Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes
Abstract
1. Introduction
2. Material and Methods
2.1. Minimal Inhibitory Concentration (MIC) Determination
2.2. Fractional Inhibitory Concentration Indices (FICI) Determination
2.3. BODIPY™-TR-Cadaverine Displacement Assay
2.4. Ursolic Acid and Ampicillin Uptake in S. aureus ATCC 33591 and COL
2.5. S. aureus Membrane Permeabilization as Fluorescence of Propidium Iodide
2.6. S. aureus Membrane Depolarization as Fluorescence of DiSC3(5)
2.7. Large Unilamellar Vesicles (LUVs) Preparation
2.8. Membrane Fluidity as DPH Anisotropy Measurements
2.9. Lipid Phases as Laurdan Generalized Polarization Studies
2.10. Membrane Permeabilisation as Calcein Release Measurements
2.11. Membrane Fusion as R18 Dequenching Measurements
2.12. Liposome Size as Dynamic Light Scattering Measurements
2.13. Statistical Analysis
3. Results
3.1. MIC and Synergistic Effect of Ursolic or Oleanolic Acids with Ampicillin
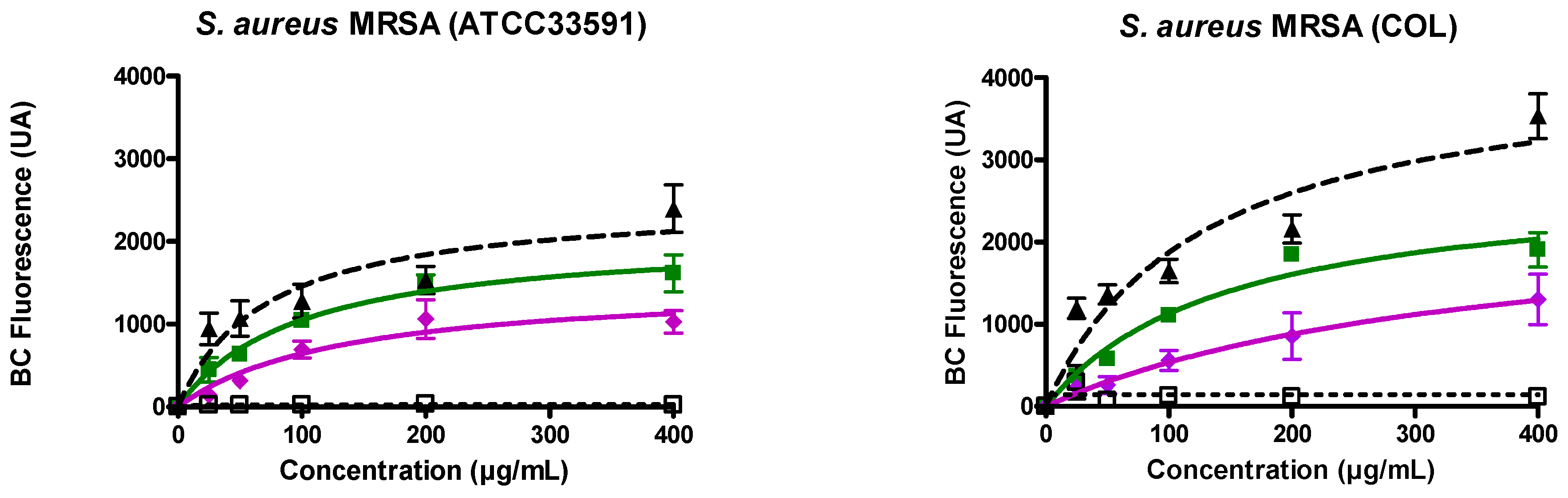
3.2. Ursolic and Oleanolic Acids Bound to Lipoteichoic Acid (LTA) of MRSA
3.3. Ursolic and Oleanolic Acids Induced MRSA Membrane Permeabilization and Depolarization
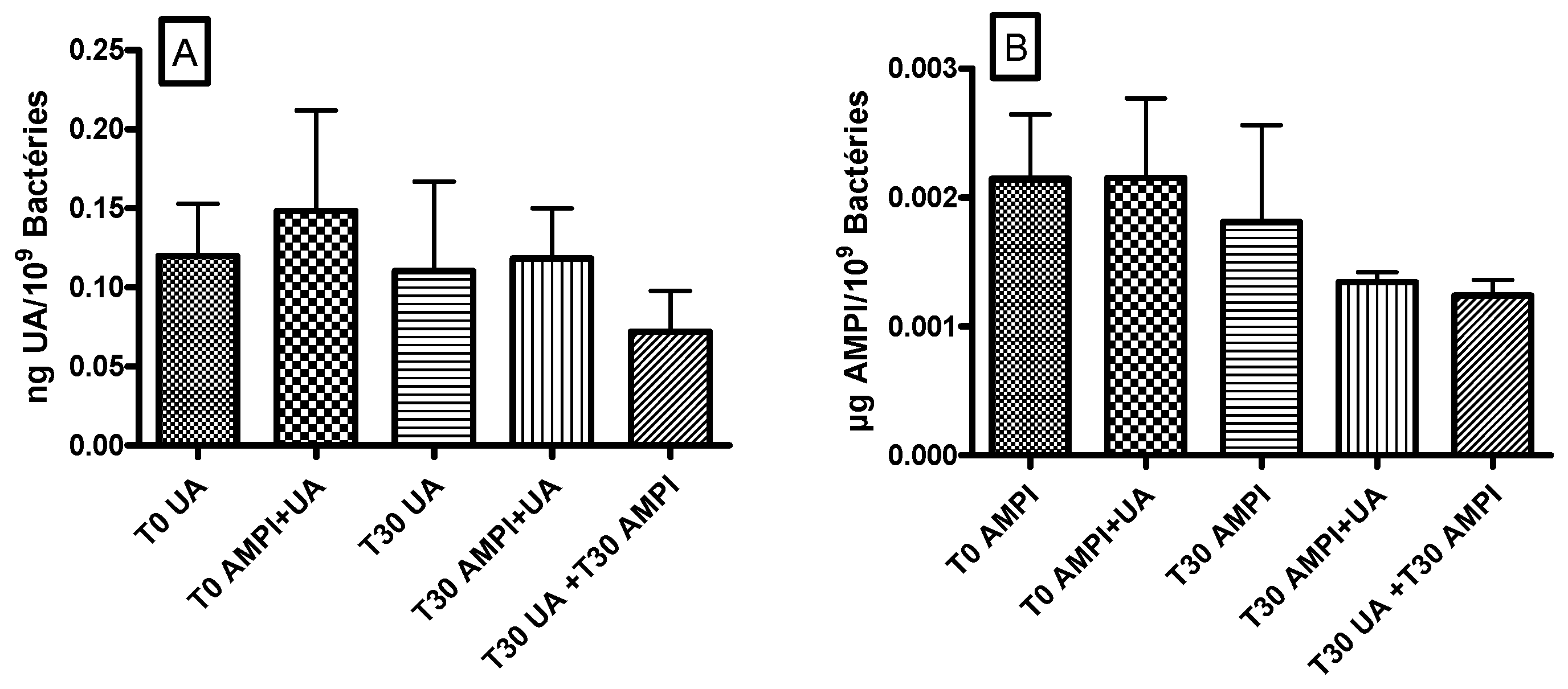
3.4. Ursolic Acid Did Not Accumulate in S. aureus and Did Not Increase the Accumulation of Ampicillin
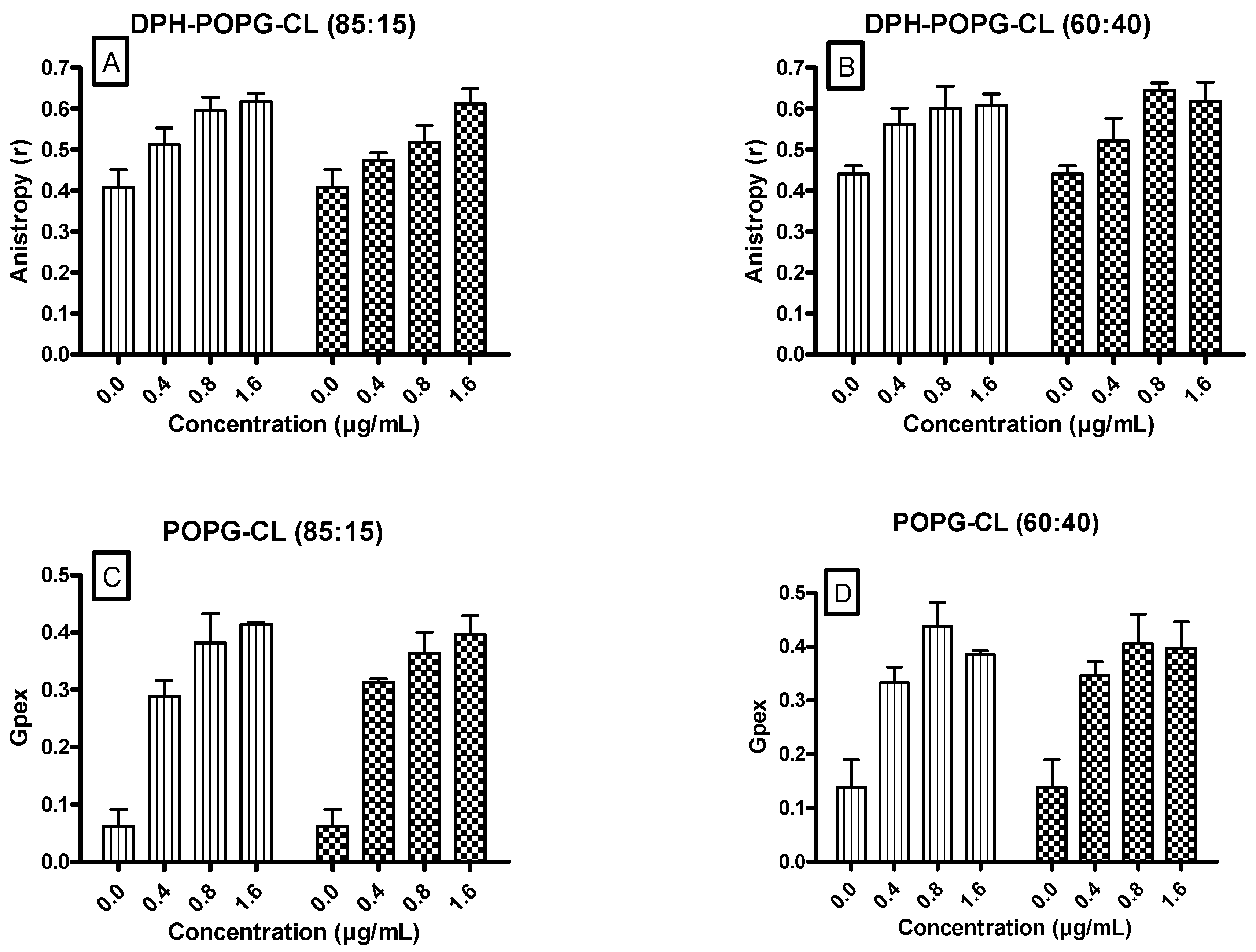
3.5. Ursolic and Oleanolic Acids Decreased the Fluidity of Lipid Membrane Models
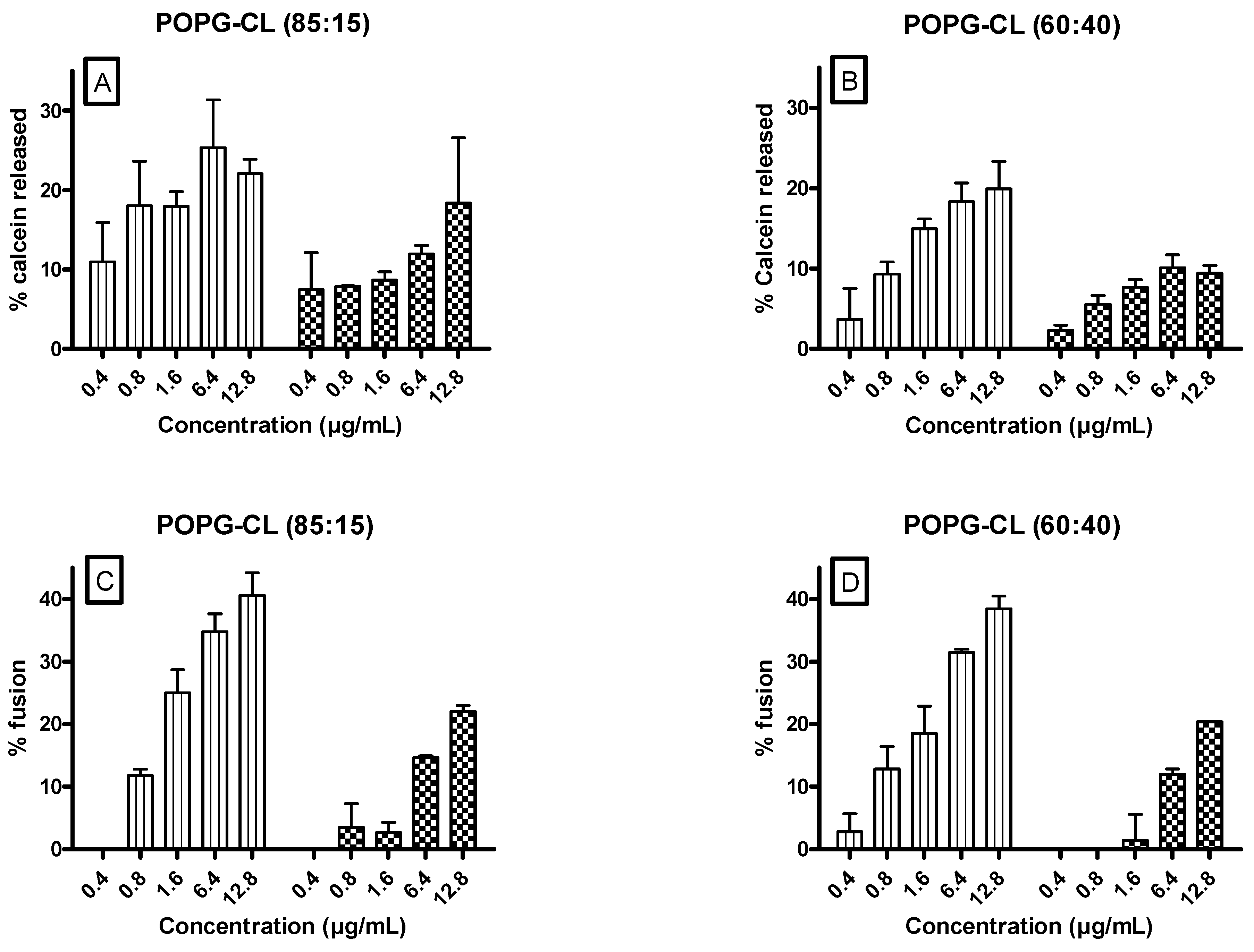
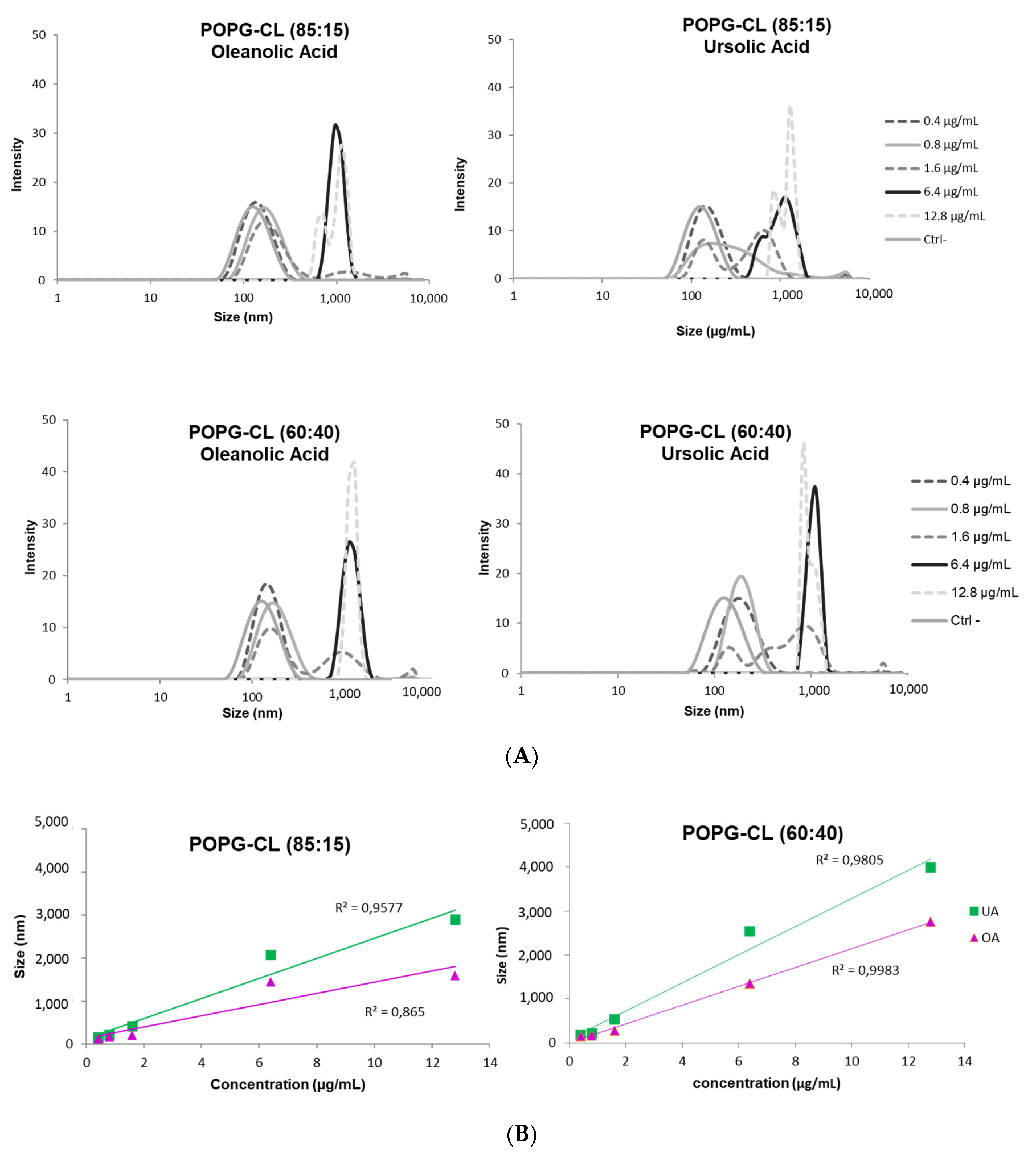
3.6. Ursolic and Oleanolic Acids Increased Permeability, Fusion and Size of LUVs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef]
- Qian, W.; Wang, W.; Zhang, J.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of ursolic acid against carbapenem-resistant Klebsiella pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, N.S.; Mohan, H.M.; Subramaniam, S.; Raman, T.; Selva, G.S.; Sivasubamanian, A.; Nagarajan, S. Ursolic acid inhibits colistin efflux and curtails colistin resistant Enterobacteriaceae. AMB. Express 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef]
- Catteau, L.; Reichmann, N.T.; Olson, J.; Pinho, M.G.; Nizet, V.; Van, B.F.; Quetin-Leclercq, J. Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and beta-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules 2017, 22, 2245. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.; Roh, B.D.; Park, S.H.; Park, J.W. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor. Dent. Endod. 2013, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, S.; Castaneda-Sanchez, J.I.; Jimenez-Arellanes, A.; Dominguez-Lopez, L.; Castro-Mussot, M.E.; Hernandez-Sanchez, J.; Luna-Herrera, J. Macrophage Activation by Ursolic and Oleanolic Acids during Mycobacterial Infection. Molecules 2015, 20, 14348–14364. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, L.; Wolska, K.I.; Janiszowska, W.; Popowska, M. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie Leeuwenhoek 2010, 97, 61–68. [Google Scholar] [CrossRef]
- Martins, A.; Couto, I.; Aagaard, L.; Martins, M.; Viveiros, M.; Kristiansen, J.E.; Amaral, L. Prolonged exposure of methicillin-resistant Staphylococcus aureus (MRSA) COL strain to increasing concentrations of oxacillin results in a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 2007, 29, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Chen, H.T.; Wu, Z.Y.; Jhan, Y.L.; Shyu, C.L.; Chou, C.H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Galati, E.M. Norfloxacin and ursolic acid: In vitro association and postantibiotic effect against Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 193–197. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, A.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Ahn, S.J.; Kook, J.K. Oleanolic acid and ursolic acid inhibit peptidoglycan biosynthesis in Streptococcus mutans UA159. Braz. J. Microbiol. 2015, 46, 613–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashizume, H.; Sawa, R.; Harada, S.; Igarashi, M.; Adachi, H.; Nishimura, Y.; Nomoto, A. Tripropeptin C blocks the lipid cycle of cell wall biosynthesis by complex formation with undecaprenyl pyrophosphate. Antimicrob. Agents Chemother. 2011, 55, 3821–3828. [Google Scholar] [CrossRef]
- Han, S.K.; Ko, Y.I.; Park, S.J.; Jin, I.J.; Kim, Y.M. Oleanolic acid and ursolic acid stabilize liposomal membranes. Lipids 1997, 32, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.; Vogler, O.; Alemany, R.; Gomez-Florit, M.; Funari, S.S.; Ruiz-Gutierrez, V.; Barcelo, F. Plant pentacyclic triterpenic acids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2011, 1808, 752–760. [Google Scholar] [CrossRef]
- de Lencastre, H.; Wu, S.W.; Pinho, M.G.; Ludovice, A.M.; Filipe, S.; Gardete, S.; Sobral, R.; Gill, S.; Chung, M.; Tomasz, A. Antibiotic resistance as a stress response: Complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb. Drug Resist. 1999, 5, 163–175. [Google Scholar] [CrossRef]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; Deboy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef]
- Galletta, M.; Reekie, T.A.; Nagalingam, G.; Bottomley, A.L.; Harry, E.J.; Kassiou, M.; Triccas, J.A. Rapid Antibacterial Activity of Cannabichromenic Acid against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; El, K.M.; Flament, A.; Dezanet, C.; Briee, F.; Van Der Smissen, P.; Decout, J.L.; Mingeot-Leclercq, M.P. Antimicrobial activity of amphiphilic neamine derivatives: Understanding the mechanism of action on Gram-positive bacteria. Biochim. Biophys. Acta Biomembr. 2019, 1861, 182998. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gupta, R.C.; Dey, A.; Malik, T.; Pandey, D.K. Validation and quantification of major biomarkers in ‘Mahasudarshan Churna’—An ayurvedic polyherbal formulation through high-performance thin-layer chromatography. BMC. Complement Med. Ther. 2020, 20, 184. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Mishra, S. Simultaneous HPTLC analysis of ursolic acid, betulinic acid, stigmasterol and lupeol for the identification of four medicinal plants commonly available in the Indian market as Shankhpushpi. J. Chromatogr. Sci. 2015, 53, 816–823. [Google Scholar] [CrossRef]
- Wojciak-Kosior, M. Separation and determination of closely related triterpenic acids by high performance thin-layer chromatography after iodine derivatization. J. Pharm. Biomed. Anal. 2007, 45, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Jusko, W.J. Fluorometric analysis of ampicillin in biological fluids. J. Pharm. Sci. 1971, 60, 728–732. [Google Scholar] [CrossRef]
- Niven, G.W.; Mulholland, F. Cell membrane integrity and lysis in Lactococcus lactis: The detection of a population of permeable cells in post-logarithmic phase cultures. J. Appl. Microbiol. 1998, 84, 90–96. [Google Scholar] [CrossRef]
- Krasne, S. Interactions of voltage-sensing dyes with membranes. I. Steady-state permeability behaviors induced by cyanine dyes. Biophys. J. 1980, 30, 415–439. [Google Scholar] [CrossRef]
- Krasne, S. Interactions of voltage-sensing dyes with membranes. II. Spectrophotometric and electrical correlates of cyanine-dye adsorption to membranes. Biophys. J. 1980, 30, 441–462. [Google Scholar] [CrossRef]
- Smith, R.M.; Jarett, L. Partial characterization of mechanism of insulin accumulation in H35 hepatoma cell nuclei. Diabetes 1990, 39, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Van, B.F.; Mingeot-Leclercq, M.P.; Schanck, A.; Brasseur, R.; Tulkens, P.M. Alterations in membrane permeability induced by aminoglycoside antibiotics: Studies on liposomes and cultured cells. Eur. J. Pharmacol. 1993, 247, 155–168. [Google Scholar]
- Lelkes, P.I.; Friedmann, P. Stabilization of large multilamellar liposomes by human serum in vitro. Biochim. Biophys. Acta 1984, 775, 395–401. [Google Scholar] [CrossRef]
- BARTLETT, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Shinitzky, M.; Barenholz, Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta 1978, 515, 367–394. [Google Scholar] [CrossRef]
- Lentz, B.R. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem. Phys. Lipids 1993, 64, 99–116. [Google Scholar] [CrossRef]
- Kaiser, R.D.; London, E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: Comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1998, 37, 8180–8190. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Parasassi, T.; De, S.G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Chong, P.L.; Wong, P.T. Interactions of Laurdan with phosphatidylcholine liposomes: A high pressure FTIR study. Biochim. Biophys. Acta 1993, 1149, 260–266. [Google Scholar] [CrossRef]
- Parasassi, T.; De, S.G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Yoshikami, S.; Henkart, P.; Blumenthal, R.; Hagins, W.A. Liposome-cell interaction: Transfer and intracellular release of a trapped fluorescent marker. Science 1977, 195, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, D.; De Boer, T.; Klappe, K.; Wilschut, J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 1984, 23, 5675–5681. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Mishra, N.N.; Bayer, A.S. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1082–1085. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Tulkens, P.M.; Brasseur, R.; Mingeot-Leclercq, M.P. Aminoglycoside antibiotics induce aggregation but not fusion of negatively-charged liposomes. Eur. J. Pharmacol. 1995, 289, 321–333. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Mingeot-Leclercq, M.P.; Brasseur, R.; Tulkens, P.M.; Schanck, A. Aminoglycoside antibiotics prevent the formation of non-bilayer structures in negatively-charged membranes. Comparative studies using fusogenic (bis(beta-diethylaminoethylether)hexestrol) and aggregating (spermine) agents. Chem. Phys. Lipids 1996, 79, 123–135. [Google Scholar] [CrossRef]
- Park, S.N.; Lim, Y.K.; Choi, M.H.; Cho, E.; Bang, I.S.; Kim, J.M.; Ahn, S.J.; Kook, J.K. Antimicrobial Mechanism of Oleanolic and Ursolic Acids on Streptococcus mutans UA159. Curr. Microbiol. 2018, 75, 11–19. [Google Scholar] [CrossRef]
- Hamza, M.; Nadir, M.; Mehmood, N.; Farooq, A. In vitro effectiveness of triterpenoids and their synergistic effect with antibiotics against Staphylococcus aureus strains. Indian J. Pharmacol. 2016, 48, 710–714. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Z.; Kang, O.H.; Mun, S.H.; Seo, Y.S.; Kong, R.; Shin, D.W.; Liu, X.Q.; Kwon, D.Y. Antimicrobial activity and synergism of ursolic acid 3-O-alpha-L-arabinopyranoside with oxacillin against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, W.; Li, B.; Zheng, X.; Liu, X.; Cen, Y.; Li, J.; Pan, X.; Cao, H.; Zheng, J.; Zhou, H. Artesunate in combination with oxacillin protect sepsis model mice challenged with lethal live methicillin-resistant Staphylococcus aureus (MRSA) via its inhibition on proinflammatory cytokines release and enhancement on antibacterial activity of oxacillin. Int. Immunopharmacol. 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Anderico, L.; Vukomanovic, M.; Parra, A.; de Alvarez, C.L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An in Vitro and in Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.; Mihaly, J.; Nemeth, C.; Wacha, A.; Bota, A. Effects of ursolic acid on the structural and morphological behaviours of dipalmitoyl lecithin vesicles. Biochim. Biophys. Acta 2015, 1848, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Flasinski, M.; Zieba, K.; Miskowiec, P. Langmuir monolayer studies of the interaction of monoamphiphilic pentacyclic triterpenes with anionic mitochondrial and bacterial membrane phospholipids—Searching for the most active terpene. Biochim. Biophys. Acta 2014, 1838, 2460–2472. [Google Scholar] [CrossRef]
- Broniatowski, M.; Mastalerz, P.; Flasinski, M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli inner membrane-Langmuir monolayer approach. Biochim. Biophys. Acta 2015, 1848, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Sanchez, E.; Galiano, V.; Villalain, J. Location of the bioactive pentacyclic triterpene ursolic acid in the membrane. A molecular dynamics study. J. Biomol. Struct. Dyn. 2017, 35, 2688–2700. [Google Scholar] [CrossRef]
- Khin, M.; Knowles, S.L.; Crandall, W.J.; Jones, D.D., Jr.; Oberlies, N.H.; Cech, N.B.; Houriet, J. Capturing the antimicrobial profile of Rosmarinus officinalis against methicillin-resistant Staphylococcus aureus (MRSA) with bioassay-guided fractionation and bioinformatics. J. Pharm. Biomed. Anal. 2021, 197, 113965. [Google Scholar] [CrossRef]
- Broniatowski, M.; Flasinski, M.; Zieba, K.; Miskowiec, P. Interactions of pentacyclic triterpene acids with cardiolipins and related phosphatidylglycerols in model systems. Biochim. Biophys. Acta 2014, 1838, 2530–2538. [Google Scholar] [CrossRef]
- Broniatowski, M.; Flasinski, M.; Wydro, P.; Fontaine, P. Grazing incidence diffraction studies of the interactions between ursane-type antimicrobial triterpenes and bacterial anionic phospholipids. Colloids Surf. B Biointerfaces 2015, 128, 561–567. [Google Scholar] [CrossRef]
- Usmani, Y.; Ahmed, A.; Faizi, S.; Versiani, M.A.; Shamshad, S.; Khan, S.; Simjee, S.U. Antimicrobial and biofilm inhibiting potential of an amide derivative [N-(2’, 4’-dinitrophenyl)-3beta-hydroxyurs-12-en-28-carbonamide] of ursolic acid by modulating membrane potential and quorum sensing against colistin resistant Acinetobacter baumannii. Microb. Pathog. 2021, 157, 104997. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial properties of amine- and guanidine-functionalized derivatives of betulinic, ursolic and oleanolic acids: Synthesis and structure/activity evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef]
- Wu, P.; Tu, B.; Liang, J.; Guo, S.; Cao, N.; Chen, S.; Luo, Z.; Li, J.; Zheng, W.; Tang, X.; et al. Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg. Chem. 2021, 109, 104692. [Google Scholar] [CrossRef]
- Marrink, S.J.; Mark, A.E. Molecular view of hexagonal phase formation in phospholipid membranes. Biophys. J. 2004, 87, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Rehal, R.; Barker, R.D.; Lu, Z.; Bui, T.T.; Deme, B.; Hause, G.; Wolk, C.; Harvey, R.D. Lipid domain formation and non-lamellar structures associated with varied lysylphosphatidylglycerol analogue content in a model Staphylococcal plasma membrane. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183571. [Google Scholar] [CrossRef] [PubMed]
- Wolk, C.; Youssef, H.; Guttenberg, T.; Marbach, H.; Vizcay-Barrena, G.; Shen, C.; Brezesinski, G.; Harvey, R.D. Phase Diagram for a Lysyl-Phosphatidylglycerol Analogue in Biomimetic Mixed Monolayers with Phosphatidylglycerol: Insights into the Tunable Properties of Bacterial Membranes. Chemphyschem 2020, 21, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Wolk, C.; Giselbrecht, J.; Chan, K.L.A.; Harvey, R.D. A combined FTIR and DSC study on the bilayer-stabilising effect of electrostatic interactions in ion paired lipids. Colloids Surf. B Biointerfaces 2018, 169, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, A.J.; Leunissen-Bijvelt, J.; De Kruijff, B.; Hope, M.; Cullis, P.R. Non-bilayer structures in membrane fusion. Ciba Found. Symp. 1984, 103, 45–59. [Google Scholar] [PubMed]
- Meher, G.; Chakraborty, H. Membrane Composition Modulates Fusion by Altering Membrane Properties and Fusion Peptide Structure. J. Membr. Biol. 2019, 252, 261–272. [Google Scholar] [CrossRef]
- Garcia-Fernandez, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Suss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Savietto, A.; de Sousa, B.A.; Martinez, D.; Berbon, M.; Roelofsen, J.R.; Hartman, A.M.; de Boer, R.; Van der Klei, I.J.; Hirsch, A.K.; et al. Flotillin-mediated membrane fluidity controls peptidoglycan synthesis and MreB movement. Elife 2020, 9, e57179. [Google Scholar] [CrossRef]
- Tiwari, K.B.; Gatto, C.; Wilkinson, B.J. Interrelationships between Fatty Acid Composition, Staphyloxanthin Content, Fluidity, and Carbon Flow in the Staphylococcus aureus Membrane. Molecules 2018, 23, 1201. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Dong, P.T.; Liang, L.; Mandal, T.; Li, J.; Ulloa, E.R.; Zhan, Y.; Jusuf, S.; Zong, C.; Seleem, M.N.; et al. Photo-Disassembly of Membrane Microdomains Revives Conventional Antibiotics against MRSA. Adv. Sci. (Weinh.) 2020, 7, 1903117. [Google Scholar] [CrossRef] [PubMed]
- Nagendra Prasad, H.S.; Karthik, C.S.; Manukumar, H.M.; Mallesha, L.; Mallu, P. New approach to address antibiotic resistance: Miss loading of functional membrane microdomains (FMM) of methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2019, 127, 106–115. [Google Scholar] [CrossRef] [PubMed]

| S. aureus | MICs (mg/L) | FICI | |||
|---|---|---|---|---|---|
| UA | OA | AMP | AMP-UA | AMP-OA | |
| ATCC33591 | 16 | 32 | 64 | 0.38–1.00 | 0.31–1.00 |
| COL | 8 | 16 | 32 | 0.38–1.00 | 0.25–1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verstraeten, S.; Catteau, L.; Boukricha, L.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics 2021, 10, 1381. https://doi.org/10.3390/antibiotics10111381
Verstraeten S, Catteau L, Boukricha L, Quetin-Leclercq J, Mingeot-Leclercq M-P. Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics. 2021; 10(11):1381. https://doi.org/10.3390/antibiotics10111381
Chicago/Turabian StyleVerstraeten, Sandrine, Lucy Catteau, Laila Boukricha, Joelle Quetin-Leclercq, and Marie-Paule Mingeot-Leclercq. 2021. "Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes" Antibiotics 10, no. 11: 1381. https://doi.org/10.3390/antibiotics10111381
APA StyleVerstraeten, S., Catteau, L., Boukricha, L., Quetin-Leclercq, J., & Mingeot-Leclercq, M.-P. (2021). Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics, 10(11), 1381. https://doi.org/10.3390/antibiotics10111381






