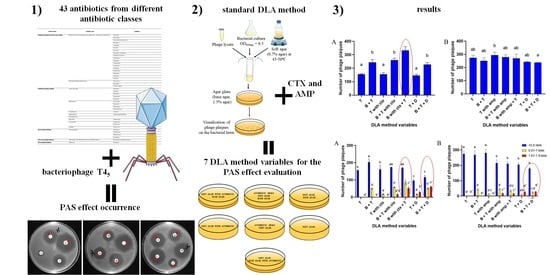
Double-Layer Agar (DLA) Modifications for the First Step of the Phage-Antibiotic Synergy (PAS) Identification
Abstract
1. Introduction
2. Results
2.1. Antibiotic Selection to Enable the Occurrence of the PAS Effect
2.2. Antibiotic Susceptibility Assay
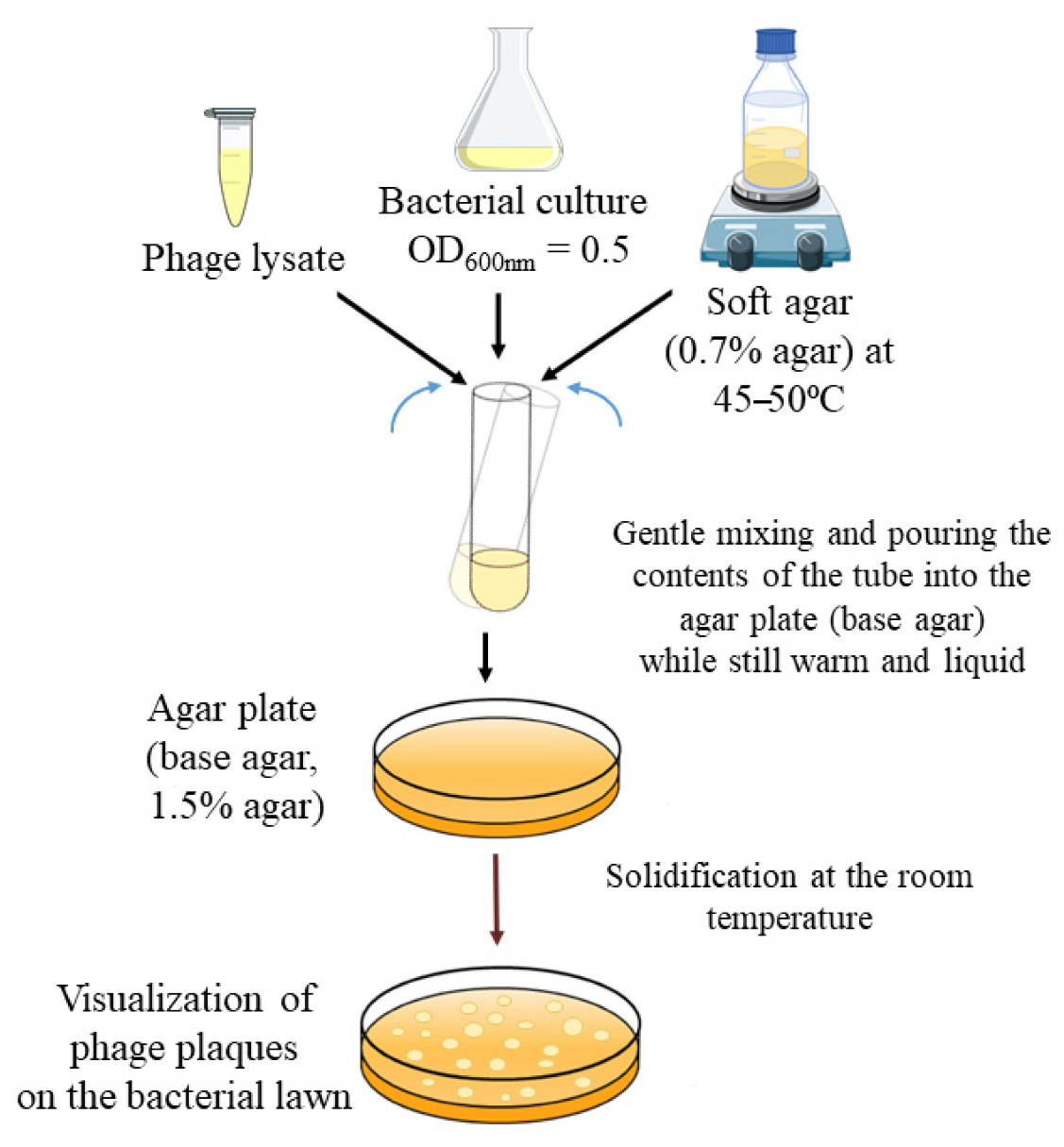
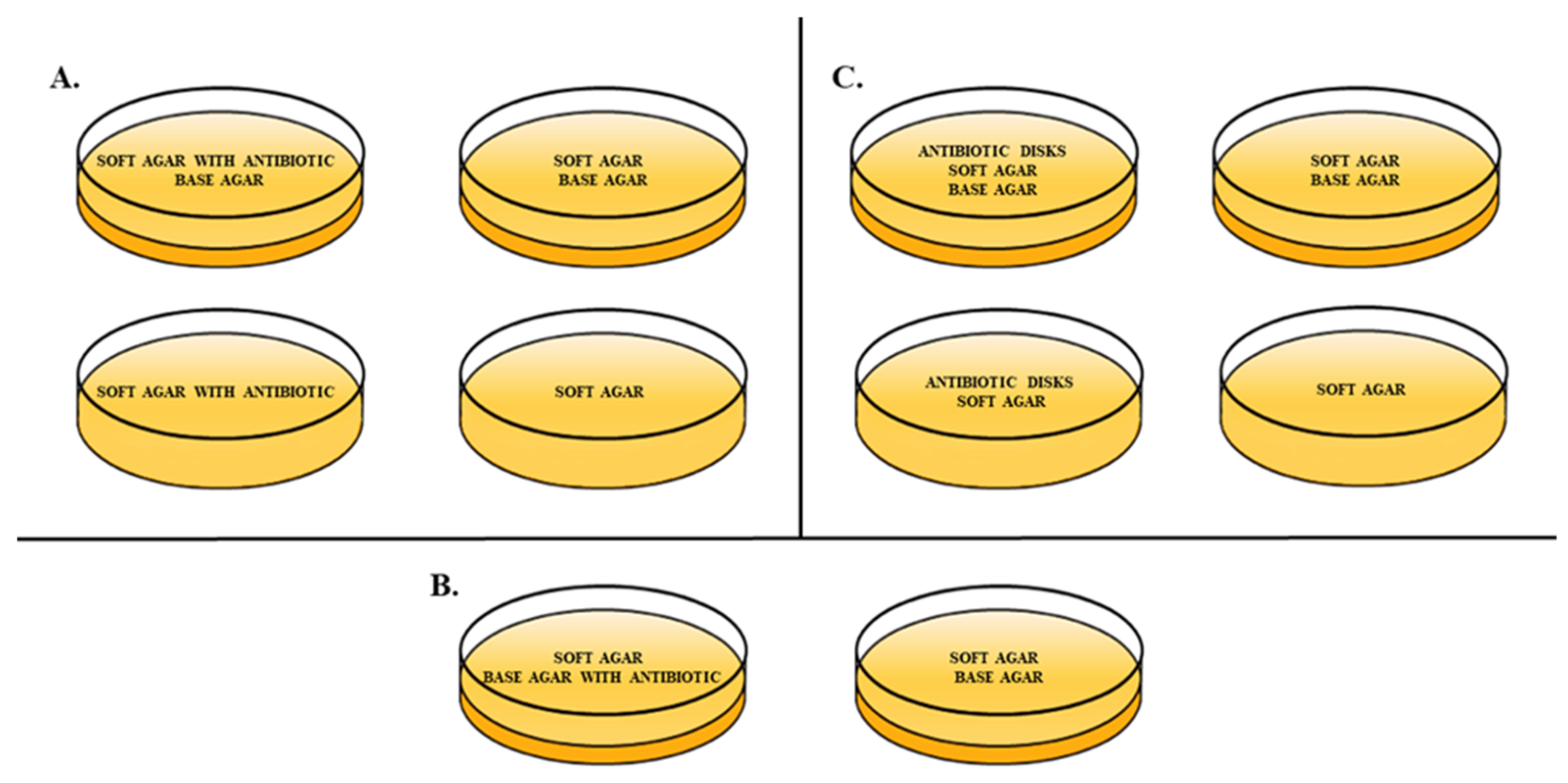
2.3. Double-Layer Agar (DLA) Method Variables
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Bacteriophage Propagation
4.2. Antibiotics Used for PAS Effect Occurrence
4.3. Antibiotics Susceptibility Assay
4.4. Double-Layer Agar (DLA) Method Variables
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Monk, A.B.; Rees, C.D.; Barrow, P.; Hagens, S.; Harper, D.R. Bacteriophage applications: Where are we now? Lett. Appl. Microbiol. 2010, 51, 363–369. [Google Scholar] [CrossRef]
- Ryan, E.M.; Alkawareek, M.Y.; Donnelly, R.F.; Gilmore, B.F. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol. Med. Microbiol. 2012, 65, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.E. Synergistic action of gentamicin and bacteriophage in a continuous culture population of Staphylococcus aureus. PLoS ONE 2012, 7, e51017. [Google Scholar] [CrossRef]
- Coulter, L.B.; McLean, R.J.; Rohde, R.E.; Aron, G.M. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 2014, 6, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.; Dennis, J.J. Burkholderia cepacia complex phage-antibiotic synergy (PAS): Antibiotics stimulate lytic phage activity. Appl. Environ. Microbiol. 2015, 81, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Kim, J.; Ding, T.; Ahn, J. Role of phage-antibiotic combination in reducing antibiotic resistance in Staphylococcus aureus. Food Sci. Biotechnol. 2016, 25, 1211–1215. [Google Scholar] [CrossRef]
- Nouraldin, A.A.M.; Baddour, M.M.; Harfoush, R.A.H.; Essa, S.A.M. Bacteriophage-antibiotic synergism to control planktonic and biofilm producing clinical isolates of Pseudomonas aeruginosa. Alex. J. Med. 2016, 52, 99–105. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prere, M.F.; Krisch, H.M. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.P. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.B.; Carvalho, C.M.; Sillankorva, S.; Nicolau, A.; Ferreira, E.C.; Azeredo, J. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Łoś, J.M.; Golec, P.; Węgrzyn, G.; Węgrzyn, A.; Łoś, M. Simple method for plating Escherichia coli bacteriophages forming very small plaques or no plaques under standard conditions. Appl. Environ. Microbiol. 2008, 74, 5113–5120. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Price, W.H. Bacteriophage formation without bacterial growth: I. Formation of Staphylococcus phage in the presence of bacteria inhibited by penicillin. J. Gen. Physiol. 1947, 31, 119. [Google Scholar] [CrossRef][Green Version]
- Krueger, A.P.; Cohn, T.; Smith, P.N.; McGuire, C.D. Observations on the effect of penicillin on the reaction between phage and staphylococci. J. Gen. Physiol. 1948, 31, 477–488. [Google Scholar] [CrossRef]
- Hadas, H.; Einav, M.; Fishov, I.; Zaritsky, A. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology 1997, 143, 179–185. [Google Scholar] [CrossRef]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr. Opin. Microbiol. 2019, 51, 46–50. [Google Scholar] [CrossRef]
- Lopes, A.; Pereira, C.; Almeida, A. Sequential combined effect of phages and antibiotics on the inactivation of Escherichia coli. Microorganisms 2018, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.P.U.; Wallace, A.T.; Murdoch, J.M. Ampicillin in treatment of certain gram-negative bacterial infections. Br. Med. J. 1963, 2, 962. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. In Bacteriophages. Methods in Molecular Biology; Clokie, M.R., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 69–76. [Google Scholar]
- Jo, A.; Ding, T.; Ahn, J. Synergistic antimicrobial activity of bacteriophages and antibiotics against Staphylococcus aureus. Food Sci. Biotechnol. 2016, 25, 935–940. [Google Scholar] [CrossRef]
- Uchiyama, J.; Shigehisa, R.; Nasukawa, T.; Mizukami, K.; Takemura-Uchiyama, I.; Ujihara, T.; Murakami, H.; Imanishi, I.; Nishifuji, K.; Sakaguchi, M.; et al. Piperacillin and ceftazidime produce the strongest synergistic phage–antibiotic effect in Pseudomonas aeruginosa. Arch. Virol. 2018, 163, 1941–1948. [Google Scholar] [CrossRef]
- Kaur, S.; Harjai, K.; Chhibber, S. Methicillin-resistant Staphylococcus aureus phage plaque size enhancement using sublethal concentrations of antibiotics. Appl. Environ. Microbiol. 2012, 78, 8227–8233. [Google Scholar] [CrossRef]
- Abedon, S.T. Detection of bacteriophages: Phage plaques. In Bacteriophages: Biology, Technology, Therapy; Harper, D., Abedon, S., Burrowes, B., McConville, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 9–10. [Google Scholar]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplanc, H.B.; Trautnera, B.W.; Ramig, R.F.; Maresso, A.W. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef] [PubMed]
- Payne, O.B.; Ericson, J.E. Chapter 2—Empiric Antimicrobials for Neonatal Sepsis. In Infectious Disease and Pharmacology; Benitz, W.E., Smith, P.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–25. [Google Scholar]
- Appleyard, R.K. Segregation of new lysogenic types during growth of a doubly lysogenic strain derived from Escherichia coli K12. Genetics 1954, 39, 440–452. [Google Scholar] [CrossRef] [PubMed]



| Antibiotic (Abbreviation and Concentration) | Probability of the PAS Effect Occurrence | Antibiotic (Abbreviation and Concentration) | Probability of the PAS Effect Occurrence | Antibiotic (Abbreviation and Concentration) | Probability of the PAS Effect Occurrence |
|---|---|---|---|---|---|
| ATM 30 | + | AMC 30 | + | UB 30 | + |
| PRL 30 | + | CTX 30 | ++ | DA 2 | / |
| CN 10 | - | CAZ 30 | ++ | VA 30 | / |
| CXM 30 | / | CPD 30 | + | CFP 75 | + |
| NOR 10 | / | SXT 25 | + | CL 30 | / |
| AML 25 | / | TGC 15 | - | TE 30 | - |
| TOB 10 | / | TPZ 36 | + | LNZ 30 | / |
| AK 30 | / | TC 75 | + | FA 10 | / |
| SYN 15 | / | MEM 10 | + | RA 5 | / |
| AUG 3 | / | FOR CYL | + | TEC 30 | / |
| CEF 30 | / | DO 30 | - | S 300 | - |
| OB 5 | / | OX 1 | / | K 30 | - |
| C 30 | / | E 15 | / | CIP 5 | + |
| FOX 30 | + | P 10 | / | MY 15 | / |
| CT 25 | + | OT 30 | / |
| Mode of Action | Antibiotic Class | Antibiotic | Antibiotic Abbreviation | Company of Origin |
|---|---|---|---|---|
| Inhibition/disruption of the cell wall synthesis | β-lactams (penicillins, cephalosporins, cephamycins, carbapenems, monobactams) | Piperacillin | PRL | Oxoid |
| Amoxicillin | AML | Oxoid | ||
| Amoxicillin/clavulanic acid | AMC | Oxoid | ||
| Penicillin G | P | Oxoid | ||
| Cloxacillin | OB | Oxoid | ||
| Oxacillin | OX | BioMaxima | ||
| Ticarcillin | TC | Oxoid | ||
| Piperacillin/tazobactam | TPZ | Oxoid | ||
| Cefacetril 30 masticef | CEF | BioMaxima | ||
| Ceftazidime | CAZ | BioMaxima | ||
| Cephalexin | CL | Emapol | ||
| Cefoperazone | CFP | Oxoid | ||
| Cefuroxime | CXM | Oxoid | ||
| Cefotaxime | CTX | BioMaxima | ||
| Cefpodoxime | CPD | Emapol | ||
| Meropenem | MEM | Oxoid | ||
| Aztreonam | ATM | BioMaxima | ||
| Cefoxitin | FOX | BioMaxima | ||
| Other (glycopeptides, polymyxins) | Vancomycin | VA | Oxoid | |
| Teicoplanin | TEC | Oxoid | ||
| Colistin sulfate | CT | Emapol | ||
| Inhibition of protein synthesis | Amino-glycosides | Gentamicin | CN | Oxoid |
| Amikacin | AK | Oxoid | ||
| Tobramycin | TOB | Oxoid | ||
| Streptomycin | S | Oxoid | ||
| Kanamycin | K | Oxoid | ||
| Tetracyclines | Doxycycline | DO | Oxoid | |
| Tigecycline | TGC | Oxoid | ||
| Tetracycline | TE | Oxoid | ||
| Oxytetracycline | OT | Oxoid | ||
| Oxazilidinones | Linezolid | LNZ | BioMaxima | |
| Streptogramins | Chinopristina/dalfopristin | SYN | BioMaxima | |
| Chloramphenicol | C | BioMaxima | ||
| Macrolides | Erythromycin | E | Oxoid | |
| Lincosamides | Clindamycin | DA | BioMaxima | |
| Lincomycin | MY | Oxoid | ||
| Fusidanes | Fusidic acid | FA | BioMaxima | |
| DNA synthesis inhibitors | Fluoroquinolones | Norfloxacin | NOR | Oxoid |
| Ciprofloxacin | CIP | Oxoid | ||
| Marbofloxacin | FOR CYL | BioMaxima | ||
| Flumequine | UB | Oxoid | ||
| Folic acid synthesis inhibitors | Sulfonamides with dihydrofolate reductase (DHFR) inhibitor | Trimethoprim-sulfamethoxazole | SXT | Oxoid |
| RNA synthesis inhibitors | Rifamycins | Rifampicin | RA | BioMaxima |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachurska, X.; Roszak, M.; Jabłońska, J.; Mizielińska, M.; Nawrotek, P. Double-Layer Agar (DLA) Modifications for the First Step of the Phage-Antibiotic Synergy (PAS) Identification. Antibiotics 2021, 10, 1306. https://doi.org/10.3390/antibiotics10111306
Stachurska X, Roszak M, Jabłońska J, Mizielińska M, Nawrotek P. Double-Layer Agar (DLA) Modifications for the First Step of the Phage-Antibiotic Synergy (PAS) Identification. Antibiotics. 2021; 10(11):1306. https://doi.org/10.3390/antibiotics10111306
Chicago/Turabian StyleStachurska, Xymena, Marta Roszak, Joanna Jabłońska, Małgorzata Mizielińska, and Paweł Nawrotek. 2021. "Double-Layer Agar (DLA) Modifications for the First Step of the Phage-Antibiotic Synergy (PAS) Identification" Antibiotics 10, no. 11: 1306. https://doi.org/10.3390/antibiotics10111306
APA StyleStachurska, X., Roszak, M., Jabłońska, J., Mizielińska, M., & Nawrotek, P. (2021). Double-Layer Agar (DLA) Modifications for the First Step of the Phage-Antibiotic Synergy (PAS) Identification. Antibiotics, 10(11), 1306. https://doi.org/10.3390/antibiotics10111306