro-Vascular Coagulopathy and Thromboembolism/Prophylactic and Therapeutic Management. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 12. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Wu, W.; Wang, A.; Liu, M. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Haliga, R.E.; Sorodoc, V.; Lionte, C.; Petris, O.R.; Bologa, C.; Coman, A.E.; Vata, L.G.; Puha, G.; Dumitrescu, G.; Sirbu, O.; et al. Acute Clinical Syndromes and Suspicion of SARS-CoV-2 Infection: The Experience of a Single Romanian Center in the Early Pandemic Period. Medicina 2021, 57, 121. [Google Scholar] [CrossRef]
- Aoki, N.; Matsuda, T.; Saito, H.; Takatsuki, K.; Okajima, K.; Takahashi, H.; Takamatsu, J.; Asakura, H.; Ogawa, N. A comparative double-blind randomized trial of activated protein C and unfractionated heparin in the treatment of disseminated intravascular coagulation. Int. J. Hematol. 2002, 75, 540–547. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Petrillo, F.; Chianese, A.; De Bernardo, M.; Zannella, C.; Galdiero, M.; Reibaldi, M.; Avitabile, T.; Boccia, G.; Galdiero, M.; Rosa, N.; et al. Inhibitory effect of ophthalmic solutions against sars-cov-2: A preventive action to block the viral transmission? Microorganisms 2021, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, L.; Viale, P.; Longo, L.; Vitale, D.C.; Drago, F. The Potential Role of Heparin in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review. Front. Pharmacol. 2020, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Obi, A.T.; Barnes, G.D.; Napolitano, L.M.; Henke, P.K.; Wakefield, T.W. Venous thrombosis epidemiology, pathophysiology, and anticoagulant therapies and trials in severe acute respiratory syndrome coronavirus 2 infection. J. Vasc. Surgery. Venous Lymphat. Disord. 2021, 9, 23. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Thimmareddygari, D.; Ramahi, A.; Atallah, L.; Baranetsky, N.G.; Slim, J. Clinical characteristics and outcome in patients with combined diabetic ketoacidosis and hyperosmolar hyperglycemic state associated with COVID-19: A retrospective, hospital-based observational case series. Diabetes Res. Clin. Pract. 2020, 166, 108279. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Kunichoff, D.; Adhikari, S.; Ahuja, T.; Amoroso, N.; Aphinyanaphongs, Y.; Cao, M.; Goldenberg, R.; Hindenburg, A.; Horowitz, J. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients With COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Miesbach, W.; Makris, M. COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clin. Appl. Thromb. Hemost. 2020, 26. [Google Scholar] [CrossRef]
- Reichert, J.A.; Hlavinka, P.F.; Stolzfus, J.C. Risk of hemorrhage in patients with chronic liver disease and coagulopathy receiving pharmacologic venous thromboembolism prophylaxis. Pharmacotherapy 2014, 34, 1043–1049. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip MJ, H.A.; Van der Meer NJ, M.; Arbous, M.S.; Gommers DA MP, J.; Kant, K.M.; Kapteina, F.H.J.; van Paassend, J.; Stalsa, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: A multicenter retrospective study from Wuhan, China. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Khan, S. Coagulopathy and Plausible Benefits of Anticoagulation Among COVID-19 Patients. Curr. Probl. Cardiol. 2020, 45, 100648. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canoglu, K.; Saylan, B. Therapeutic dosing of low-molecular-weight heparin may decrease mortality in patients with severe COVID-19 infection. Ann. Saudi Med. 2020, 40, 462. [Google Scholar] [CrossRef]
- Fontana, P.; Casini, A.; Robert-Ebadi, H.; Glauser, F.; Righini, M.; Blondon, M. Venous thromboembolism in COVID-19: Systematic review of reported risks and current guidelines. Swiss Med. Wkly. 2020, 150, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C. The management of venous thromboembolism in hospitalized patients with COVID-19. Blood Adv. 2020, 4, 4028. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Gasperetti, A.; Mancone, M.; Curnis, A.; Mascioli, G.; Mitacchione, G.; Busana, M.; Sabato, F.; Gobbi, C.; Antinori, S.; et al. Oral anticoagulation and clinical outcomes in COVID-19: An Italian multicenter experience. Int. J. Cardiol. 2021, 323, 276–280. [Google Scholar] [CrossRef] [PubMed]
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Variables | Anticoagulant Group (n = 130) | Non-Anticoagulant Group (n = 61) | p-Value | Cases (n = 47) | Controls (n = 47) | p-Value |
| Biochemical parameters | ||||||
| Age | 47.40 + 13.54 | 54.37 + 11.89 | 0.00 | 53.87 + 12.57 | 52.20 + 15.69 | 0.56 |
| Platelets × 103 cells/L | 246.42 + 95.05 | 125.46 + 82.89 | 0.01 | 201.57 + 25.15 | 187.34 + 32.59 | 0.21 |
| D-dimers (ng/mL) | 367.97 + 91.17 | 122.31 + 32.21 | 0.01 | 245.92 + 95.26 | 221.3 + 82.24 | 0.38 |
| Trop T (ng/mL) | 0.33 + 0.24 | 0.08 + 0.64 | 0.02 | 0.20 + 0.14 | 0.18 + 0.34 | 0.22 |
| Comorbidities | 0.00 | |||||
| Yes | 82.3% (107/130) | 57.4% (35/61) | 63.8% (30/47) | 65.9% (31/47) | 0.62 | |
| No | 17.7% (23/130) | 42.6% (26/61) | 36.2% (17/47) | 34.1% (16/47) | ||
| Diabetes mellitus | 0.00 | |||||
| Yes | 66.2% (86/130) | 47.5% (29/61) | 46.8% (22/47) | 48.9% (23/47) | 0.16 | |
| No | 33.8% (44/130) | 52.5% (32/61) | 53.2% (25/47) | 51.1% (24/47) | ||
| Hypertension | 0.00 | |||||
| Yes | 60% (78/130) | 39.3% (24/61) | 46.8% (22/47) | 48.9% (23/47) | 0.58 | |
| No | 40% (52/130) | 60.7% (37/61) | 53.2% (25/47) | 51.1% (24/47) | ||
| Disease severity | 0.05 | |||||
| Moderate disease | 30% (39/130) | 42.6% (26/61) | 31.9% (15/47) | 31.9% (15/47) | 1.00 | |
| Severe disease | 70% (91/130) | 57.4% (35/61) | 68.1% (32/47) | 68.1% (32/47) | ||
| Survival analysis | 0.00 | |||||
| Survived | 84.6% (110/130) | 54.1% (33/61) | 74.4% (35/47) | 38.3% (18/47) | <0.0001 | |
| Died | 15.4% (20/130) | 45.9% (28/61) | 25.5% (12/47) | 61.7% (29/47) | ||
| Variables | OR (95% CI) | p-Value |
|---|---|---|
| Age | 1.03 (1.01–1.05) | 0.00 |
| Platelet count | 0.81 (0.74–0.89) | 0.00 |
| Troponin T levels | 1.09 (1.03–2.01) | 0.01 |
| Comorbid conditions | 0.32 (0.12–0.83) | 0.02 |
| Enoxaparin (therapeutic dose) | 0.26 (0.11–0.65) | 0.00 |
| Enoxaparin (prophylactic dose) | 0.19 (0.06–0.64) | 0.00 |
| Heparin (therapeutic dose) | 0.60 (0.20–1.74) | 0.34 |
| Heparin (prophylactic dose) | 0.05 (0.01–0.26) | 0.00 |
| Oral anticoagulants | 0.26 (0.05–1.22) | 0.09 |
| Non-anticoagulant group | 0.27 (0.06–1.17) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, Z.; Khan, A.A.; Khalid, S.; Asghar, M.; Muhammad, K.; Waheed, Y. Beneficial Effects of Anticoagulants on the Clinical Outcomes of COVID-19 Patients. Antibiotics 2021, 10, 1394. https://doi.org/10.3390/antibiotics10111394
Jamil Z, Khan AA, Khalid S, Asghar M, Muhammad K, Waheed Y. Beneficial Effects of Anticoagulants on the Clinical Outcomes of COVID-19 Patients. Antibiotics. 2021; 10(11):1394. https://doi.org/10.3390/antibiotics10111394
Chicago/Turabian StyleJamil, Zubia, Azmat Ali Khan, Samreen Khalid, Muhammad Asghar, Khalid Muhammad, and Yasir Waheed. 2021. "Beneficial Effects of Anticoagulants on the Clinical Outcomes of COVID-19 Patients" Antibiotics 10, no. 11: 1394. https://doi.org/10.3390/antibiotics10111394
APA StyleJamil, Z., Khan, A. A., Khalid, S., Asghar, M., Muhammad, K., & Waheed, Y. (2021). Beneficial Effects of Anticoagulants on the Clinical Outcomes of COVID-19 Patients. Antibiotics, 10(11), 1394. https://doi.org/10.3390/antibiotics10111394










