Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent
Abstract
:1. Introduction
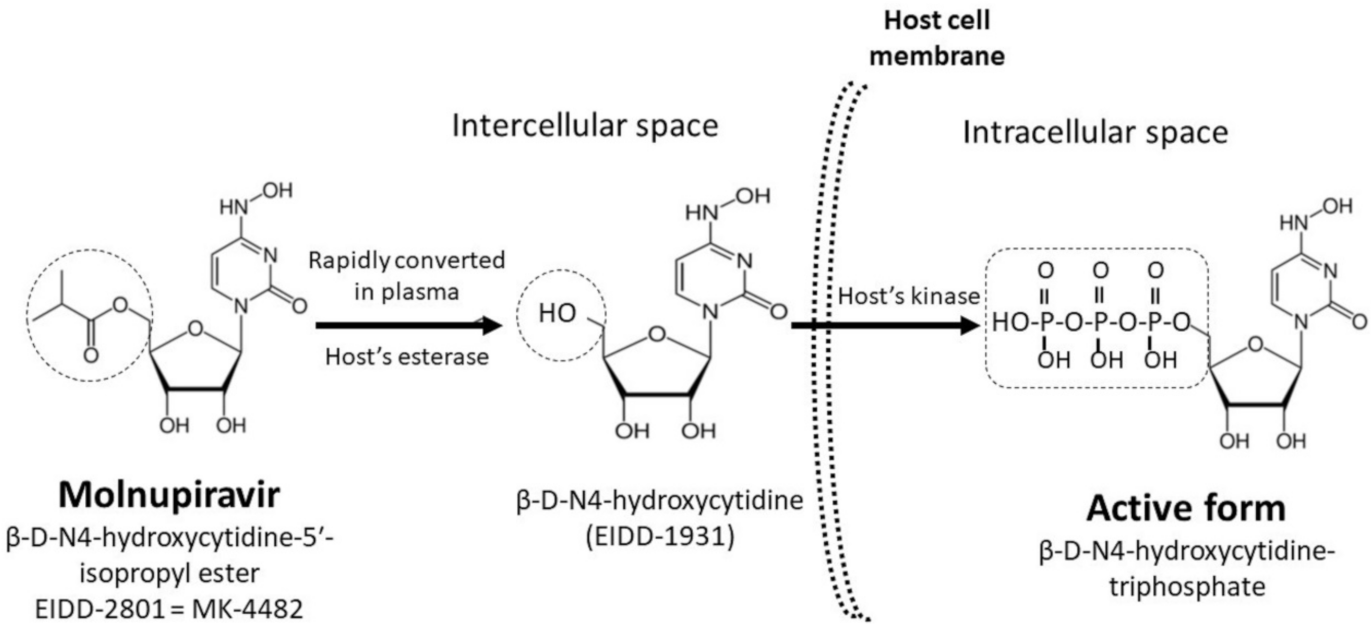
2. Molnupiravir
2.1. Animal Models
2.2. Resistant Barrier
| Compound Name | β-d-N4-hydroxycytidine-5′-isopropyl ester (EIDD-2801) |
| Active form | β-d-N4-hydroxycytidine-triphosphate |
| Molecular weight | 329.31 Da |
| Classification of Antiviral Agents | Inhibitor of RNA-dependent RNA polymerase |
| Antiviral mechanism [20,21] | Inhibits viral replication by incorporation into the viral genome and causes the accumulation of mutations |
| In vitro activity against virus types [16,17,18,19] | Coronaviruses, Venezuelan equine encephalitis virus, respiratory syncytial virus, Ebola virus, influenza A and B viruses, and Chikungunya virus |
| Administration Route | Oral |
| Anti-SARS-CoV-2 Activity (Vero Cell Line) [24] | |
| IC (inhibitory concentration)50 | 0.3 μM |
| CC (cytotoxic concentration)50 | >10 μM |
| Selectivity index | >100 |
| Pharmacodynamic Properties (Single Dosing of 800 mg) [29] | |
| AUClast (mean) | 8720 ng·h/mL |
| AUCinf (mean) | 8720 ng·h/mL |
| Cmax (mean) | 3640 ng/mL |
| tmax | 1.00 h |
| t1/2 (mean) | 1.29 h |
| Ae0–24 (mean) | 18.0 mg |
| Fe0–24 (mean) | 2.86% |
| Resistance | 1. High resistant barrier across the coronavirus genome [19]; 2. Low potential for mutations in cellular RNA of the host [28]. |
| Clinical Applications | |
| Prophylactic efficacy | Evidenced in animal models [26] |
| Therapeutic efficacy | For patients with asymptomatic or mild severity of COVID-19, as evidenced by phase-I and II trials [30,31,32] |
3. Clinical Trials of Molnupiravir
3.1. Phase I
3.2. Phase II
3.3. Phase III
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Shen, F.-M.; Chen, F.; Lin, Z. Origin and evolution of the 2019 novel coronavirus. Clin. Infect. Dis. 2020, 71, 882–883. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.-W.; To, K.K.-W.; Tse, H.; Jin, D.-Y.; Yuen, K.-Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Zumla, A.I.; Al-Hakeem, R.F.; Al-Rabeeah, A.A.; Stephens, G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 2013, 368, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zheng, B.; Li, Y.; Poon, L.; Xie, Z.; Chan, K.; Li, P.; Tan, S.; Chang, Q.; Xie, J. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- WHO. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 16 June 2021).
- Vetter, P.; Vu, D.L.; L’Huillier, A.G.; Schibler, M.; Kaiser, L.; Jacquerioz, F. Clinical features of Covid-19. BMJ 2020, 369, 1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Kandeel, M.; Yamamoto, M.; Tani, H.; Kobayashi, A.; Gohda, J.; Kawaguchi, Y.; Park, B.K.; Kwon, H.-J.; Inoue, J.-I.; Alkattan, A. Discovery of new fusion inhibitor peptides against SARS-CoV-2 by targeting the spike S2 subunit. Biomol. Ther. 2021, 29, 282. [Google Scholar] [CrossRef]
- Vicenti, I.; Zazzi, M.; Saladini, F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin. Ther. Pat. 2021, 31, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Caccuri, F.; Bugatti, A.; Zani, A.; Vanoni, M.; Bonfanti, P.; Cazzaniga, M.E.; Perno, C.F.; Messa, C.; Alberghina, L. Methotrexate inhibits SARS-CoV-2 virus replication “in vitro”. J. Med. Virol. 2021, 93, 1780–1785. [Google Scholar] [CrossRef]
- Amin, S.A.; Jha, T. Fight against novel coronavirus: A perspective of medicinal chemists. Eur. J. Med. Chem. 2020, 201, 112559. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, C.-H.; Wang, C.-Y.; Chen, K.-H.; Wang, Y.-H.; Hsueh, P.-R. Clinical efficacy and safety of remdesivir in patients with COVID-19: A systematic review and network meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2021, 76, 1962–1968. [Google Scholar] [CrossRef]
- Reynard, O.; Nguyen, X.-N.; Alazard-Dany, N.; Barateau, V.; Cimarelli, A.; Volchkov, V.E. Identification of a new ribonucleoside inhibitor of ebola virus replication. Viruses 2015, 7, 6233–6240. [Google Scholar] [CrossRef]
- Toots, M.; Yoon, J.-J.; Hart, M.; Natchus, M.G.; Painter, G.R.; Plemper, R.K. Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model. Transl. Res. 2020, 218, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Urakova, N.; Kuznetsova, V.; Crossman, D.K.; Sokratian, A.; Guthrie, D.B.; Kolykhalov, A.A.; Lockwood, M.A.; Natchus, M.G.; Crowley, M.R.; Painter, G.R. β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 2018, 92, e01965-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, M.L.; Pruijssers, A.J.; Chappell, J.D.; Gribble, J.; Lu, X.; Andres, E.L.; Bluemling, G.R.; Lockwood, M.A.; Sheahan, T.P.; Sims, A.C. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019, 93, e01348-19. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Santiago, B.I.; Beltran, T.; Stuyver, L.; Chu, C.K.; Schinazi, R.F. Metabolism of the anti-hepatitis C virus nucleoside β-d-N4-hydroxycytidine in different liver cells. Antimicrob. Agents Chemother. 2004, 48, 4636–4642. [Google Scholar] [CrossRef] [Green Version]
- Painter, G.R.; Bowen, R.A.; Bluemling, G.R.; DeBergh, J.; Edpuganti, V.; Gruddanti, P.R.; Guthrie, D.B.; Hager, M.; Kuiper, D.L.; Lockwood, M.A. The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus infection. Antivir. Res. 2019, 171, 104597. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toots, M.; Yoon, J.-J.; Cox, R.M.; Hart, M.; Sticher, Z.M.; Makhsous, N.; Plesker, R.; Barrena, A.H.; Reddy, P.G.; Mitchell, D.G. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019, 11, eaax5866. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Stevens, L.J. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [Green Version]
- Wahl, A.; Gralinski, L.E.; Johnson, C.E.; Yao, W.; Kovarova, M.; Dinnon, K.H.; Liu, H.; Madden, V.J.; Krzystek, H.M.; De, C. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 2021, 591, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Wolf, J.D.; Plemper, R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021, 6, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; De Jonghe, S.; Maes, P.; Weynand, B.; Neyts, J. Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model. J. Infect. Dis. 2021, 224, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 100770. [Google Scholar] [CrossRef]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.C.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02428-20. [Google Scholar] [CrossRef]
- Khoo, S.H.; FitzGerald, R.; Fletcher, T.; Ewings, S.; Jaki, T.; Lyon, R.; Downs, N.; Walker, L.; Tansley-Hancock, O.; Greenhalf, W. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: A phase 1, dose-escalating, randomised controlled study. medRxiv 2021. [Google Scholar]
- Painter, W.; Sheahan, T.; Baric, R.; Holman, W.; Donovan, J.; Fang, L.; Alabanza, P.; Eron, J.; Goecker, E.; Coombs, R. Reduction in infectious SARS-COV-2 in treatment study of covid-19 with molnupiravir. Top. Antivir. Med. 2021, 304–305. [Google Scholar]
- Fischer, W.A.; Eron, J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.S.; Mollan, K.R.; Wolfe, C.R. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Griffiths, G.; Fitzgerald, R.; Jaki, T.; Corkhill, A.; Marwood, E.; Reynolds, H.; Stanton, L.; Ewings, S.; Condie, S.; Wrixon, E. AGILE-ACCORD: A randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: A structured summary of a study protocol for a randomised platform trial. Trials 2020, 21, 1–3. [Google Scholar]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/home. (accessed on 3 October 2021).
- Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed on 3 October 2021).
| Phase | Registered No. | Participants | Study Site | Study Design | Primary Aims | Principal Results | Reference |
|---|---|---|---|---|---|---|---|
| I | NCT04392219 | 64 healthy volunteers | United Kingdom | Randomized, double-blind, placebo-controlled, single-center | Effects of single or multiple doses and food intake on pharmacokinetics | Similar pharmacokinetics after the administration of single or multiple doses; limited effects of food intake on absorption | [29] |
| I | NCT04746183 | 18 adults within 5 days of COVID-19 symptom onset | United Kingdom | Dose-escalating, open-label, randomized-controlled, single-center | Safety and tolerability of multiple ascending doses to recommend a dose for the phase-II trial | Well tolerated at 400, 600, or 800 mg doses | [30] |
| II | NCT04405739 | 78 adults with onset of COVID-19 signs or symptoms within 7 days | United States | Double-blind, randomized, placebo-controlled, multicenter trial | Rates of viral clearance, by viral cultures in nasopharyngeal sites, as efficacies of molnupiravir | Compared to the placebo, the efficacies were significantly different on day 5, but not on day 3 after administration | [31] |
| II | NCT04405570 | 202 outpatients with onsets of COVID-19 symptoms within 7 days | United States | Double-blind, randomized, placebo-controlled, multicenter trial | Efficacies of molnupiravir based on the proportions of undetectable SARS-CoV-2 and alterations in the viral load in nasopharyngeal swabs detected using RT-PCR | Compared to the placebo, the effect appears significant on days 3 and 5 after administration | [32] |
| Registered No. | Participants | Study Site | Study Design * | Primary Outcome * | Reference |
|---|---|---|---|---|---|
| NCT04575597 | 1850 non-hospitalized adults with mild or moderate COVID-19 | The United States, Canada, Brazil, Mexico, Chile, Colombia, Japan, Taiwan, Philippines, Israel, Germany, France, Poland, Spain, Sweden, United Kingdom, Russian, Ukraine, South Africa (total 141 locations) | Double-blind, randomized-controlled, multicenter | 1. Time-to-sustained recovery (up to 29 days); 2. Percentage of participants experiencing adverse events (up to 7 months); 3. Percentage of withdrawal participants due to adverse events (up to 6 days). | [34] |
| NCT04575584 | 304 hospitalized adults with mild, moderate, or severe COVID-19 | The United States, Canada, Brazil, Mexico, Chile, Colombia, South Korea, Philippines, Israel, France, Poland, Spain, United Kingdom, Russian, Ukraine, South Africa (total 89 locations) | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Hsieh, C.-C.; Ko, W.-C. Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent. Antibiotics 2021, 10, 1294. https://doi.org/10.3390/antibiotics10111294
Lee C-C, Hsieh C-C, Ko W-C. Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent. Antibiotics. 2021; 10(11):1294. https://doi.org/10.3390/antibiotics10111294
Chicago/Turabian StyleLee, Ching-Chi, Chih-Chia Hsieh, and Wen-Chien Ko. 2021. "Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent" Antibiotics 10, no. 11: 1294. https://doi.org/10.3390/antibiotics10111294
APA StyleLee, C.-C., Hsieh, C.-C., & Ko, W.-C. (2021). Molnupiravir—A Novel Oral Anti-SARS-CoV-2 Agent. Antibiotics, 10(11), 1294. https://doi.org/10.3390/antibiotics10111294







