How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Microbiological Tests
2.1.1. Minimum Inhibitory Concentration (MICs) and Minimum Bactericidal Concentration (MBCs)
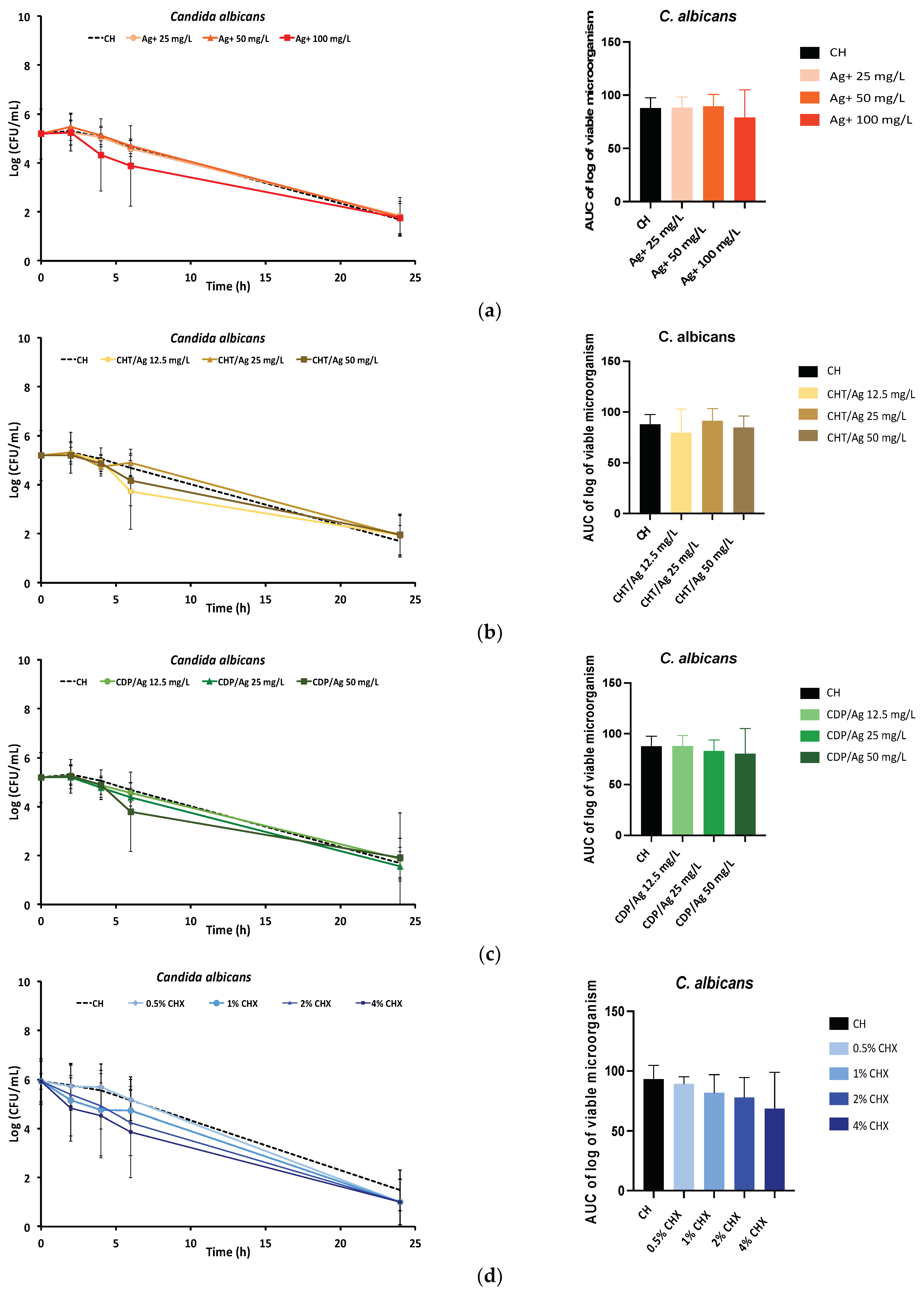
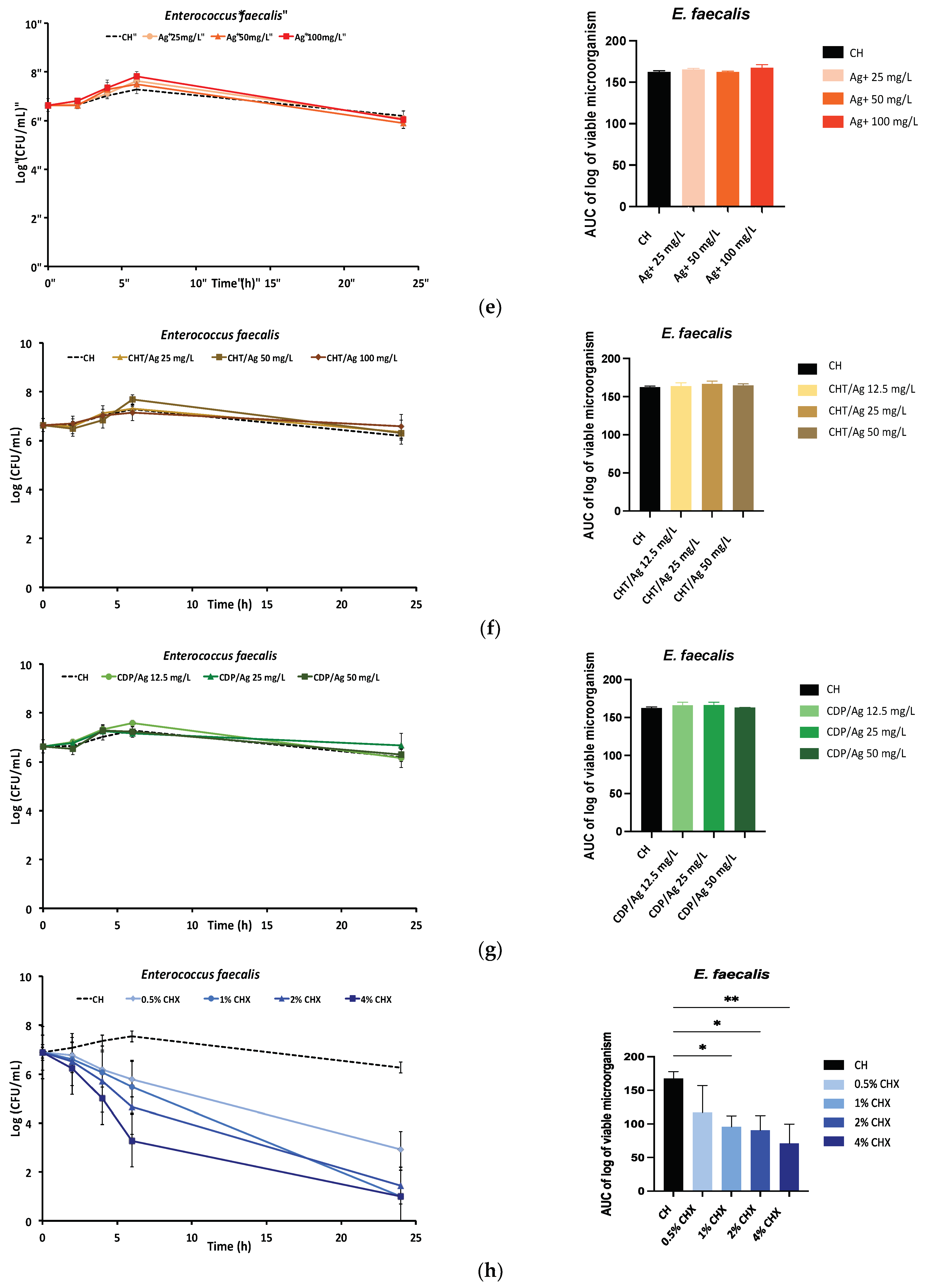
2.1.2. Time-Kill Kinetics Assay
2.1.3. Drug Release Measurements
2.2. Physical Properties
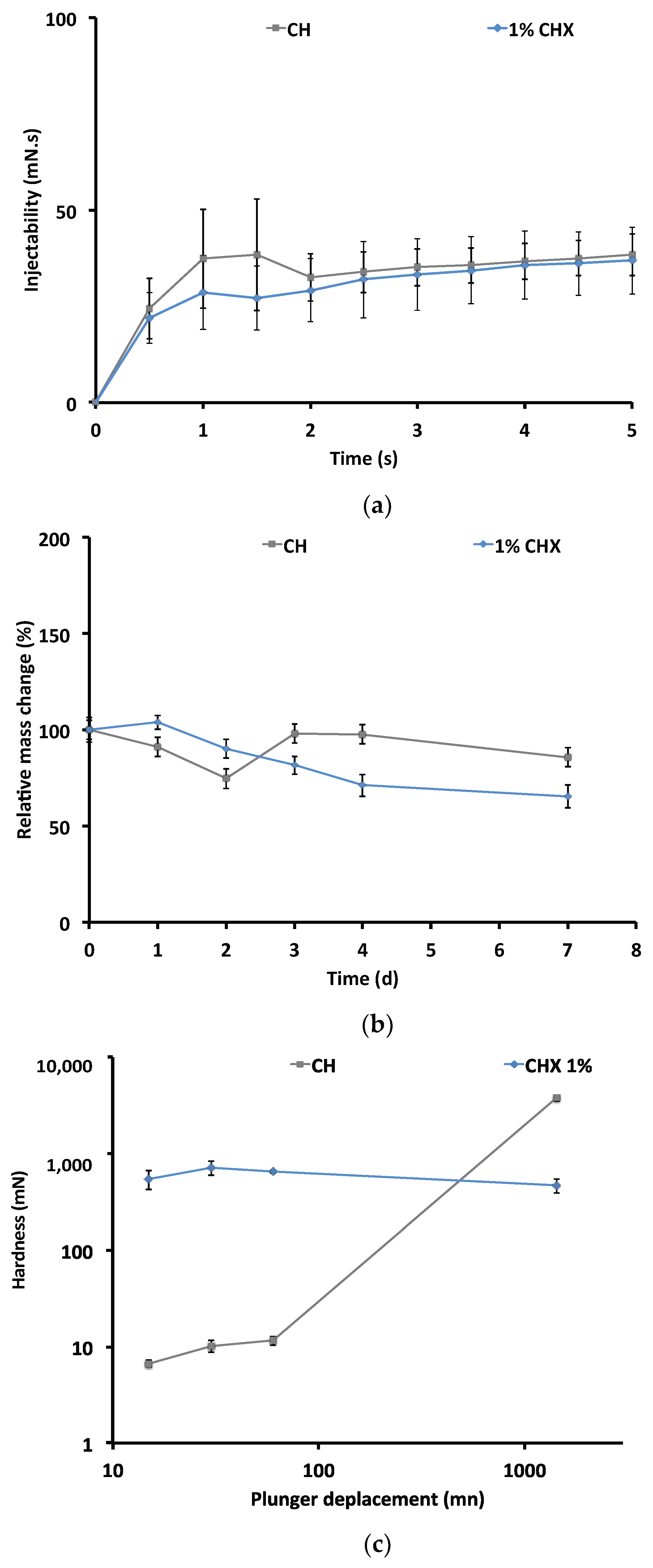
2.2.1. Injectability
2.2.2. Mass Change
2.2.3. Puncture Resistance Test
2.3. Rheological Properties
3. Discussion
4. Materials and Methods
4.1. Media, Molecules, and Formulations
4.1.1. Cultures and Media
4.1.2. Preparation of Antimicrobial Agents
4.1.3. Formulation of the Calcium Hydroxide Pastes
4.2. Antimicrobial Properties
4.2.1. Minimal Inhibitory Concentrations and Minimal Bactericidal Concentrations
4.2.2. Time-Kill Kinetics Assay
4.2.3. Drug Release Measurements
4.3. Physical Properties
4.3.1. Injectability
4.3.2. Monitoring Changes in Dynamic Mass
4.3.3. Penetration Resistance Test
4.4. Rheological Properties
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siqueira, J.F.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301. [Google Scholar] [CrossRef]
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233–239. [Google Scholar] [CrossRef]
- Nair, P.N.R. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G. Ecology of the root canal flora. J. Endod. 1992, 18, 427–430. [Google Scholar] [CrossRef]
- Abouelenien, S.S.; Ibrahim, S.M.; Shaker, O.G.; Ahmed, G.M. Evaluation of postoperative pain in infected root canals after using double antibiotic paste versus calcium hydroxide as intra-canal medication: A randomized controlled trial. F1000Research 2018, 7, 1768. [Google Scholar] [CrossRef]
- Arruda, M.E.F.; Neves, M.A.S.; Diogenes, A.; Mdala, I.; Guilherme, B.P.S.; Siqueira, J.F.; Rôças, I.N. Infection control in teeth with apical periodontitis using a triple antibiotic solution or calcium hydroxide with chlorhexidine: A Randomized Clinical Trial. J. Endod. 2018, 44, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Ghatole, K.; Gowdra, R.H.G.; Azher, S.; Sabharwal, S.; Singh, V.T.; Sundararajan, B.V. Enhancing the antibacterial activity of the gold standard intracanal medicament with incorporation of silver zeolite: An in vitro study. J. Int. Soc. Prev. Community Dent. 2016, 6, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef] [PubMed]
- Sukawat, C.; Srisuwan, T. A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J. Endod. 2002, 28, 102–104. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Tewari, S.; Tagg, J.; Chikindas, M.L.; Popov, I.V.; Tiwari, S.K. Can Probiotics Emerge as Effective Therapeutic Agents in Apical Periodontitis? A Review. Probiotics Antimicrob. Proteins 2021, 13, 299–314. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: A literature review–Part I. In vitro studies. Restor. Dent. Endod. 2014, 39, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B.; Tjäderhane, L.; Santos, J.M.; Pascon, E.; Grad, H.; Lawrence, H.P.; Friedman, S. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 618–624. [Google Scholar] [CrossRef]
- Punathil, S.; Moyin, S.; Bhat, S.S.; Hedge, S.; Pai, A.; James, J. Comparison of Antibacterial Effect of Calcium Hydroxide Combined with Chlorhexidine and Povidone-Iodine Against Enterococcus faecalis in Dentinal Tubules of Human Incisors: An In Vitro Comparative Study. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. S1), S448–S452. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Baumgartner, J.C.; Khemaleelakul, S.; Xia, T. Efficacy of calcium hydroxide: Chlorhexidine paste as an intracanal medication in bovine dentin. J. Endod. 2003, 29, 338–339. [Google Scholar] [CrossRef]
- Pereira, T.C.; Vasconcelos, L.R.D.S.M.; Graeff, M.S.Z.; Ribeiro, M.C.M.; Duarte, M.A.H.; de Andrade, F.B. Intratubular decontamination ability and physicochemical properties of calcium hydroxide pastes. Clin. Oral Investig. 2019, 23, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.D.A.; Vianna, M.E.; Sena, N.T.; Zaia, A.A.; Ferraz, C.C.R.; de Souza Filho, F.J. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 544–550. [Google Scholar]
- Ercan, E.; Dalli, M.; Dülgergil, C.T. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e27–e31. [Google Scholar] [CrossRef]
- Delgado, R.J.R.; Gasparoto, T.H.; Sipert, C.R.; Pinheiro, C.R.; Moraes, I.G.; Garcia, R.B.; Bramante, C.M.; Campanelli, A.P.; Bernardineli, N. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J. Endod. 2010, 36, 1389–1393. [Google Scholar] [CrossRef]
- Prabhakar, A.R.; Hadakar, S.G.; Raju, O.S. Comparative evaluation of pH and antibacterial effect of various calcium hydroxide combinations on E. faecalis and its effect on root strength: An in vitro study. Contemp. Clin. Dent. 2012, 3, 42–47. [Google Scholar] [CrossRef]
- Almyroudi, A.; Mackenzie, D.; McHugh, S.; Saunders, W.P. The effectiveness of various disinfectants used as endodontic intracanal medications: An in vitro study. J. Endod. 2002, 28, 163–167. [Google Scholar] [CrossRef]
- Schäfer, E.; Bössmann, K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J. Endod. 2005, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lynne, R.E.; Liewehr, F.R.; West, L.A.; Patton, W.R.; Buxton, T.B.; McPherson, J.C. In vitro antimicrobial activity of various medication preparations on E. faecalis in root canal dentin. J. Endod. 2003, 29, 187–190. [Google Scholar]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef]
- Charannya, S.; Duraivel, D.; Padminee, K.; Poorni, S.; Nishanthine, C.; Srinivasan, M.R. Comparative Evaluation of Antimicrobial Efficacy of Silver Nanoparticles and 2% Chlorhexidine Gluconate When Used Alone and in Combination Assessed Using Agar Diffusion Method: An In vitro Study. Contemp. Clin. Dent. 2018, 9 (Suppl. S2), S204–S209. [Google Scholar] [CrossRef]
- Ahrari, F.; Eslami, N.; Rajabi, O.; Ghazvini, K.; Barati, S. The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sanguis to colloidal solutions of different nanoparticles applied as mouthwashes. Dent. Res. J. 2015, 12, 44–49. [Google Scholar]
- Yousefshahi, H.; Aminsobhani, M.; Shokri, M.; Shahbazi, R. Anti-bacterial properties of calcium hydroxide in combination with silver, copper, zinc oxide or magnesium oxide. Eur. J. Transl. Myol. 2018, 28, 7545. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Vivani, R.; Donnadio, A.; Vischini, E.; Russo, A.; Visai, L.; Arciola, C.R.; Montanaro, L.; Nocchetti, M. Antibacterial Properties of a Novel Zirconium Phosphate-Glycinediphosphonate Loaded with Either Zinc or Silver. Materials 2019, 12, 3184. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Torab, A.; Hosseini, O.; Abbasi, T.; Abdollahi, A.A.; Divband, B. Antibacterial Effect of Two Nano Zinc Oxide Gel Preparations Compared to Calcium Hydroxide and Chlorhexidine Mixture. Iran. Endod. J. 2018, 13, 305–311. [Google Scholar] [PubMed]
- Nunes, B.S.; Rosendo, R.A.; Filho, A.A.O.; Fook, M.V.L.; de Sousa, W.J.B.; Barbosa, R.C.; Pina, H.N.; da Silva Neto, J.E.; Amoah, S.K.S.; Fontana, S.E.; et al. Chitosan-Based Biomaterial, Calcium Hydroxide and Chlorhexidine for Potential Use as Intracanal Medication. Materials 2021, 14, 488. [Google Scholar] [CrossRef]
- Revathi, T.; Thambidurai, S. Cytotoxic, antioxidant and antibacterial activities of copper oxide incorporated chitosan-neem seed biocomposites. Int. J. Biol. Macromol. 2019, 139, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Mogrovejo-Valdivia, A.; Rahmouni, O.; Tabary, N.; Maton, M.; Neut, C.; Martel, B.; Blanchemain, N. In vitro evaluation of drug release and antibacterial activity of a silver-loaded wound dressing coated with a multilayer system. Int. J. Pharm. 2019, 556, 301–310. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Song, J.; Zhang, S.; Wang, M.; Guo, Y.; Dong, C. Colorimetric detection of riboflavin by silver nanoparticles capped with β-cyclodextrin-grafted citrate. Colloids Surf. B Biointerfaces 2016, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Carapeto, A.P.; Ferraria, A.M.; do Rego, A.M.B. Unraveling the reaction mechanism of silver ions reduction by chitosan from so far neglected spectroscopic features. Carbohydr. Polym. 2017, 174, 601–609. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Blanchemain, N.; Karrout, Y.; Tabary, N.; Bria, M.; Neut, C.; Hildebrand, H.F.; Siepmann, J.; Martel, B. Comparative study of vascular prostheses coated with polycyclodextrins for controlled ciprofloxacin release. Carbohydr. Polym. 2012, 90, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Lopez, M.; Tabary, N.; Neut, C.; Chai, F.; Betbeder, D.; Herkt, C.; Cazaux, F.; Gaucher, V.; Martel, B.; et al. Preparation and characterization of novel chitosan and β-cyclodextrin polymer sponges for wound dressing applications. Carbohydr. Polym. 2017, 173, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Degoutin, S.; Tabary, N.; Cazaux, F.; Maton, M.; Gaucher, V.; Janus, L.; Neut, C.; Chai, F.; Martel, B.; et al. Triclosan loaded electrospun nanofibers based on a cyclodextrin polymer and chitosan polyelectrolyte complex. Int. J. Pharm. 2016, 513, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, M.J.; Maton, M.; Benzine, Y.; Tabary, N.; Baptiste, E.J.; Gargouri, M.; Bria, M.; Blanchemain, N.; Karoutt, Y. Ciprofloxacin loaded vascular prostheses functionalized with poly-methylbeta- cyclodextrin: The importance of in vitro release conditions. J. Drug Deliv. Sci. Technol. 2019, 53, 101166. [Google Scholar] [CrossRef]
- Vermet, G.; Degoutin, S.; Chai, F.; Maton, M.; Flores, C.; Neut, C.; Danjou, P.E.; Blanchemain, N.; Martel, B. Cyclodextrin modified PLLA parietal reinforcement implant with prolonged antibacterial activity. Acta Biomater. 2017, 53, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Vermet, G.; Degoutin, S.; Chai, F.; Maton, M.; Bria, M.; Danel, C.; Hildebrand, H.F.; Blanchemain, N.; Martel, B. Visceral mesh modified with cyclodextrin for the local sustained delivery of ropivacaine. Int. J. Pharm. 2014, 476, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Tomson, R.M.E.; Polycarpou, N.; Tomson, P.L. Contemporary obturation of the root canal system. Br. Dent. J. 2014, 216, 315–322. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Kim, D.; Nguyen, M.; Hsiao, G.; Mito, R.; Kang, M.K.; Chugal, N.; Chi, W. The Antimicrobial Effect of Silver Ion Impregnation into Endodontic Sealer against Streptococcus mutans. Open Dent. J. 2008, 2, 18–23. [Google Scholar]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Duran, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Pikos, M.A.; Sculean, A.; Miron, R.J. Cytotoxicity and Gene Expression Changes of a Novel Homeopathic Antiseptic Oral Rinse in Comparison to Chlorhexidine in Gingival Fibroblasts. Materials 2020, 13, E3190. [Google Scholar] [CrossRef]
- Vestergaard, M.; Skive, B.; Domraceva, I.; Ingmer, H.; Franzyk, H. Peptide/β-Peptoid Hybrids with Activity against Vancomycin-Resistant Enterococci: Influence of Hydrophobicity and Structural Features on Antibacterial and Hemolytic Properties. Int. J. Mol. Sci. 2021, 22, 5617. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, H.; Wang, Y.; Nie, B.; Yang, H.; Yuan, W.; Qu, X.; Yue, B. Antibacterial and antibiofilm effects of flufenamic acid against methicillin-resistant Staphylococcus aureus. Pharmacol. Res. 2020, 160, 105067. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Farreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against Gram-positive and Gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Aghatabay, N.M.; Neshat, A.; Karabiyik, T.; Somer, M.; Haciu, D.; Dülger, B. Synthesis, characterization and antimicrobial activity of Fe(II), Zn(II), Cd(II) and Hg(II) complexes with 2,6-bis(benzimidazol-2-yl) pyridine ligand. Eur. J. Med. Chem. 2007, 42, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Apip, C.; Meléndrez, M.F.; Domínguez, M.; Sánchez-Sanhueza, G.; Marzialetti, T.; Catalán, A. Dual antifungal activity against Candida albicans of copper metallic nanostructures and hierarchical copper oxide marigold-like nanostructures grown in situ in the culture medium. J. Appl. Microbiol. 2021, 130, 1883–1892. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef]
- Nozari, A.; Karimkhani, A.; Motamedifar, M.; Arasteh, P. The antimicrobial effects of zinc oxide-calcium hydroxide mixture fillers: Determining the ideal mixture ratio. Iran. J. Microbiol. 2019, 11, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Zain, N.M. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Cummins, D. The effects of triclosan and zinc citrate, alone and in combination, on a community of oral bacteria grown in vitro. J. Dent. Res. 1993, 72, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sun, Q.; Duan, M.; Liu, D.; Fan, W. Establishment and characterization of silver-resistant Enterococcus faecalis. Folia Microbiol. 2020, 65, 721–733. [Google Scholar] [CrossRef]
- Kulatunga, D.; Dananjaya, S.; Godahewa, G.I.; Lee, J.; De Zoysa, M. Chitosan silver nanocomposite (CAgNC) as an antifungal agent against Candida albicans. Med. Mycol. 2017, 55, 213–222. [Google Scholar] [CrossRef]
- Freire, P.L.L.; Albuquerque, A.J.R.; Farias, I.A.P.; da Silva, T.G.; Aguiar, J.S.; Galembeck, A.; Flores, M.A.P.; Sampaio, F.C.; Stamford, T.C.M.; Rosenblatt, A. Antimicrobial and cytotoxicity evaluation of colloidal chitosan–silver nanoparticles–fluoride nanocomposites. Int. J. Biol. Macromol. 2016, 93, 896–903. [Google Scholar] [CrossRef]
- Sy, K.; Flamme, J.; Maquet, H.; Chai, F.; Neut, C.; Siepmann, F.; Agossa, K. Antimicrobial effect and physical properties of an injectable ‘active oxygen’ gel for the treatment of periodontitis. Am. J. Dent. 2020, 33, 305–309. [Google Scholar]
- Saatchi, M.; Shokraneh, A.; Navaei, H.; Maracy, M.R.; Shojaei, H. Antibacterial effect of calcium hydroxide combined with chlorhexidine on Enterococcus faecalis: A systematic review and meta-analysis. J. Appl. Oral Sci. 2014, 22, 356–365. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Ingle, J.I.; Bakland, L.K.; Baumgartner, J.C. Endodontics, 6th ed.; BC Decker: Bloomington, IN, USA, 2008; p. 996. [Google Scholar]
- Siqueira, J.F.; de Uzeda, M. Intracanal medicaments: Evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J. Endod. 1997, 23, 167–169. [Google Scholar] [CrossRef]
- Tülü, G.; Kaya, B.Ü.; Çetin, E.S.; Köle, M. Antibacterial effect of silver nanoparticles mixed with calcium hydroxide or chlorhexidine on multispecies biofilms. Odontology 2021, 109, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, A.; Hervella, P.; Leroux, J.-C.; Pratsinis, S.E.; Sotiriou, G.A. Toxicity of silver nanoparticles in macrophages. Small 2013, 9, 2576–2584. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta 2015, 1850, 299–306. [Google Scholar] [CrossRef]
- Waltimo, T.M.; Sirén, E.K.; Orstavik, D.; Haapasalo, M.P. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int. Endod. J. 1999, 32, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, P.H.; Carnietto, C.; Weckwerth, A.C.V.B.; Duarte, M.A.H.; Kuga, M.C.; Vivan, R.R. In vitro susceptibility of oral Candida albicans strains to different pH levels and calcium hydroxide saturated aqueous solution. Braz. Dent. J. 2012, 23, 192–198. [Google Scholar] [CrossRef]
- Sen, B.H.; Safavi, K.E.; Spångberg, L.S. Growth patterns of Candida albicans in relation to radicular dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 68–73. [Google Scholar] [CrossRef]
- Haapasalo, H.K.; Sirén, E.K.; Waltimo, T.M.; Ørstavik, D.; Haapasalo, M.P. Inactivation of local root canal medicaments by dentine: An in vitro study. Int. Endod. J. 2000, 33, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, J.L. Grossman’s Endodontic Practice–13th Edition. J. Conserv. Dent. 2016, 19, 494. [Google Scholar] [CrossRef]
- Calt, S.; Serper, A. Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J. Endod. 1999, 25, 431–433. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, Y.-K.; Zhu, Q.; Shon, W.J.; Lee, W.C.; Kum, K.Y.; Baek, S.H.; Lee, I.B.; Lim, B.-S.; Bae, K.S. Comparison of the rheological properties of four root canal sealers. Int. J. Oral Sci. 2015, 7, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bauza, G.A.; Rached-Junior, F.J.A.; Souza-Gabriel, A.E.; Sousa-Neto, M.D.; Miranda, C.E.S.; Silva-Sousa, Y.T.C. Physicochemical properties of methacrylate resin-based root canal sealers. J. Endod. 2010, 36, 1531–1536. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Favieri, A.; Gahyva, S.M.; Moraes, S.R.; Lima, K.C.; Lopes, H.P. Antimicrobial activity and flow rate of newer and established root canal sealers. J. Endod. 2000, 26, 274–277. [Google Scholar] [CrossRef]
- De Freitas, J.V.; Ebert, J.; Mazzi-Chaves, J.F.; de Sousa-Neto, M.D.; Lohbauer, U.; Baratto-Filho, F. Do Contaminating Substances Influence the Rheological Properties of Root Canal Sealers? J. Endod. 2020, 46, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.E.; Ormaechea, M.F.; Picca, M.; Canzobre, M.C.; Ubios, A.M. Rheological properties and biocompatibility of endodontic sealers. Int. Endod. J. 2003, 36, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Agossa, K.; Lizambard, M.; Rongthong, T.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F. Physical key properties of antibiotic-free, PLGA/HPMC-based in-situ forming implants for local periodontitis treatment. Int. J. Pharm. 2017, 521, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Lin, C.; Zhang, Q.; Gong, L.; Wang, Y.; Zhang, X. Antibacterial Properties of Small-Size Peptide Derived from Penetratin against Oral Streptococci. Materials 2021, 14, 2730. [Google Scholar] [CrossRef]
- Abedini, A.; Roumy, V.; Mahieux, S.; Gohari, A.; Farimani, M.M.; Rivière, C.; Samaillie, J.; Sahpaz, S.; Bailleul, F.; Neut, C.; et al. Antimicrobial activity of selected Iranian medicinal plants against a broad spectrum of pathogenic and drug multiresistant micro-organisms. Lett. Appl. Microbiol. 2014, 59, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Lizambard, M.; Menu, T.; Fossart, M.; Bassand, C.; Agossa, K.; Huck, O.; Neut, C.; Siepmann, F. In-situ forming implants for the treatment of periodontal diseases: Simultaneous controlled release of an antiseptic and an anti-inflammatory drug. Int. J. Pharm. 2019, 572, 118833. [Google Scholar] [CrossRef]
- Winkler, B.; Margerison, J.K. Mechanical properties of the bovine claw horn during lactation. J. Dairy Sci. 2012, 95, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Durand, C.; Lopez, M.; Cazaux, F.; Martel, B.; Blanchemain, N.; Chai, F. Influence of the Soluble−Insoluble Ratios of Cyclodextrins Polymers on the Viscoelastic Properties of Injectable Chitosan−Based Hydrogels for Biomedical Application. Polymers 2019, 11, 214. [Google Scholar] [CrossRef]


| Antimicrobials Agents | Enterococcus faecalis MIC/MBC (mg/L) | Candida albicans MIC/MBC (mg/L) |
|---|---|---|
| Cu2+ | >3200 | >3200 |
| CHT/Cu | >3200 | >3200 |
| CDP/Cu | >3200 | >3200 |
| Zn2+ | 3200 | 3200 |
| CHT/Zn | 3200/>3200 | 3200/>3200 |
| CDP/Zn | 3200 | 3200 |
| Ag+ | 50/>200 | 50 |
| CHT/Ag | 50/>100 | 25/50 |
| CDP/Ag | 25/>100 | 25/50 |
| CHX | 2/16 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sy, K.; Agossa, K.; Maton, M.; Chijcheapaza-Flores, H.; Martel, B.; Siepmann, F.; Deveaux, E.; Blanchemain, N.; Neut, C. How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study. Antibiotics 2021, 10, 1352. https://doi.org/10.3390/antibiotics10111352
Sy K, Agossa K, Maton M, Chijcheapaza-Flores H, Martel B, Siepmann F, Deveaux E, Blanchemain N, Neut C. How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study. Antibiotics. 2021; 10(11):1352. https://doi.org/10.3390/antibiotics10111352
Chicago/Turabian StyleSy, Kadiatou, Kevimy Agossa, Mickaël Maton, Henry Chijcheapaza-Flores, Bernard Martel, Florence Siepmann, Etienne Deveaux, Nicolas Blanchemain, and Christel Neut. 2021. "How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study" Antibiotics 10, no. 11: 1352. https://doi.org/10.3390/antibiotics10111352
APA StyleSy, K., Agossa, K., Maton, M., Chijcheapaza-Flores, H., Martel, B., Siepmann, F., Deveaux, E., Blanchemain, N., & Neut, C. (2021). How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study. Antibiotics, 10(11), 1352. https://doi.org/10.3390/antibiotics10111352








