Abstract
Escherichia coli plays an important role in biofilm formation across a wide array of disease and ecological settings. Insulin can function as an adjuvant in the regulation of biofilm levels. The modulation of insulin-regulated biofilm formation by environmental conditions has not been previously described. In the present study, the effects that various environmental growth conditions and nutrients have on insulin-modulated levels of biofilm production were measured. Micropipette tips were incubated with E. coli ATCC® 25922™ in a Mueller Hinton broth (MH), or a yeast nitrogen base with 1% peptone (YNBP), which was supplemented with glucose, lactose, galactose and/or insulin (Humulin®-R). The incubation conditions included a shaking or static culture, at 23 °C or 37 °C. After incubation, the biofilm production was calculated per CFU. At 23 °C, the presence of insulin increased biofilm formation. The amount of biofilm formation was highest in glucose > galactose >> lactose, while the biofilm levels decreased in shaking cultures, except for galactose (3-fold increase; 0.1% galactose and 20 μU insulin). At 37 °C, regardless of condition, there was more biofilm formation/CFU under static conditions in YNBP than in MH, except for the MH containing galactose. E. coli biofilm formation is influenced by aeration, temperature, and insulin concentration in combination with the available sugars.
1. Introduction
Biofilms are comprised of cell aggregates and extracellular polymer material that play an essential role in nearly half of all infectious processes [,]. Biofilm-associated microbes exhibit decreased sensitivity to antimicrobials, as compared to planktonic cells. This effect of biofilm on susceptibility is further exacerbated by variations in antibiotic concentration throughout a biofilm, resulting in microbial cells being exposed to sub-inhibitory drug levels due to their drug diffusion properties [,,,,]. An additional factor that can contribute to the phenotypic antimicrobial resistance of bacteria in a biofilm is the physiologic status of the organisms. The biofilm formation phenotype is modulated in response to a variety of environmental signals, including temperature, available carbohydrates, and aeration levels [,]. In addition, quorum signaling compounds regulate the processing of the environmental signals that regulate the displayed phenotype [,].
Insulin, a polypeptide quorum signaling chemical that is synthesized by vertebrates, fungi, members of Protista, and bacteria, has a broad host specificity. E. coli-derived insulin induces responses in mammalian cells and tissues analogous to that of human insulin [,,,,,,,]. Human-derived insulin functions as a quorum chemical signaling molecule that regulates E. coli biofilm formation on uroepithelial cells, catheter material, plastic, and glass [,,,]. Human insulin affects E. coli biofilm formation and exhibits global rheostat-like regulation of all stages, including motility, adherence, cell-population growth, as well as biofilm building and maturation [,,]. The changes in cell wall architecture relate to alterations that have been demonstrated to result in enhanced virulence. Similar to observations of insulin’s interaction with mammalian cells, the changes appear to correspond with the uptake of glucose and the binding of insulin to the cell []. However, the effect of insulin is bi-functional. In conditions where insulin is in the presence of glucose, the virulence factors associated with colonization are up-regulated. In the absence of glucose, insulin appears to act as a warning signal, supporting the planktonic state by functioning as a chemo-repellant, which results in movement away from surfaces. However, in the presence of glucose, chemoattraction is enhanced by the promotion of the initial stage in the establishment of a biofilm, i.e., surface adherence. Furthermore, while insulin alone under the host’s physiologic conditions has no effect on the level of biofilm formation or cell growth, both are significantly enhanced in the presence of glucose. These findings were measured both for E. coli ATCC®25922™, a standard control strain, and clinical uropathogens [,,]. In addition, the cell surface remodeling, e.g., hydrophobicity and capsule production, occurred so that it corresponds with alterations in biofilm levels. These findings indicate that insulin with glucose, as would be present in the urine of individuals with type 2 diabetes, can trigger the expression of biofilm formation and thus increase the risk of urinary tract infections [].
E. coli plays a role in biofilm-associated disease production, as well as biofouling, in both industrial and agricultural settings, which can serve as reservoirs for antimicrobial-resistant strains []. Thus, the understanding of how insulin modulates the behavior of biofilm formation across these diverse environmental conditions is important and could be exploited to reduce biofilm formation, since insulin appears to have a bifunctionality, depending on the presence of sugar. The focus of this study is to quantify the effect that physiologically relevant concentrations of insulin have on biofilm formation, and the effect that this has on growth in nutrient-rich, complex medium vs. nutrient-minimal medium, under various environmental conditions, in response to the presence of sugars present in vivo through metabolism and nutritional exposure, i.e., glucose, galactose, and lactose.
2. Results
2.1. Insulin Effects on Biofilm Formation
Biofilm formation in the presence of insulin alone (human physiologic concentrations; 20 µU and 200 µU), was similar to the levels measured for the growth in the media-alone homologous control, as had been previously reported (Figure 1) [,,,]. However, in YNBP, a minimal nutrient medium, biofilm levels were significantly (p < 0.05) increased (>5-fold), compared to the levels of growth measured in MH, a complex nutrient-rich medium. This enhanced biofilm response of E. coli to a minimal-nutrient growth medium has been previously reported and is presumably due to an increase in the global gene expression that occurs after growth in a minimal medium [,,,,]. In addition, the levels of biofilm formation after growth in YNBP (23 °C; Figure 1B) were similar, regardless of the culture’s conditions (shaking vs. static). However, significantly higher levels (37 °C; p < 0.05; Figure 1D) were produced under shaking growth conditions. Interestingly, the reverse pattern of biofilm formation was measured after growth in MH medium (Figure 1A,C), although the level of biofilm produced per cell was significantly reduced when compared to the YNBP grown cells, regardless of environmental growth condition.
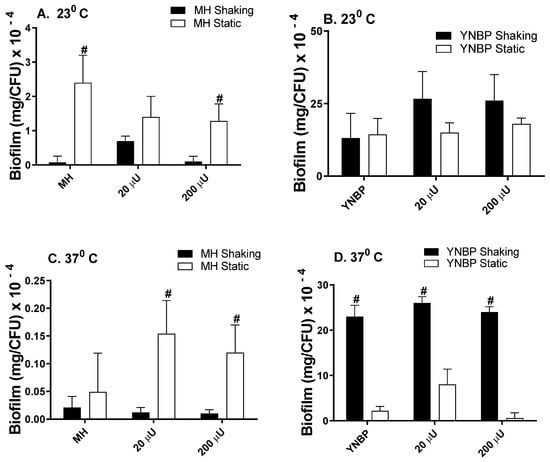
Figure 1.
Effect of Humulin® insulin (20 µU and 200 µU) on biofilm formation after growth (24 h) in a Mueller Hinton broth (Panels (A,C)) and yeast nitrogen base with 1% peptone (Panels (B,D)) at ambient (23 °C; Panels (A,B)) and human body temperature (37 °C; Panels (C,D)) in shaking (dark bars) and static (light bars) cultures. Results were expressed as total amount of biofilm (mg) per CFU. Results are the Mean ± SEM; # indicates significant difference (p < 0.05) between homologous media static and shaking culture, with or without insulin.
2.2. Insulin Modulation of Glucose Effects on Biofilm Formation
In the presence of insulin, glucose enhanced biofilm formation compared to insulin or glucose alone, regardless of incubation temperature or aeration conditions (Figure 2A–D). As observed in the biofilm formation response to insulin alone, biofilm levels were highest in response to insulin and glucose when grown in a YNBP vs. an MH medium. The glucose-insulin mediated biofilm formation level was highest for the YNBP (23 °C) with 0.1% glucose and 20 µU/mL of insulin (5280 mg/CFU × 10−4; Figure 2B). This biofilm level was 220-fold higher than that measured for the level of biofilm formation incubated under homologous conditions at 37 °C (Figure 2A), and 140-fold higher than that in a YNBP with 20 µU/mL insulin alone (Figure 1B and Figure 2B). After incubation at 37 °C, regardless of glucose or insulin concentration, biofilm levels/CFU were higher for static incubation. Interestingly, as observed for insulin alone, incubation at 37 °C in both an MH and a YNBP media was the least permissive for biofilm formation. Previous studies have demonstrated that, under physiologic conditions, insulin increased the transport of glucose compared to glucose alone []. This may be of particular importance with regards to populations at an increased risk of urinary tract infections, including diabetics, individuals with gestational diabetes, and those with severe trauma, all of whom exhibit an increased secretion of insulin in the urine [,,,,]. The differential enhancement of biofilm formation at room temperature (23 °C) may also play a role in E. coli contamination of external catheters and urine collection bags. In addition, colonization of surface wound infections, particularly those caused by extended-spectrum antimicrobial-resistant E. coli, may benefit from modulation of the conditions associated with biofilm formation, since insulin and glucose dysmetabolism occurs in response to trauma [,]. Furthermore, E. coli as a fecal coliform persists in both temperate and tropical environments [,]. Under these environmental conditions, the production of biofilm could promote the levels of fecal contamination and the development of antibiotic resistant strains, which could enter the food chain, affecting both humans and animals.
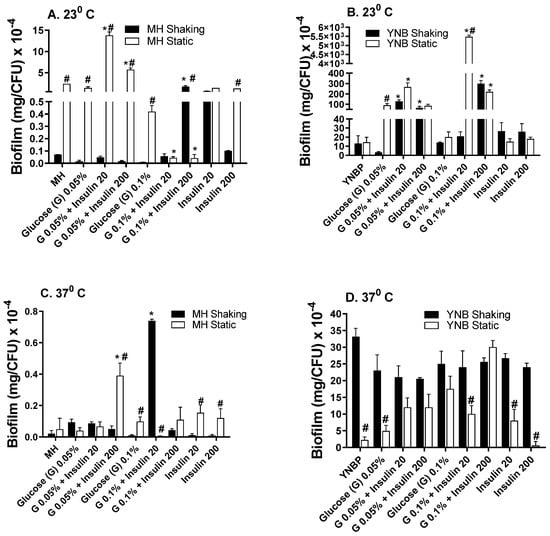
Figure 2.
Effect of Humulin® insulin (20 µU and 200 µU) on biofilm formation upon growth (24 h) in Mueller Hinton broth (Panels (A,C)) or yeast nitrogen base with 1% peptone (Panels (B,D)) with glucose (0.05% and 0.1%) at ambient (23 °C; Panels (A,B)) and human body temperature (37 °C; Panels (C,D)) in shaking (dark bars) and static (light bars) cultures. Results were expressed as total amount of biofilm (mg) per CFU. Results are the Mean ± SEM; * indicates significant difference from medium with glucose alone control (p < 0.05). # indicates significant difference between homologous media static and shaking culture, with or without insulin.
2.3. Galactose Induces Maximal Biofilm Formation
Galactose has been demonstrated to be essential for exopolysaccharide biosynthesis during biofilm formation and is important for the establishment of biofilm-associated intracellular populations in uropathogenic E. coli [,]. Galactose, a stereoisomer of glucose, elicited the highest overall level of biofilm/CFU when compared to glucose, lactose or insulin alone (Figure 3A–D). For growth in MH-galactose, temperature had the greatest effect on biofilm formation with biofilm levels from 1 × 103 to 1 × 104 higher than that measured after growth at ambient temperature (23 °C). At 37 °C, insulin significantly (p < 0.001) enhanced biofilm formation from 200- to >300-fold more than galactose or insulin alone. Maximal biofilm formation occurred in response to 0.1% galactose with 200 μU insulin. Interestingly, the higher levels of biofilm were produced at 37 °C in aMH containing galactose. This finding of enhanced biofilm formation in a rich, complex medium, MH, vs. the minimal YNBP is in contrast to the pattern of biofilm formation in YNBP alone, or containing glucose or lactose. This may be due to the differential expression of a galactose-specific lectin in addition to biofilm material. In E. coli, the galactose-specific lectin is responsible for aggregative behavior in pathogenic E. coli, e.g., EHEC and EAEC [,].
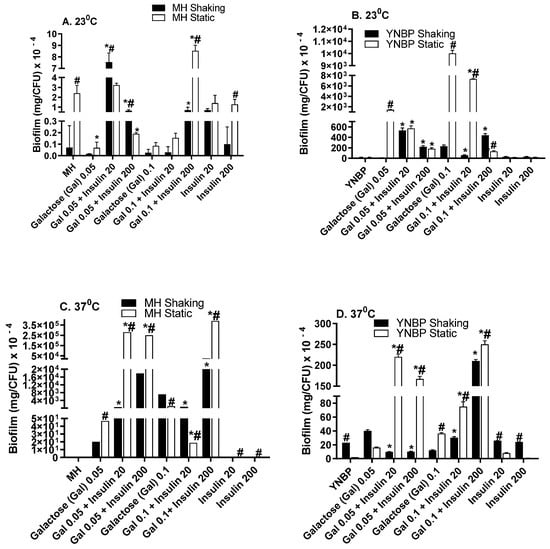
Figure 3.
Effect of Humulin® insulin (20 µU and 200 µU) on biofilm formation upon growth in medium (Muller Hinton broth, Panels (A,C); yeast nitrogen base with 1% peptone, Panels (B,D)) with galactose (0.05% and 0.1%) at ambient (23 °C; Panels (A,B)) and human body temperature (37 °C; Panels (C,D)) in shaking (dark bars) and static (light bars) cultures. Results were expressed as the total amount of biofilm (mg) per CFU. Results are the Mean ± SEM of three experiments with an n = 6 for each experiment. * indicates a significant difference from the medium with the galactose alone control (p < 0.05). # indicates a significant difference between the homologous media with or without insulin after a static and shaking culture.
2.4. Lactose Is Inhibitory for Biofilm Formation as Compared to Glucose and Galactose
In contrast to the pattern of biofilm formation measured in response to glucose or galactose, the presence of lactose (disaccharide composed of glucose and galactose) in MH was generally inhibitory for biofilm formation (Figure 4A–D), with biofilm levels ranging from approximately two-fold to 125-fold less than that measured for insulin alone under homologous incubation conditions. The single condition wherein the lactose promotion of biofilm formation exceeded that of glucose (five-fold higher) was the YNBP-lactose (0.05%; 23 °C; static) with 200 µU/mL of insulin. Some previous studies have indicated that high lactose levels (2.3%) enhance biofilm formation, which appears to be associated with the maturation of biofilm formation. However, the concentrations tested were not relevant to human physiology [,]. Converse findings have also been reported for E. coli biofilm formation in response to lactose []. It is possible that since both studies only measured total biofilm formation and not biofilm/CFU, the apparent differences may be the result of differences in population sizes, and/or exposure to autologous insulin as E. coli’s quorum signaling chemical.
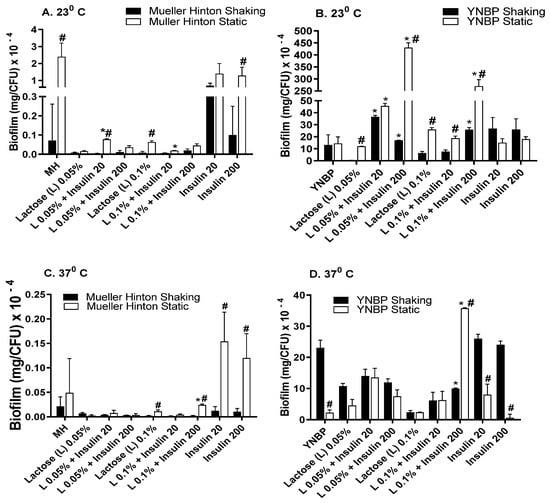
Figure 4.
Effect of Humulin® insulin (20 µU and 200 µU) on biofilm formation upon growth in medium (Muller Hinton broth, Panels (A,C); yeast nitrogen base with 1% peptone, Panels (B,D)) with lactose (0.05% and 0.1%) at ambient (23 °C; Panels (A,B)) and human body temperature (37 °C; Panels (C,D)) in shaking (dark bars) and static (light bars) cultures. Results were expressed as total amount of biofilm (mg) per CFU. Results are the Mean ± SEM of three experiments with an n = 6 for each experiment. * indicates significant difference from medium with lactose alone control (p < 0.05). # indicates significant difference between homologous media with or without insulin after static and shaking culture.
3. Discussion
A fundamental factor influencing E. coli’s biofilm formation and subsequent response to antimicrobials is its response to inter-cellular chemical communication signals, i.e., quorum sensing. To date, most studies have focused on the effects of auto-signaling between members of a microbial population, via N-acylhomoserine lactones (AHL) and 2-alkyl-4-quinolone (PQS) derivatives [,]. However, various hormones, including insulin, also function as quorum signaling molecules [,,,]. Upon entry into the host, pathogens are continuously exposed to various concentrations of insulin [,,,]. Of the array of quorum signals that have been described from either endogenous or exogenous sources, insulin may be the most versatile [,]. Insulin appears to exhibit a differential regulatory effect on E. coli behavior, resulting in the control of biofilm formation at concentrations below that which is required for the support of microbial growth [,,,].
An essential function of quorum compounds is to regulate microbial phenotype, including biofilm formation, in response to environmental conditions [,,]. The formation of biofilms is significantly affected by not only quorum compounds, but various environmental conditions, including available nutrients []. This interaction can be complex and diverse. It has been well established that, in response to other quorum signaling molecules, the biofilm structure is dependent on available nutrients [,,]. For example, for other microbes, e.g., Bacillus subtilis, the sugar-nucleotide UDP-galactose is toxic for planktonic cells but enhances biofilm formation []. The complexity of biofilm building is thus not only under the control of nutrients and available quorum signals, but further expression of the biofilm phenotype is affected by environmental growth conditions []. Biofilms are important in the enabling of the long-term colonization of a variety of surfaces, both abiotic and biotic. Biofilms also play a significant role in protecting organisms from the effects of antimicrobial agents [,].
The results from these studies on insulin’s effects may at least partially resolve some of the conflicting findings with regards to glucose and other sugars, with respect to their effect on the catabolite repression/induction of virulence factors, as well as the lack of effect that sugars alone have on E. coli biofilm formation on an abiotic surface [,,]. While significant work has been carried out to examine carbohydrates and catabolite repression, including that of biofilm regulation, the role interkingdom and non-lactone or quinolone signaling molecules play in modulating catabolite repression has not been examined []. E. coli’s response to human-r insulin is nutrition-specific. The amounts of E. coli biofilm formation were highest in galactose > glucose >> lactose in a manner that was insulin-concentration-specific. This indicates that not only can E. coli respond to endogenous insulin, but, as a pathogen, it also responds to exogenous insulin. The variation in response results in a broad phenotype that enhances survival regardless of the environmental specifics, although nutritionally poor environments, such as a YNBP, can be biofilm-promoting []. In addition, the potential to cause phenotypic switching in a microbial population would provide a mechanism for the removal of unwanted biofilms, which could serve as a source of drug-resistant organisms. The observation that a nutrient-rich medium, i.e., Mueller Hinton Broth, at physiological temperature minimally enhanced biofilm formation in response to glucose and lactose is of potential utility, and indicates that there is a possibility for minimizing biofilm through patient management, particularly regarding insulin concentrations.
The potential to cause phenotypic switching in a microbial population through the use of insulin requires further exploration, since there is some evidence that in the absence of sugars there is an inhibition and potential reversion of sessile state cells to the planktonic state (data not shown). Since physiologically, in the human host, galactose is primarily converted to glucose, galactose would be expected to play a minimal role in the biofilm formation associated with chronic infections. The potential role that galactose plays in resident gut microbiome-associated biofilm formation and microbial population stability has yet to be determined. In individuals with chronic infections, modulating dietary intake to reduce the levels of available sugars, especially galactose, and regulating insulin levels during antibiotic therapy, may be effective in modulating in vivo biofilm formation. The use of insulin to affect the sociobiology of E. coli, a model organism, would allow for the development of systems that could be exploited to affect the biofouling of indwelling catheters, as well as the prevention of healthcare-associated infections of abiotic surfaces and tissue, particularly external catheters, urine collection bags, and surface wounds. The modulation of insulin sugar levels may be of benefit in situations where the biofilm has been formed by extended spectrum antimicrobial resistant E. coli, since insulin and high glucose levels are present in serous fluid due to trauma [,]. Whether the MDR E. coli exhibit increased biofilm formation in response to insulin is under investigation [,,,,].
Shear stress, which may affect biofilm levels in shaking cultures, has a variable effect on E. coli biofilm formation, depending on available nutrient and growth conditions. Shaking YNBP (minimal medium) growth conditions were more permissive for biofilm formation than static growth conditions, in contrast to the opposite pattern measured for biofilm formation in nutrient-rich conditions (MH medium), with the exception of galactose. This finding may be useful in modulating in situ sepsis, and the biofilm formation associated with wounds, urinary tract, or catheter-associated infections [,].
Although the effect of insulin and glucose under human physiologic conditions has been reported, the influence of insulin in combination with mono- and di-saccharides on E. coli biofilm formation, under various incubation conditions, had not been previously examined [,]. In this study, we identified the extent to which insulin, as a quorum signaling molecule, regulates biofilm formation in response to various environmental stressors, including nutrient and oxygenation availability, and growth temperature. The findings indicate that the regulation of biofilm formation mediated by insulin is dependent on the carbon source and the environmental conditions, including temperature and type of aeration (static vs. shaking culture).
4. Materials and Methods
Bacteria and culture conditions. E. coli ATCC® 25922™, a K12 highly stable, wild type, quality control isolated for antibiotic susceptibility testing and biofilm studies, was used for all assays and maintained at −80 °C. Overnight (18 h) cultures (23 °C or 37 °C; shaking, 200 revolutions/min or static aeration) were prepared by inoculating two nutritionally different broth media to an Absorbance at 600 nm of 0.01 from an overnight tryptic soy agar culture (BD Difco, Franklin Lakes, NJ, USA). The two media used were Mueller Hinton broth (MH; BD Difco, Franklin Lakes, NJ, USA), a rich, complex medium, and Yeast Nitrogen Base (YNBP; BD Difco, Franklin Lakes, NJ, USA), a minimal-nutrient semi-defined medium, which lacks amino acids, carbohydrates and ammonium sulfate, to which 1% Bacto-Peptone (BD Difco, Franklin Lakes, NJ, USA) was added. Both MH and YNBP were supplemented with various physiologically relevant concentrations of glucose (0.05% (50 mg/dL-fasting glucose level) and 0.1% (100 mg/dL-bedtime glucose level; Sigma-Aldrich, St. Louis, MO, USA), galactose (0.05% and 0.1%; Sigma-Aldrich, St. Louis, MO, USA) or lactose (0.05% and 0.1%; Sigma-Aldrich, St. Louis, MO, USA), and/or insulin (Humulin®-R; 20 µU and 200 µU; Local retail pharmacy). These starter cultures were used to inoculate (0.01 Abs600nm) homologous media incubated under homologous conditions. The controls consisted of cultures grown under homologous growth conditions in MH or YNBP medium alone without supplements.
Biofilm assay: Biofilm formation after growth in various media and environmental growth conditions was determined. To assess the potential effect of the environmental source, e.g., food and water, as well as the in situ temperature, both the effects of ambient (23 °C) and human body temperature (37 °C) on biofilm formation were measured [,]. The amount of biofilm per CFU was measured using polypropylene micropipette tips (Sigma, St. Louis, MO, USA). This system is novel, using an inexpensive, readily available, and highly standardized abiotic material. In addition, it has the advantage of supporting direct measurement of biofilm (mg) formed per CFU, which is determined by measuring tip-associated CFU, incubated in parallel cultures. Overnight inoculum (18 h) was prepared as described above (23 °C or 37 °C; shaking, 200 revolutions/min on an Innova 2000 Platform Shaker, New Brunswick Scientific, Edison, NJ, USA; or static aeration) and was used to inoculate homologous media (3 × 104 CFU/mL; 13 × 100 mm borosilicate test tubes; 3 mL/tube; Fisher Scientific, Waltham, MA, USA) (Figure 5A). Pre-weighed 200 µL sterile polypropylene micropipette tips were aseptically placed into each tube and incubated under homologous conditions for 24 h. Previous determinations assessing E. coli growth under each of the conditions confirmed that there was no further increase in CFU after 24 h (data not shown). Under these conditions the top one-third of the tip was exposed above the level of the medium to allow for ease of manipulation. After incubation, half of the tips (n = 6) were suspended on racks to air dry, with care exercised to ensure that the biofilm was undisturbed (Figure 5B).
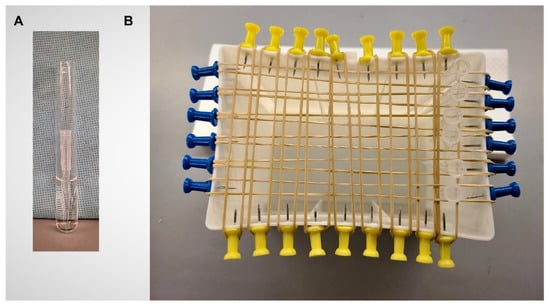
Figure 5.
Biofilm-determination methodology. (A) Pipet tip in 3 mL of medium (13 × 100 mm borosilicate test tubes). Tip can be easily removed with forceps; (B) Tip drying rack is constructed from upcycled plastic pipet tip boxes. Tips are suspended between rubber bands that are held in place with push pins inserted into tip box plastic. Insertion of the pins is facilitated by heating of the metal push pin tip.
The pipet tips were then reweighed for a final dry weight determination. The remaining tips (n = 6) were sonicated (Bransonic B12 Bath Sonicator, Branson Cleaning Company, Shelton, CN, USA) to disperse micropipette tip-attached biofilm (7 min, 23 °C). The CFU/tip was determined by spread-plating (0.1 mL) of sonicate, and its serial dilutions, onto tryptic soy agar, then counting resultant colonies. Removal of attached biofilm by sonication procedure was verified by rolling the pipet tip onto media to show that no residual organisms remained. All assays were repeated at least twice. Biofilm production was calculated as the amount of biofilm produced (Δ tip mass) per tip-associated CFU.
Statistical analysis. Data were analyzed using InStat (GraphPad Prism). Significance (p < 0.05) was determined using ANOVA and Student–Newman–Kuels post-hoc analysis.
5. Conclusions
These findings demonstrate that insulin, an important quorum signaling peptide, regulates biofilm expression across a variety of environmental conditions. This regulation is insulin concentration specific. Interestingly, galactose with insulin induces the highest level of biofilm formation per cell regardless of the temperature or additional available nutrients, and thus would be anticipated to be the most detrimental with regards to antibiotic efficacy. This finding may begin to explain the E. coli sepsis reported in individuals with galactosemia. The ability of E. coli to respond to insulin with the plasticity demonstrated in this study indicates that the potential utility of insulin instrument coatings must be carefully considered, taking into account the specific environment (nutrient rich, e.g., blood, or minimal nutrient availability, e.g., urine) that is targeted. These findings also open the potential for the development of microbial traps to decrease the bacterial load for better antimicrobial efficacy.
Author Contributions
Conceptualization, B.J.P.; methodology, N.P., J.C.C. and B.J.P.; validation, N.P., J.C.C. and B.J.P.; formal analysis, B.J.P.; investigation, N.P., J.C.C. and B.J.P.; resources, B.J.P.; data curation, B.J.P.; writing—original draft preparation, B.J.P.; writing—review and editing, B.J.P.; visualization, B.J.P.; supervision, B.J.P.; project administration, B.J.P.; funding acquisition, B.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Midwestern University Office of Research and Sponsored Programs.
Acknowledgments
The authors wish to thank the Midwestern University Office of Research and Sponsored Programs for the funding of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ramey, B.E.; Koutsoudis, M.; von Bodman, S.B.; Fuqua, C. Biofilm formation in plant–microbe associations. Curr. Opin. Microbiol. 2004, 7, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Cerqueira, L.; Oliveira, J.A.; Nicolau, A.; Azevedo, N.F.; Vieira, M.J. Biofilm formation with mixed cultures of Pseudomonas aeruginosa/Escherichia coli on silicone using artificial urine to mimic urinary catheters. Biofouling 2013, 29, 829–840. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Hatakeyama, T.; Ma, Z. Antimicrobial susceptibility and sub-MIC biofilm formation of Moraxella catarrhalis clinical isolates under anaerobic conditions. Adv. Microbiol. 2015, 5, 244. [Google Scholar] [CrossRef][Green Version]
- Farzam, F.; Plotkin, B. Effect of sub-MICs of antibiotics on the hydrophobicity and production of acidic polysaccharide by Vibrio vulnificus. Chemotherapy 2001, 47, 184–193. [Google Scholar] [CrossRef]
- Surette, M.G.; Bassler, B.L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1998, 95, 7046–7050. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-García, L.; Blasco, L.; Trastoy, R.; García-Contreras, R.; Wood, T.K.; Tomás, M. Quorum sensing systems and persistence. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: New York, NY, USA, 2018; pp. 17–27. [Google Scholar]
- Konaklieva, M.; Plotkin, B. Chemical communication--do we have a quorum? Mini-Rev. Med. Chem. 2006, 6, 817–825. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Shiloach, J.; Heffron, R.; Rubinovitz, C.; Tanenbaum, R.; Roth, J. Insulin-related material in microbes: Similarities and differences from mammalian insulins. Can. J. Biochem. Cell Biol. 1985, 63, 839–849. [Google Scholar] [CrossRef]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M. Evolutionary origins of vertebrate hormones: Substances similar to mammalian insulins are native to unicellular eukaryotes. Proc. Natl. Acad. Sci. USA 1980, 77, 6184–6188. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M.A. Insulin or a closely related molecule is native to Escherichia coli. J. Biol. Chem. 1981, 256, 6533–6536. [Google Scholar] [CrossRef]
- Christensen, S.; Quie, H.; Kemp, K.; Rasmussen, L. Insulin produces a biphasic response in Tetrahymena thermophila by stimulating cell survival and activating proliferation in two separate concentration intervals. Cell Biol. Int. 1996, 20, 437–444. [Google Scholar] [CrossRef]
- Conlon, J. Molecular evolution of insulin in non-mammalian vertebrates. Am. Zool. 2000, 40, 200–212. [Google Scholar]
- Fawell, S.E.; Lenard, J. A specific insulin receptor and tyrosine kinase activity in the membranes of Neurospora crassa. Biochem. Biophys. Res. Commun. 1988, 155, 59–65. [Google Scholar] [CrossRef]
- Kole, H.K.; Muthukumar, G.; Lenard, J. Purification and properties of a membrane-bound insulin binding protein, a putative receptor, from Neurospora crassa. Biochememistry 1991, 30, 682–688. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Lesniak, M.; Roth, J. Insulin in insects and annelids. Diabetes 1981, 30, 70–76. [Google Scholar] [CrossRef]
- Kirby, D.; Raino, C.; Rabor, S.F., Jr.; Wasson, C.; Plotkin, B. Semi-automated method for multi-tasking measurement of microbial growth, capsule, and biofilm formation. Adv. Microbiol. 2012, 2, 623–628. [Google Scholar] [CrossRef][Green Version]
- Klosowska, K.; Plotkin, B. Human insulin modulation of Escherichia coli adherence and chemotaxis. Am. J. Infect. Dis. 2006, 2, 197–200. [Google Scholar] [CrossRef]
- Plotkin, B.; Wu, Z.; Ward, K.; Nadella, S.; Green, J.; Rumnani, B. Effect of human insulin on the formation of catheter-associated E. coli biofilms. Open J. Urol. 2014, 4, 49–56. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Viselli, S.M. Effect of insulin on microbial growth. Curr. Microbiol. 2000, 41, 60–64. [Google Scholar] [CrossRef]
- Poursina, F.; Sepehrpour, S.; Mobasherizadeh, S. Biofilm formation in nonmultidrug-resistant Escherichia coli isolated from patients with urinary tract infection in Isfahan, Iran. Adv. Biomed. Res. 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Crémet, L.; Corvec, S.; Batard, E.; Auger, M.; Lopez, I.; Pagniez, F.; Dauvergne, S.; Caroff, N. Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diagn. Microbiol. Infect. Dis. 2013, 75, 252–255. [Google Scholar] [CrossRef]
- Ostrowska, K.; Strzelczyk, A.; Rozalski, A.; Staczek, P. Bacterial biofilm as a cause of urinary tract infection-pathogens, methods of prevention and eradication. Postepy Hig. Med. Dosw. 2013, 67, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Cloete, T.E.; Brözel, V.S.; Von Holy, A. Practical aspects of biofouling control in industrial water systems. Int. Biodeterior. Biodegrad. 1992, 29, 299–341. [Google Scholar] [CrossRef]
- Tao, H.; Bausch, C.; Richmond, C.; Blattner, F.R.; Conway, T. Functional genomics: Expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 1999, 181, 6425–6440. [Google Scholar] [CrossRef]
- Adnan, M.; Morton, G.; Singh, J.; Hadi, S. Contribution of rpoS and bolA genes in biofilm formation in Escherichia coli K-12 MG1655. Mol. Cell. Biochem. 2010, 342, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Constan, L.; Mako, M.; Juhn, D.; Rubenstein, A.H. The excretion of proinsulin and insulin in urine. Diabetologia 1975, 11, 119–123. [Google Scholar] [CrossRef][Green Version]
- Meguid, M.M.; Aun, F.; Soeldner, J.S. Effect of severe trauma on urine loss of insulin. Surgery 1976, 79, 177–181. [Google Scholar] [PubMed]
- Rubenstein, A.H. The significance of immunoassayable insulin in urine. J. Am. Med. Assoc. 1969, 209, 254–256. [Google Scholar] [CrossRef]
- Rubenstein, A.H.; Lowy, C.; Welborn, T.A.; Fraser, T.R. Urine insulin in normal subjects. Metabolism 1967, 16, 234–244. [Google Scholar] [CrossRef]
- Trayner, I.M.; Welborn, T.A.; Rubenstein, A.; Fraser, T.R. Serum and urine insulin in late pregnancy and in a few pregnant latent diabetics. J. Endocrinol. 1967, 37, 443–453. [Google Scholar] [CrossRef]
- Carter, E.A. Insulin resistance in burns and trauma. Nutr. Rev. 1998, 56, S170. [Google Scholar] [CrossRef]
- Hrynyk, M.; Neufeld, R.J. Insulin and wound healing. Burns 2014, 40, 1433–1446. [Google Scholar] [CrossRef]
- Byappanahalli, M.; Whitman, R.; Shively, D.; Sadowsky, M.; Ishii, S. Population structure, persistence, and seasonality of autochthonous Escherichia coli in temperate, coastal forest soil from a Great Lakes watershed. Environ. Microbiol. 2006, 8, 504–513. [Google Scholar] [CrossRef]
- Silcox, C.A.; Robinson, B.A.; Willoughby, T.C. Concentrations of Escherichia coli in Streams in the Kankakee and Lower Wabash River Watersheds in Indiana, June-September 1999; US Department of the Interior: Denver, CO, USA; US Geological Survey: Denver, CO, USA, 2001; Volume 1.
- Conover, M.S.; Hadjifrangiskou, M.; Palermo, J.J.; Hibbing, M.E.; Dodson, K.W.; Hultgren, S.J. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 2016, 7, 2. [Google Scholar] [CrossRef]
- Chai, Y.; Beauregard, P.B.; Vlamakis, H.; Losick, R.; Kolter, R. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio 2012, 3, e00184-12. [Google Scholar] [CrossRef]
- Grover, V.; Ghosh, S.; Sharma, N.; Chakraborti, A.; Majumdar, S.; Ganguly, N.K. Characterization of a galactose specific adhesin of enteroaggregative Escherichia coli. Arch. Biochem. Biophys. 2001, 390, 109–118. [Google Scholar] [CrossRef]
- Coutiño-Rodríguez, R.; Hernández-Cruz, P.; Giles-Ríos, H. Lectins in fruits having gastrointestinal activity: Their participation in the hemagglutinating property of Escherichia coli 0157: H7. Arch. Med. Res. 2001, 32, 251–257. [Google Scholar] [CrossRef]
- Arola, H.; Tamm, A. Metabolism of lactose in the human body. Scand. J. Gastroenterol. 1994, 29, 21–25. [Google Scholar] [CrossRef]
- Bas, S.; Kramer, M.; Stopar, D. Biofilm surface density determines biocide effectiveness. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Samrakandi, M.M.; Roques, C.; Michel, G. Influence of trophic conditions on exopolysaccharide production: Bacterial biofilm susceptibility to chlorine and monochloramine. Can. J. Microbiol. 1997, 43, 751–758. [Google Scholar] [CrossRef]
- Cloak, O.M.; Solow, B.T.; Briggs, C.E.; Chen, C.Y.; Fratamico, P.M. Quorum sensing and production of autoinducer-2 in Campylobacter spp., Escherichia coli O157:H7, and Salmonella enterica serovar Typhimurium in foods. Appl. Environ. Microbiol. 2002, 68, 4666–4671. [Google Scholar] [CrossRef]
- Plotkin, B.; Konaklieva, M. Possible role of sarA in dehydroepiandosterone (DHEA)-mediated increase in Staphylococcus aureus resistance to vancomycin. Chemotherapy 2007, 53, 181–184. [Google Scholar] [CrossRef]
- Plotkin, B.; Morejon, A.; Laddaga, R.; Viselli, S.; Thijo, J.; Schreckenberger, P. Induction of increased resistance to vancomycin in Staphylococcus aureus clinical isolates (MSSA, MRSA) by dehydroepiandosterone (DHEA). Lett. Appl. Microbiol. 2005, 40, 249–254. [Google Scholar] [CrossRef]
- May, A.K.; Kauffmann, R.M.; Collier, B.R. The place for glycemic control in the surgical patient. Surg. Infect. 2011, 12, 405–418. [Google Scholar] [CrossRef]
- Messenger, B.; Clifford, M.; Morgan, L. Glucose-dependent insulinotropic polypeptide and insulin-like immunoreactivity in saliva following sham-fed and swallowed meals. J. Endocrinol. 2003, 177, 407–412. [Google Scholar] [CrossRef]
- Nikolic, D.M. Effects of Candida on insulin secretion of human adult pancreatic islets and possible onset of diabetes. Br. J. Biomed. Sci. 2014, 71, 73–78. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Reaven, G.M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes 1977, 26, 680–688. [Google Scholar] [CrossRef]
- Plotkin, B.; Erickson, Q.; Roose, R.; Viselli, S. Effect of androgens and glucocorticoids on microbial growth and antimicrobial susceptibility. Curr. Microbiol. 2003, 47, 514–520. [Google Scholar] [CrossRef]
- Feraco, D.; Blaha, M.; Khan, S.; Green, J.M.; Plotkin, B.J. Host environmental signals and effects on biofilm formation. Microb. Pathog. 2016, 99, 253–263. [Google Scholar] [CrossRef]
- Hastings, J.; Greenberg, E. Quorum sensing:the explanation of a curious phenomenon reveals a common characteristic of bacteria. J. Bacteriol. 1999, 181, 2667–2668. [Google Scholar] [CrossRef]
- Horswill, A.; Stoodley, P.; Stewart, P.; Parsek, M. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Anal. Bioanal. Chem. 2007, 387, 371–380. [Google Scholar] [CrossRef]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef]
- Rice, S.A.; Koh, K.S.; Queck, S.Y.; Labbate, M.; Lam, K.W.; Kjelleberg, S. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J. Bacteriol. 2005, 187, 3477–3485. [Google Scholar] [CrossRef]
- Christensen, B.B.; Haagensen, J.A.; Heydorn, A.; Molin, S. Metabolic commensalism and competition in a two-species microbial consortium. Appl. Environ. Microbiol. 2002, 68, 2495–2502. [Google Scholar] [CrossRef]
- Khangholi, M.; Jamalli, A. The effects of sugars on the biofilm formation of Escherichia coli 185p on stainless steel and polyethylene terephthalate surfaces in a laboratory model. Jundishapur J. Microbiol. 2016, 9, e40137. [Google Scholar] [CrossRef]
- Jackson, D.; Simecka, J.; Romeo, T. Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 2002, 184, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Suzuki, K.; Oakford, L.; Simecka, J.; Hart, M.; Romeo, T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kremling, A.; Bettenbrock, K.; Gilles, E.D. Analysis of global control of Escherichia coli carbohydrate uptake. BMC Syst. Biol. 2007, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Schembri, M.A.; Kjærgaard, K.; Klemm, P. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 2003, 48, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Haque, A.; Ali, A.; Mohsin, M.; Bashir, S.; Tariq, A.; Afzal, A.; Iftikhar, T.; Sarwar, Y. Relationship of drug resistance to phylogenetic groups of E. coli isolates from wound infections. J. Infect. Dev. Ctries. 2009, 3, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Toliver-Kinsky, T.E.; Williams, F.N.; Song, J.; Cui, W.; Herndon, D.N.; Jeschke, M.G. Insulin increases resistance to burn wound infection-associated sepsis. Crit. Care Med. 2010, 38, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.; Brammah, S.; Wills, E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010, 36, 49–56. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; del Prete, M.S.; Barchiesi, F.; D'Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Sisay, M.; Worku, T.; Edessa, D. Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: A meta-analysis of laboratory-based cross-sectional studies. BMC Pharmacol. Toxicol. 2019, 20, 35. [Google Scholar] [CrossRef]
- Sierra, M.V.; Gomez, N. Structural characteristics and oxygen consumption of the epipelic biofilm in three lowland streams exposed to different land uses. WaterAirSoil Pollut. 2007, 186, 115–127. [Google Scholar] [CrossRef]
- Lyon, D.R.; Ziegler, S.E. Carbon cycling within epilithic biofilm communities across a nutrient gradient of headwater streams. Limnol. Oceanogr. 2009, 54, 439–449. [Google Scholar] [CrossRef]
- Byappanahalli, M.; Fowler, M.; Shively, D.; Whitman, R. Ubiquity and Persistence of Escherichia coli in a Midwestern Coastal Stream. Appl. Environ. Microbiol. 2003, 69, 4549–4555. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).