Abstract
Ceftazidime/avibactam uniquely demonstrates activity against both KPC and OXA-48-like carbapenemase-expressing Enterobacterales. Clinical resistance to ceftazidime/avibactam in KPC-producers was foreseen in in-vitro resistance studies. Herein, we assessed the resistance selection propensity of ceftazidime/avibactam in K. pneumoniae expressing OXA-48-like β-lactamases (n = 10), employing serial transfer approach. Ceftazidime/avibactam MICs (0.25–4 mg/L) increased to 16–256 mg/L after 15 daily-sequential transfers. The whole genome sequence analysis of terminal mutants showed modifications in proteins linked to efflux (AcrB/AcrD/EmrA/Mdt), outer membrane permeability (OmpK36) and/or stress response pathways (CpxA/EnvZ/RpoE). In-vitro growth properties of all the ceftazidime/avibactam-selected mutants were comparable to their respective parents and they retained the ability to cause pulmonary infection in neutropenic mice. Against these mutants, we explored the activities of various combinations of β-lactams (ceftazidime or cefepime) with structurally diverse β-lactamase inhibitors or a β-lactam enhancer, zidebactam. Zidebactam, in combination with either cefepime or ceftazidime, overcame ceftazidime/avibactam resistance (MIC range 0.5–8 mg/L), while cefepime/avibactam was the second best (MIC: 0.5–16 mg/L) in yielding lower MICs. The present work revealed the possibility of ceftazidime/avibactam resistance in OXA-48-like K. pneumoniae through mutations in proteins involved in efflux and/or porins without concomitant fitness cost mandating astute monitoring of ceftazidime/avibactam resistance among OXA-48 genotypes.
1. Introduction
The carbapenem non-susceptibility in Enterobacterales is mainly imparted by the ‘triad’ of carbapenemases, viz., Klebsiella pneumoniae carbapenemases (KPCs), metallo-β-lactamases (MBLs) and OXA-48-like. Lately, OXA-48-like carbapenemases have captured the attention due to their wider geographic dissemination leading to their ascendency in several parts of the globe, including the Middle East, North Africa, some European countries and the Indian sub-continent [1]. Among the reported multiple plasmid-encoded blaOXA-48-like variants, the prototype blaOXA-48-like, its four-amino-acid variant-blaOXA-181 and the five-amino-acid variant-blaOXA-232 are predominant. Compared to the other two carbapenemases in the triad, OXA-48-like β-lactamases possess a weaker carbapenemase activity; however, when they co-exist with impermeability, extended-spectrum β-lactamases (ESBLs) and/or AmpC, a clinically-relevant resistance to carbapenems is observed [1,2,3,4]. In particular, such a multiplicity of resistance mechanisms, usually prevalent in K. pneumoniae harboring OXA-48-like, presents a therapeutic challenge to diverse antibiotic classes [1,5,6].
Disappointingly, recently approved carbapenem based combinations, imipenem/relebactam and meropenem/vaborbactam, do not cover OXA-48-like-producing K. pneumoniae due to the inability of respective β-lactamase inhibitors to inhibit these enzymes [7,8]. Though cephalosporins are stable to OXA-48-like, the coverage of this resistotype by cephalosporin-based combinations is contingent to the β-lactam’s vulnerability towards co-expressed β-lactamases, which in turn is linked with partner β-lactamase inhibitor’s ability to restore the activity of partner β-lactam to a therapeutically meaningful level. This resonates in the activity of ceftazidime/avibactam against OXA-48-like-expressing K. pneumoniae but not for ceftolozane/tazobactam [8,9]. Thus, currently, ceftazidime/avibactam is the only approved β-lactam (BL)/β-lactamase inhibitor (BLI) [BL/BLI] combination with coverage of OXA-48-like-expressing organisms. Recently, registered cefiderocol has been reported to be active against isolates expressing OXA-48-like [10]; however, in the absence of definitive diagnosis of the ‘type’ of carbapenem-resistant Enterobacterales (CRE) in most clinical settings, treatment decisions need to factor a wide scatter in cefiderocol MIC90 for isolates with New Delhi metallo-β-lactamases (NDM) often exceeding the susceptibility breakpoint [10,11]. Among the pipeline agents, cefepime/taniborbactam, cefepime/enmetazobactam and cefepime/zidebactam have been shown to possess in-vitro and in-vivo activity against pathogens expressing this resistance mechanism [12,13,14].
In regions with predominant prevalence of KPC, such as the USA, the clinical use of ceftazidime/avibactam is mainly directed towards confirmed or suspected cases of KPC infection. Unfortunately, within a few years of its usage, reports of on-therapy resistance began to multiply in KPC-expressing K. pneumoniae which was associated with mutations in KPC leading to enhanced hydrolytic activity for ceftazidime rendering avibactam’s inhibitory activity inadequate [15,16,17,18,19]. The early signals of propensity of resistance development with ceftazidime/avibactam against KPC were detected in the in-vitro studies that showed relative ease of acquisition of mutational resistance in single and multiple drug-passage studies [20]. However, in-vitro studies assessing the propensity of resistance development to ceftazidime/avibactam against OXA-48-like resistotype are extremely limited, specifically those employing ‘real-world’ clinical isolates of K. pneumoniae.
To the best of our knowledge, this is the first ever study assessing the phenotypic and genotypic aspects of in-vitro resistance development to ceftazidime/avibactam against clinical isolates of K. pneumoniae harboring blaOXA-181 or blaOXA-232 and their in-vivo relevance. Additionally, to explore the interplay between β-lactams and β-lactamase inhibitors in tackling potential acquisition of ceftazidime/avibactam resistance in OXA-48-like pathogens, we evaluated the activities of structurally and mechanistically diverse BLIs as well as β-lactam enhancer, zidebactam, when partnered with cefepime or ceftazidime.
2. Results
2.1. Serial Transfer Study
The OXA-48-like-expressing K. pneumoniae isolates utilized in this study were susceptible to ceftazidime/avibactam as shown in Table 1 (MICs 0.25 to 4 mg/L; susceptibility breakpoint 8 mg/L). Figure 1 shows the day-wise changes in the MICs of ceftazidime/avibactam during the 15 sequential passages. The rate of MIC increase was variable in the range of 3 to 15 passages to raise the MICs above 8 mg/L; generally higher passage numbers were required for strains with lower baseline MICs. The MICs after the last passage ranged from 16 to 256 mg/L, all resistant to ceftazidime/avibactam. The resistance to ceftazidime/avibactam in the terminal mutants was stable after 15 drug-free passages (MICs 16—>128 mg/L). Furthermore, all the terminal mutants grew well, comparable to their respective parent isolates in M9 minimal medium and cation-adjusted Mueller–Hinton broth (CAMHB), suggesting lack of fitness cost (Supplementary Figures S1 and S2).

Table 1.
Characteristics of K. pneumoniae expressing OXA-48-like β-lactamases employed in serial transfer study.
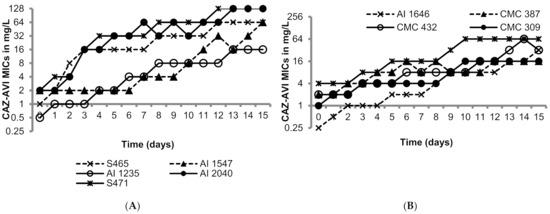
Figure 1.
Serial transfer studies of ceftazidime/avibactam against ten OXA-48-like-expressing K. pneumoniae. (A) OXA-48 producing K. pneumoniae isolates had ceftazidime-avibactam MICs ranging from 0.5 to 1 mg/L. (B) OXA-48 producing K. pneumoniae isolates had ceftazidime-avibactam MICs ranging from of 0.5 to 4 mg/L.
2.2. Whole Genome Sequencing
Table 2 shows the mutations identified through whole genome sequencing analysis of ceftazidime/avibactam-selected terminal mutants when compared to the respective parent isolates. Broadly, changes were observed in proteins linked to efflux (AcrB/AcrD/EmrA/Mdt), outer membrane permeability (OmpK36) and/or stress response pathways (CpxA/EnvZ/RpoE). In one of the mutants (derived from K. pneumoniae S465), point mutation in AmpC β-lactamase was detected that might have increased its hydrolytic activity towards ceftazidime leading to elevated ceftazidime/avibactam MICs. Furthermore, two blaOXA-181-producing isolates (K. pneumoniae AI1547 and K. pneumoniae S471) selected point mutations leading to conversion of blaOXA-181 to blaOXA-232 in addition to the mutations in proteins related to efflux and outer membrane permeability, respectively. Current and previously reported studies provide support to the fact that ceftazidime/avibactam retains activity against all variants of blaOXA-48-like such as blaOXA-232, blaOXA-162 etc. expressing K. pneumoniae [21] and therefore, the observed change in OXA-enzyme type (from 181 to 232) may not be a standalone cause for increase in ceftazidime/avibactam MICs. Lastly, ceftazidime/avibactam selected AI1235 mutant showed mutation in MrdA (penicillin binding protein [PBP] 2) as previously reported [22]. This suggests PBP2 sensitization, most probably, by avibactam, which is known to have a weak affinity towards PBP2; however, the role of this mutation in elevating ceftazidime/avibactam MIC for this isolate is unlikely since as compared to E. coli, the intrinsic potency of avibactam against K. pneumoniae is of a lower order.

Table 2.
List of mutations identified in terminal mutants obtained from ceftazidime/avibactam serial exposure.
2.3. In-Vivo Infectivity and Resistance
In order to assess the in-vivo relevance of the raised ceftazidime/avibactam MICs, the magnitude of protective doses (measured as efficacy dose 50/90, ED50/90) of ceftazidime/avibactam were compared for parent vs. mutant (for two parent-mutant pairs) in a mice peritonitis model. Untreated control animals succumbed within 24 h of infection, suggesting optimal infectivity of both parent and mutants. Against two parent strains, ceftazidime in combination with avibactam (4:1 dose ratio) showed ED50 of 6 to 14.9 mg/kg which increased to 34.4 to 49.5 mg/kg for their respective mutants, thus reflecting the ceftazidime/avibactam resistance acquired by the terminal mutants (Figure 2). Similar, increase in ED90 of ceftazidime/avibactam was also observed in mutants (80.7–102.5 mg/kg) compared to parent strains (20.9–34.6 mg/kg). All the animals in meropenem treatment groups of both parent and mutants showed mortality within 24 h of infection suggesting optimal carbapenemase expression.
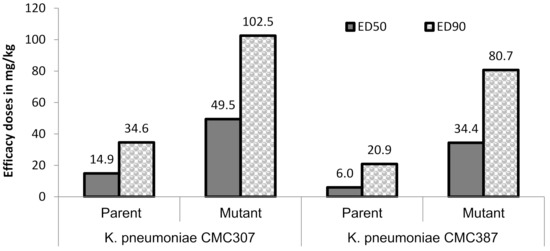
Figure 2.
Efficacy of ceftazidime/avibactam in murine systemic infection model. Ceftazidime was administered (b.i.d) in multiple doses of 3.12, 6.25, 12.5, 25, 50, 100 mg/kg in combination with avibactam 4:1 dose of 0.78, 1.56, 3.12, 6.25, 12.5, 25 mg/kg, respectively. The lower limit-upper limit for ceftazidime ED50 and ED90 were 10.1–22.0 mg/kg (parent), 32.7–68.2 mg/kg (mutant) and 24.9–47.9 mg/kg (parent), 60–180.7 mg/kg (mutant), respectively, against K. pneumoniae CMC307. Similarly, against K. pneumoniae CMC387, they were 3.4–10.7 mg/kg, 24.3–48.8 mg/kg and 9.7–45.2 mg/kg, 48.4–134.7 mg/kg, respectively. All the animals in meropenem arm succumbed to death at the highest dose (100 mg/kg) tested.
2.4. Susceptibility Profile of Serial Transfer Mutants to Combinations of Ceftazidime or Cefepime with β-Lactamase Inhibitors/β-Lactam Enhancer
To assess the interplay between β-lactam and β-lactamase inhibitors/β-lactam enhancer in tackling the acquired resistance to ceftazidime/avibactam in OXA-48-like expressing K. pneumoniae, we determined the MICs of three cefepime based combinations under clinical development, two exploratory cefepime/BLI combinations, as well as various exploratory combinations of ceftazidime with BLI or β-lactam enhancer (Table 3). Carbapenems were included in the MIC studies to determine the collateral effect of acquired resistance on their activity, if any.

Table 3.
Comparative activity of cefepime/ceftazidime when combined with various BLIs against ceftazidime-avibactam-resistant terminal mutants of blaOXA-181 and blaOXA-232 expressing K. pneumoniae recovered from serial transfer studies.
The MICs of carbapenems for the mutants were generally comparable to respective parents; limited to 1 to 2 dilution-fold change in MICs. The lone exception was the mutant of parent AI1646 for which MICs of meropenem and imipenem substantially dropped to susceptibility range, a phenomenon commonly observed with KPC harboring K. pneumoniae that acquired resistance to ceftazidime/avibactam [23]. Overall for all parent strains, avibactam lowered the MICs of imipenem to near baseline, while for mutants, the combination MICs were within 1 to 2 dilution folds compared to parent isolates (highest MIC being 1 mg/L except for the isolate CMC307).
There was a near complete cross-resistance carried to all ceftazidime based exploratory combinations except for ceftazidime/zidebactam (MIC range: 0.5–2 mg/L for parents and 0.5–8 mg/L for mutants), wherein zidebactam is a β-lactam enhancer. In case of cefepime based combinations, overall MICs were lower compared to the respective ceftazidime-based combination and among all, cefepime/zidebactam (MIC range: 0.5–2 mg/L for parents and 0.5–8 mg/L for mutants) and cefepime/avibactam (MIC range: 0.25–2 mg/L for parents and 0.5–16 mg/L for mutants) were the most potent.
From the therapeutic perspectives, none of the ceftazidime-BLI combinations yielded MICs of ≤8 mg/L, a hypothetical cut-off applied based on ceftazidime/avibactam breakpoint, and in contrast, majority of mutants showed cefepime/avibactam MICs of ≤8 mg/L, employing cefepime’s susceptibility dose-dependent breakpoint. In combination with zidebactam, both cefepime and ceftazidime yielded MICs ≤ 8 mg/L against all the ceftazidime/avibactam-resistant mutants.
3. Discussion
The launch of ceftazidime/avibactam in 2015 aptly generated a high anticipation among the clinicians owing to its activity against challenging resistance mechanisms expressed in Enterobacterales; AmpC, OXA-48-like and KPC β-lactamases, which are not amenable to then available β-lactam/β-lactamase-inhibitor combinations. However, within 5 years of ceftazidime/avibactam’s clinical use, several disturbing reports, revealing its vulnerability to on-therapy resistance selection in KPC-expressing pathogens, began appearing [16,24,25]. Corroborating this trend, in-vitro resistance selection studies published earlier have pointed towards the risk of clinical resistance to ceftazidime/avibactam, thus signifying the relevance of such in-vitro resistance selection studies [20,22].
In view of the therapeutic value of ceftazidime/avibactam for the infections caused by OXA-48-like producers, we sought to decipher the propensity of in-vitro resistance selection to ceftazidime/avibactam in this resistotype. To the best of our knowledge, this is the first-ever study, assessing multiple aspects of in-vitro resistance development in clinical isolates of OXA-181- and OXA-232-expressing K. pneumoniae. The investigations carried out in this study include genetic basis for the acquired resistance and its impact, if any, on in-vitro fitness as well as in-vivo infectivity/resistance. Further, to gain insights on the interplay between various β-lactams and β-lactamase inhibitors in overcoming ceftazidime/avibactam resistance in OXA-48-like pathogens, MICs of three cefepime based combinations under clinical development, two exploratory cefepime/BLI combinations, as well as various exploratory combinations of ceftazidime with BLI or β-lactam enhancer, were determined. The BLIs used in the study belonged to three different chemotypes: diazabicyclooctane, boronate and β-lactam.
One of the findings of this study was that the selected resistance to ceftazidime/avibactam, emanating from mutations in genes encoding AmpC, efflux, porins and stress response pathways, remained stable despite multiple consecutive drug-free passages, suggesting potential for ease of survival and dissemination of such high-risk resistant clones. Previous studies showed that mutations in these stress response pathway proteins could modulate efflux/impermeability [26,27,28,29], and hence, changes in porin (down-regulation) and efflux (up-regulation) protein expressions stand out as the possible mechanisms for ceftazidime/avibactam resistance. In a similar serial transfer study reported by Livermore et al. in 2015, stably raised MICs of ceftazidime/avibactam against KPC-expressing Enterobacterales were linked with mutations in Ω loop of KPC [20]. However, in contrast, our study did not show mutations in blaOXA-48-like; rather, mutations were mostly associated with efflux and porin encoding genes (barring a point mutation converting blaOXA-181 to catalytically comparable blaOXA-232). In another study by Livermore et al., exposure of ceftazidime/avibactam to de-repressed AmpC harboring E. coli and K. pneumoniae, led to the selection of mutants with stably elevated MICs (4–64 mg/L) and genomic studies showed several modifications in AmpC [22]. In the same study, ceftazidime/avibactam-resistant mutants of ESBL producers (ceftazidime/avibactam MICs 0.5–16 mg/L) mostly had changes affecting permeability, efflux or β-lactamase quantity; only one had an altered β-lactamase. However, in another study, polymorphism in blaCTX-M-14 has been implicated in clinical resistance to ceftazidime/avibactam which was due to augmented ceftazidimase activity of the mutated enzyme [30]. Thus, overall, it seems that established resistance imparting strategies involving mutation in β-lactamase genes as well as efflux and porins are the drivers of ceftazidime/avibactam resistance in OXA-48-like expressing strains.
In context to our study, it is pertinent to discuss a sole recent publication from Fröhlich et al. studying the propensity of resistance development in E. coli constructs expressing OXA-48 [31]. Similar to our study, Fröhlich and colleagues also described the ease of resistance selection upon exposure to ceftazidime/avibactam. However, unlike our study, mutations were reported in blaOXA-48 which resulted in increased hydrolysis of ceftazidime and reduced inhibitory activity of avibactam. This divergent observation could be linked to several factors such as differences in method of resistance selection (agar method vs. broth) and more importantly, the genus harboring blaOXA-48-like enzymes (E. coli vs. K. pneumoniae). Moreover, the two studies differed in terms of gene/s depicting the mutations (blaOXA-48 vs. blaCMY, efflux pumps and outer membrane porins). More pertinently, the present study employed clinical isolates harboring OXA-48-like enzymes along with impermeability and AmpC/ESBL, and therefore, possibly is more relevant to the real-world scenario.
From the evolutionary viewpoint, the success of Gram-negative pathogens in countering β-lactams through unleashing mutant β-lactamase gene could be judged by the fact that 2800 discrete β-lactamases [32] with wide range of substrate specificities have already been reported. Likewise, mutations in genes encoding/regulating porins and efflux pumps have also been successfully deployed with minimal fitness cost trade-off. This observation was substantiated in our study, as ceftazidime/avibactam-resistant mutants did not show any growth defects in-vitro as well as in-vivo Moreover, these mutants showed uncompromised ability to cause pulmonary infection in neutropenic mice and as anticipated, required elevated ceftazidime/avibactam doses for the protection of infected mice. Thus, ceftazidime/avibactam resistance observed in-vitro also manifested in-vivo, pointing towards the significance of our findings.
Since therapeutic options for infections caused by OXA-48-like phenotypes are limited, we investigated the potential of several combinations of chemically diverse BLIs/β-lactam enhancer zidebactam with cefepime or ceftazidime. In general, ceftazidime/avibactam-resistant mutants showed cross resistance to all the cephalosporin based BL/BLI combinations employed in this study, suggesting that despite deployment of structurally and mechanistically diverse BLIs, the BL/BLI mechanism of action has an intrinsic limitation in overcoming mutational resistance impacting other members of its own class (β-lactam antibiotics). Yet, as compared to ceftazidime, cefepime combinations tended to show lower MICs irrespective of partner BLI used in the combination. For instance, among them, cefepime/avibactam was the most potent combination, a benefit accruing from mechanistically advantageous features of cefepime as a partner β-lactam antibiotic. It has been reported that cefepime is bestowed with a β-lactamase stability, multiple PBP binding action and optimal permeation in Gram-negatives [33]. Other BLIs such as relebactam, enmetazobactam, taniborbactam and vaborbactam yielded inconsistent MICs in combination with either cefepime or ceftazidime. Previously, against mutant KPC-expressing Enterobacterales, a combination of avibactam with ceftaroline has been shown to possess better in-vitro activity as compared to its combination with ceftazidime [20]. This was ascribed to the ability of ceftaroline to better withstand the KPC-mediated hydrolysis. The role of partner antibiotic was also evident in our study, which showed that when imipenem was paired with avibactam, the majority of mutants turned susceptible to the combination. Thus, avibactam’s well-established feature of inhibition of OXA-48-like β-lactamases could be better harnessed in combination with imipenem, as compared to cephalosporins.
Finally, it was only the β-lactam enhancer, zidebactam, which provided a narrow range of low MICs regardless of the partner cephalosporin used. This is an outcome of the reported unconventional mechanism of action of zidebactam that operates through PBP2 binding and in combination with PBP3 binding β-lactams, which overcomes multiple mechanisms of resistance that adversely impact range of BL/BLI combinations [34,35,36,37]. For instance, unlike newer BLIs, the MICs of zidebactam in combination with relatively less potent partner ceftazidime were still below 8 mg/L against all the mutants.
In conclusion, the present study reveals the risk of resistance development with ceftazidime/avibactam against blaOXA-48-like-expressing K. pneumoniae, thereby mandating careful monitoring. Moreover, the study showed that acquisition of ceftazidime/avibactam resistance in K. pneumoniae is mediated through diverse mutational events in genes encoding efflux/impermeability without concomitant fitness cost suggesting possibility of successful survival and dissemination of such mutants. Sub-optimal activity of newer BLI based combinations points towards the multiplicity of resistance mechanisms in ceftazidime/avibactam selected mutants and limitations of BL/BLI approach in overcoming such resistance. The study also revealed the ability of β-lactam enhancer zidebactam in overcoming resistance to diverse BL/BLIs. This observation reinforces the need to continue efforts towards identifying novel Gram-negative therapies with unconventional mechanism of action.
4. Materials and Methods
4.1. Media, Antibiotics and Strains
Mueller–Hinton agar (MHA), cation-adjusted Mueller–Hinton broth (CAMHB) and agar were procured from Difco (Becton Dickinson, Franklin Lakes, NJ, USA. Tryptone soy broth was from Hi-media, India and was used for Tryptic soy agar (TSA) preparation. Ceftazidime, cefepime, imipenem and meropenem were acquired from commercial manufacturers. Various β-lactamase inhibitors and β-lactam enhancer (zidebactam) used in this study were kind gifts from Wockhardt Research Center, India. Ten K. pneumoniae included in the study were collected from Indian tertiary care hospitals during 2018, and were genomically characterized for blaOXA-181/blaOXA-232, ESBLs and class C β-lactamases, based on WGS. The species-level identification for all the parent strains and their respective mutants was undertaken by using VITEK 2.
4.2. Antimicrobial Susceptibility Testing
MIC of ceftazidime/avibactam and various comparator antibiotics (range used: 0.06 to 128 mg/L) was determined following the reference broth microdilution method as described in the Clinical & Laboratory Standards Institute [38,39]. Reference strains such as K. pneumoniae ATCC 700603, K. pneumoniae ATCC 1705 and P. aeruginosa ATCC 27853 were included during each MIC testing. For the sake of comparison purposes, parent and mutant strains were adjudged for their susceptibility to cefepime-based combinations employing susceptibility dose-dependent (SDD) breakpoint of cefepime and for ceftazidime-based combinations, susceptibility breakpoint of ceftazidime-avibactam was employed (both ≤8 mg/L).
4.3. Serial Transfer Studies
The study was initiated by determining macro-broth (2 mL volume of CAMHB) MICs for ceftazidime/avibactam against ten OXA-181- or OXA-232-expressing K. pneumoniae isolates. This was followed by sequential (every 24 h) transfer of inoculum (2–5 × 105 CFU/mL) from 0.25× MIC for the next round of MIC determination [40]. Such sequential transfer was undertaken for 15 days in duplicate. After completion of serial passage cycles, cultures recovered after 5, 10 and 15 day passage were subjected to one drug free passage and preserved at −80 °C.
In order to assess the stability of resistance acquired after sequential ceftazidime/avibactam exposures, mutants were subjected to 15 drug-free passages on TSA followed by determination of MICs of the selecting agents.
4.4. In-Vitro Growth Assessments of Mutants
Comparative growth profiles of terminal mutants of ceftazidime/avibactam with their respective parent strains were determined in M9 minimal media (prepared by mixing NH4Cl, 5 g/L; KH2PO4, 15 g/L; Na2HPO4·7H2O, 64 g/L; NaCl, 2.5 g/L supplemented with glucose, 0.4%; CaCl2, 0.1 mM; MgSO4, 2 mM & pH 7.2) and CAMHB. Overnight grown bacterial cultures were diluted (1:10) in MHB and grown at 37 °C at 180 rpm till the cultures reach mid-log phase. The cultures were then diluted in M9 minimal medium and CAMHB (approximately 105 CFU/mL) and grown under ambient conditions. The growth was monitored by viable cell enumeration on TSA at various time intervals till 12 h.
4.5. Molecular Characterization of Resistance Mechanisms
In order to identify the mutational changes in genes encoding β-lactam impacting enzymatic as well as non-enzymatic resistance, whole genome sequencing (WGS) was performed for terminal mutants and their respective parent strains. In short, genomic DNA (gDNA) was extracted by the alkaline lysis method and quantified by spectrophotometry (Schimadzu). gDNA libraries were prepared by using NEBNext Ultra DNA Library Preparation Kit. Such gDNA libraries were enriched on Agilent DNA HS chip and sequenced by Illumina MiSeq platform (San Diego, CA, USA). Genome sequences were assembled by SOAPdenovo2 v2.0.4 software. The genome sequence of each mutant was compared with the respective parent to identify single nucleotide polymorphisms (SNPs) by genome analysis tool kit. The whole genome sequencing data of all the parent and their respective mutant (except mutant of K. pneumoniae S471 under submission) isolates is deposited at DDBJ/ENA/GenBank under the accession JAHUX(R to Z)000000000 and JAHUY(A to J)000000000.
4.6. Infectivity and Resistance Studies in Murine Peritonitis Model Employing Ceftazidime/Avibactam-Resistant Mutants
In order to assess the impact of in-vitro resistance to ceftazidime/avibactam in blaOXA-48-like-harbouring K. pneumoniae on in-vivo infectivity and expression of resistance, murine peritonitis model was employed. Healthy male or female Swiss albino mice (18–20 g) were utilized for the in-vivo studies. In-vivo study protocols were reviewed and approved by Wockhardt’s animal ethics committee, registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Male/female Swiss albino mice were infected through intra-peritoneal injection with bacterial inoculum (1–3 × 106 CFU/mL) in 0.5 mL volume prepared in 5% hog gastric mucin (Sigma-Aldrich, St. Louis, MO, USA). Both parent and its respective ceftazidime/avibactam selected mutants of two K. pneumoniae strains (CMC307 and CMC387) were employed in this study. Two hours post-infection, animals (n = 6 per group) were dosed subcutaneously with ceftazidime in combination with avibactam at 4:1 ratio at different doses (dose rage of ceftazidime-3.12, 6.25, 12.5, 25, 50, 100 mg/kg; avibactam-0.78, 1.56, 3.12, 6.25, 12.5, 25 mg/kg) (1 dose per group) in 0.2 mL saline. Similarly, meropenem at 100 mg/kg dose (n = 6 animals) was administered. All the antibiotics were administered twice with 5 h interval for one day. Groups of animals (n = 6 per group) infected with parent and respective mutants without drug treatment were treated as placebo control to help assess the effect of in-vitro resistance on in-vivo infectivity.
In this model of infection, post-infection the mice from untreated group developed septicemia and became moribund within 24 h of infection unless they received adequate dose of antibiotic therapy. The efficacy of the antibiotic used in this study was measured using survival as the endpoint, with observation continued for 7 days post one day of antibiotic treatment.
The 50% effective dose (ED50) for the ceftazidime/avibactam treatment arm, were reported in terms of unit dose of ceftazidime. For instance, a 4:1 ceftazidime/avibactam combination ED50 of 20 mg/kg represents 20 mg/kg ceftazidime + 5 mg/kg avibactam. The ED50 values were calculated by log-probit analysis [41].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10111318/s1. Figure S1. Comparative growth kinetics of terminal mutants and their respective parent strains of blaOXA-48-like -harboring K. pneumoniae in CAMHB medium; Figure S2. Comparative growth kinetics of terminal mutants and their respective parent strains of blaOXA-48-like -harboring K. pneumoniae in M9 minimal medium.
Author Contributions
Conceptualization: S.P. and A.S.K.; methodology: S.P.; in-vitro and in-vivo data analyses: S.P.; whole genome sequence analyses: Y.D.B.; writing—original draft preparation: S.P.; writing—review and editing: K.K., K.W. and B.V.; supervision: A.S.K., K.K. and B.V. All authors have read and agreed to the published version of the manuscript.
Funding
The experiments and data analyses undertaken in this study were part of routine work and no external funding was received for the same.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
This study was conducted by S.P. supporting research at the Department of Clinical Microbiology, Christian Medical College, Vellore and Wockhardt Research Centre, India.
References
- Pitout, J.D.D.; Peirano, G.; Kock, M.M.; Strydom, K.A.; Matsumura, Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019, 33, e00102-19. [Google Scholar] [CrossRef]
- Carrër, A.; Poirel, L.; Eraksoy, H.; Cagatay, A.A.; Badur, S.; Nordmann, P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 2008, 52, 2950–2954. [Google Scholar] [CrossRef] [PubMed]
- Kalpoe, J.S.; Al Naiemi, N.; Poirel, L.; Nordmann, P. Detection of an Ambler class D OXA-48-type β-lactamase in a Klebsiella pneumoniae strain in The Netherlands. J. Med. Microbiol. 2011, 60, 677–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Shankar, C.; Mathur, P.; Venkatesan, M.; Pragasam, A.K.; Anandan, S.; Khurana, S.; Veeraraghavan, B. Rapidly disseminating blaOXA-232 carrying Klebsiella pneumoniae belonging to ST231 in India: Multiple and varied mobile genetic elements. BMC Microbiol. 2019, 19, 137. [Google Scholar] [CrossRef]
- Shanthi, M.; Sekar, U.; Arunagiri, K.; Bramhne, H.G. OXA-181 Beta Lactamase is not a Major Mediator of Carbapenem Resistance in Enterobacteriaceae. J. Clin. Diagn. Res. JCDR 2013, 7, 1986–1988. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of Beta-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e01443-17. [Google Scholar] [CrossRef]
- Haidar, G.; Clancy, C.J.; Chen, L.; Samanta, P.; Shields, R.K.; Kreiswirth, B.N.; Nguyen, M.H. Identifying Spectra of Activity and Therapeutic Niches for Ceftazidime-Avibactam and Imipenem-Relebactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e00642-17. [Google Scholar] [CrossRef] [PubMed]
- Alatoom, A.; Elsayed, H.; Lawlor, K.; AbdelWareth, L.; El-Lababidi, R.; Cardona, L.; Mooty, M.; Bonilla, M.-F.; Nusair, A.; Mirza, I. Comparison of antimicrobial activity between ceftolozane–tazobactam and ceftazidime–avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int. J. Infect. Dis. 2017, 62, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Sadouki, Z.; Vickers, A.; Livermore, D.M.; Woodford, N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2020, 64, e01582-20. [Google Scholar] [CrossRef]
- Kidd, J.M.; Livermore, D.M.; Nicolau, D.P. The difficulties of identifying and treating Enterobacterales with OXA-48-like carbapenemases. Clin. Microbiol. Infect. 2020, 26, 401–403. [Google Scholar] [CrossRef]
- Mushtaq, S.; Vickers, A.; Doumith, M.; Ellington, M.J.; Woodford, N.; Livermore, D.M. Activity of β-lactam/taniborbactam (VNRX-5133) combinations against carbapenem-resistant Gram-negative bacteria. J. Antimicrob. Chemother. 2021, 76, 160–170. [Google Scholar] [CrossRef]
- Morrissey, I.; Magnet, S.; Hawser, S.; Shapiro, S.; Knechtle, P. In Vitro Activity of Cefepime-Enmetazobactam against Gram-Negative Isolates Collected from U.S. and European Hospitals during 2014–2015. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Thomson, K.S.; AbdelGhani, S.; Snyder, J.W.; Thomson, G.K. Activity of Cefepime-Zidebactam against Multidrug-Resistant (MDR) Gram-Negative Pathogens. Antibiotics 2019, 8, 32. [Google Scholar] [CrossRef]
- Humphries, R.M.; Yang, S.; Hemarajata, P.; Ward, K.W.; Hindler, J.A.; Miller, S.A.; Gregson, A. First Report of Ceftazidime-Avibactam Resistance in a KPC-3-Expressing Klebsiella pneumoniae Isolate. Antimicrob. Agents Chemother. 2015, 59, 6605–6607. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, K.; Koivula, I.; Ilmavirta, H.; Puranen, S.; Kallonen, T.; Lyytikäinen, O.; Jalava, J. Emergence of ceftazidime-avibactam-resistant Klebsiella pneumoniae during treatment, Finland, December 2018. Eur. Surveill. 2019, 24, 1900256. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Clancy, C.J.; Shields, R.K.; Hao, B.; Cheng, S.; Nguyen, M.H. Mutations in bla(KPC-3) That Confer Ceftazidime-Avibactam Resistance Encode Novel KPC-3 Variants That Function as Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02534-16. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, Q.; Hu, H.; Hong, B.; Wu, X.; Du, X.; Akova, M.; Yu, Y. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin. Microbiol. Infect. 2020, 26, 124.e121–124.e124. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Warner, M.; Jamrozy, D.; Mushtaq, S.; Nichols, W.W.; Mustafa, N.; Woodford, N. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob. Agents Chemother. 2015, 59, 5324–5330. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Bradford, P.A.; Stone, G.G.; de Jonge, B.L.M.; Sahm, D.F. In Vitro Activity of Ceftazidime-Avibactam and Aztreonam-Avibactam against OXA-48-Carrying Enterobacteriaceae Isolated as Part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob. Agents Chemother. 2018, 62, e00592-18. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Doumith, M.; Jamrozy, D.; Nichols, W.W.; Woodford, N. Selection of mutants with resistance or diminished susceptibility to ceftazidime/avibactam from ESBL- and AmpC-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. In Vitro Selection of Meropenem Resistance among Ceftazidime-Avibactam-Resistant, Meropenem-Susceptible Klebsiella pneumoniae Isolates with Variant KPC-3 Carbapenemases. Antimicrob. Agents Chemother. 2017, 61, e00079-17. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis. 2016, 63, 1615–1618. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.B.; Singh, B.B.; Priyadarshi, N.; Chauhan, N.K.; Rajamohan, G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella pneumoniae. PLoS ONE 2014, 9, e96288. [Google Scholar] [CrossRef]
- Tsai, Y.-K.; Fung, C.-P.; Lin, J.-C.; Chen, J.-H.; Chang, F.-Y.; Chen, T.-L.; Siu, L.K. Klebsiella pneumoniae Outer Membrane Porins OmpK35 and OmpK36 Play Roles in both Antimicrobial Resistance and Virulence. Antimicrob. Agents Chemother. 2011, 55, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, A.Y.; Li, Y.; Patidar, R.; Yerex, K.; Ma, X.; Kumar, A.; Duan, K. Two Component Regulatory Systems and Antibiotic Resistance in Gram-Negative Pathogens. Int. J. Mol. Sci. 2019, 20, 1781. [Google Scholar] [CrossRef]
- Both, A.; Büttner, H.; Huang, J.; Perbandt, M.; Belmar Campos, C.; Christner, M.; Maurer, F.P.; Kluge, S.; König, C.; Aepfelbacher, M.; et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J. Antimicrob. Chemother. 2017, 72, 2483–2488. [Google Scholar] [CrossRef]
- Fröhlich, C.; Sørum, V.; Thomassen, A.M.; Johnsen, P.J.; Leiros, H.-K.S.; Samuelsen, Ø. OXA-48-Mediated Ceftazidime-Avibactam Resistance Is Associated with Evolutionary Trade-Offs. mSphere 2019, 4, e00024-19. [Google Scholar] [CrossRef]
- Egorov, A.; Rubtsova, M.; Grigorenko, V.; Uporov, I.; Veselovsky, A. The Role of the Omega-Loop in Regulation of the Catalytic Activity of TEM-Type beta-Lactamases. Biomolecules 2019, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Endimiani, A.; Perez, F.; Bonomo, R.A. Cefepime: A reappraisal in an era of increasing antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 805–824. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Huband, M.; Jones, R.N.; Flamm, R.K. WCK 5222 (Cefepime-Zidebactam) Antimicrobial Activity against Clinical Isolates of Gram-Negative Bacteria Collected Worldwide in 2015. Antimicrob. Agents Chemother. 2017, 61, e00072-17. [Google Scholar] [CrossRef] [PubMed]
- Moya, B.; Barcelo, I.M.; Cabot, G.; Torrens, G.; Palwe, S.; Joshi, P.; Umarkar, K.; Takalkar, S.; Periasamy, H.; Bhagwat, S.; et al. In Vitro and In Vivo Activities of β-Lactams in Combination with the Novel β-Lactam Enhancers Zidebactam and WCK 5153 against Multidrug-Resistant Metallo-β-Lactamase-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2019, 63, e00128-19. [Google Scholar] [CrossRef]
- Moya, B.; Bhagwat, S.; Cabot, G.; Bou, G.; Patel, M.; Oliver, A. Effective inhibition of PBPs by cefepime and zidebactam in the presence of VIM-1 drives potent bactericidal activity against MBL-expressing Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1474–1478. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; Bouchillon, S.K.; Sahm, D.F. In Vitro Activity of WCK 5222 (Cefepime-Zidebactam) against Worldwide Collected Gram-Negative Bacilli Not Susceptible to Carbapenems. Antimicrob. Agents Chemother. 2020, 64, e01432-20. [Google Scholar] [CrossRef]
- Drusano, G.L. Pharmacokinetic optimisation of β-lactams for the treatment of ventilator-associated pneumonia. Eur. Respir. Rev. 2007, 16, 45–49. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791-vii. [Google Scholar] [CrossRef]
- Oh, J.; Schuch, R. Low Propensity of Resistance Development in vitro in Staphylococcus aureus with Lysin CF-301. In Proceedings of the ASM Microbe, New Orleans, LA, USA, 1–5 June 2017. [Google Scholar]
- Finney, D. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).