Evaluation of Dietary Curcumin Nanospheres in a Weaned Piglet Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals and Reagents
2.3. Preparation of Curcumin Nanospheres (CN)
2.4. Addition of CN in the Diets of Piglets
2.5. Animals and Experimental Design
2.6. Sample Collection and Analyses of Piglets
2.6.1. Growth Parameters
2.6.2. Serum Biochemical Composition
2.6.3. Serum Proteome Analysis
Sample Preparation
Gel Electrophoresis
Nano-LC-MS/MS Analysis
Data Search and Criteria for Protein Identification
Gene Ontology (GO) and Enrichment Pathway Analyses
Protein–Protein Interaction (PPI) Network Analysis
2.6.4. Fecal Noxious Gases and Pathogenic Bacteria Contents
2.7. Statistical Analysis
3. Results
3.1. Growth and Feed Intake of Piglets
3.2. Serum Biochemistry of Piglets
3.3. Serum Proteomics Analysis
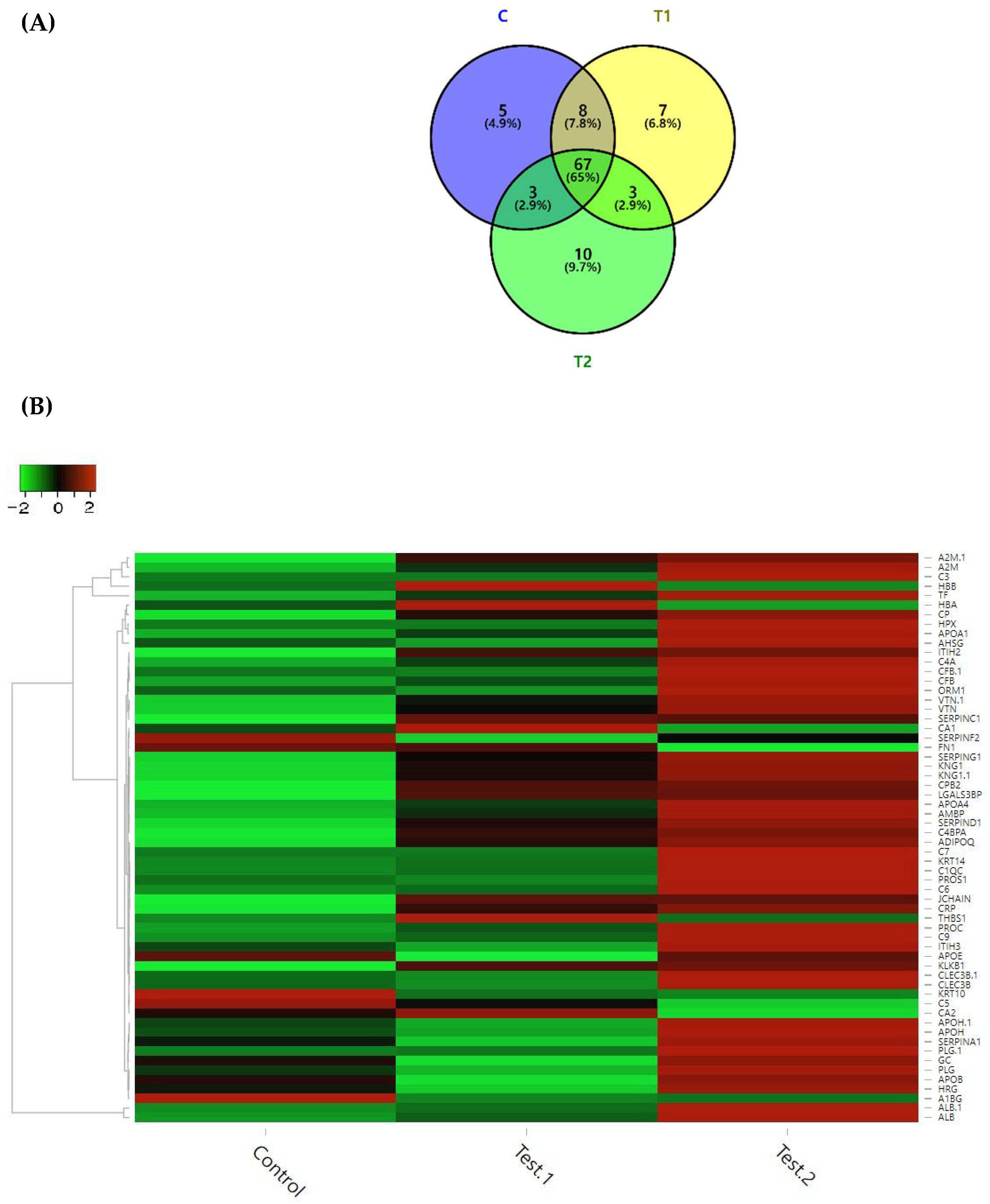
3.3.1. Identification of Differentially Expressed Proteins (DEPs)
3.3.2. Gene Ontology (GO) Terms of DEPs
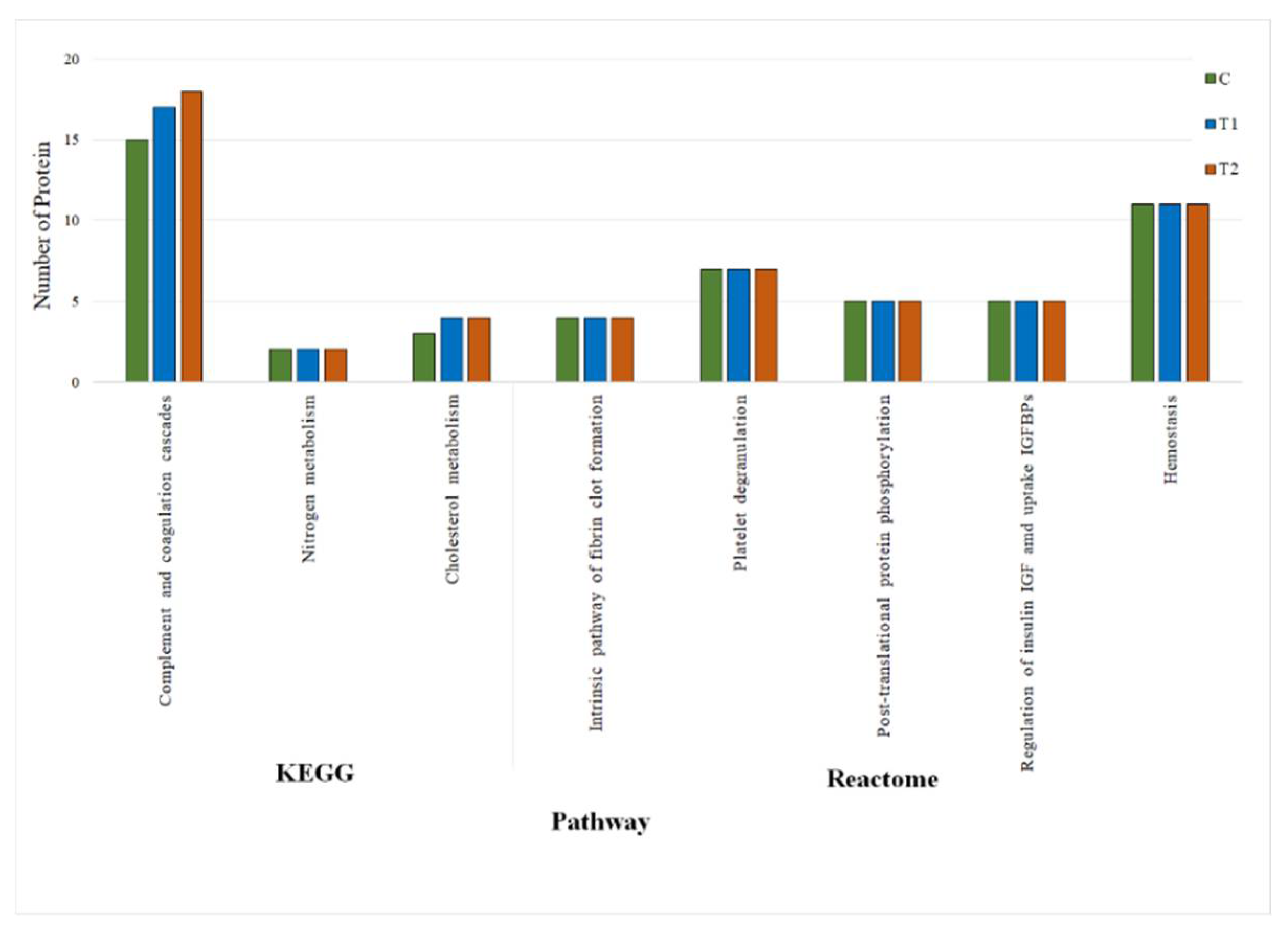
3.3.3. KEGG and REACTOME Pathway Analyses of DEPs
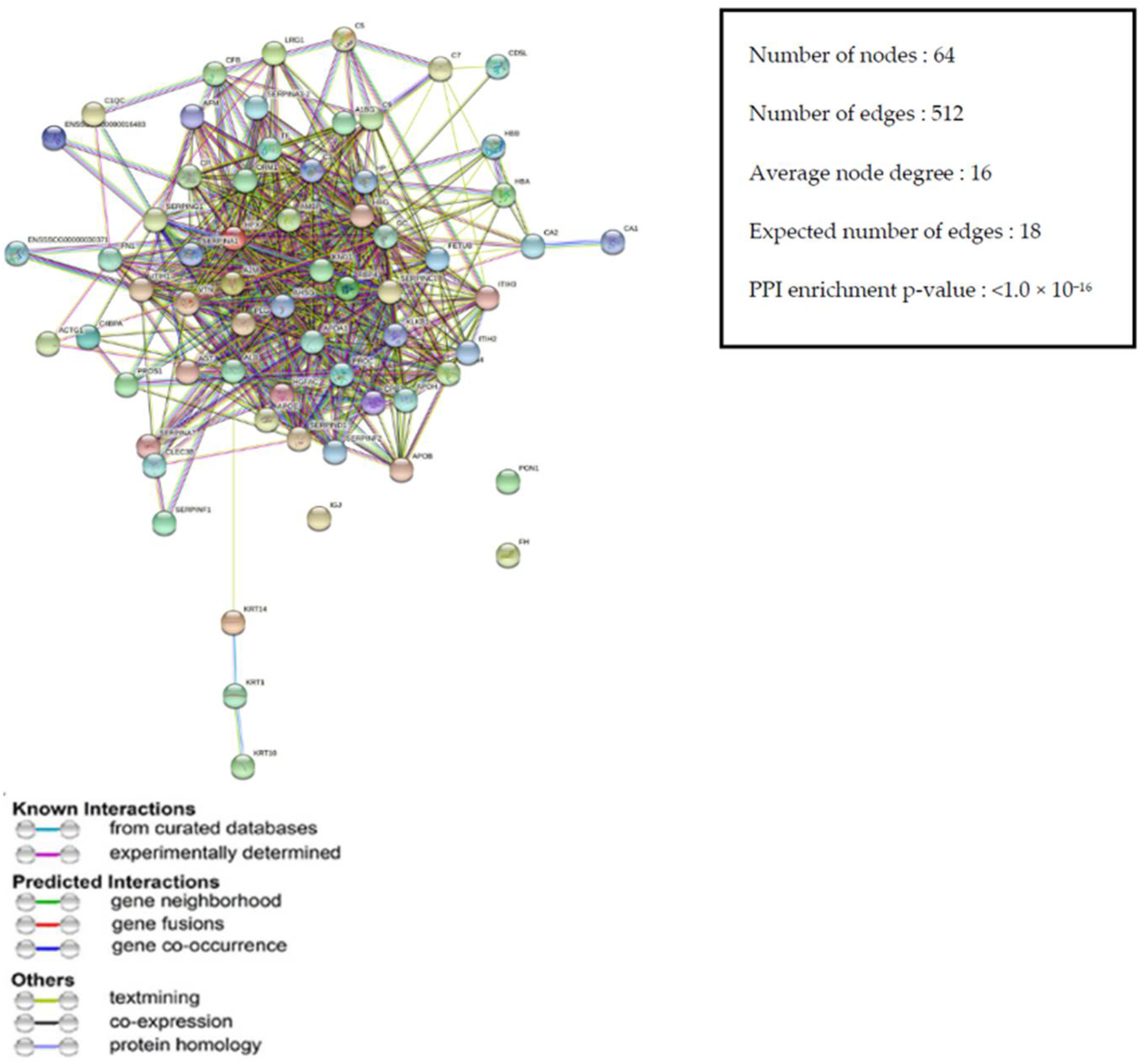
3.3.4. Protein–Protein Interaction (PPI) Network of DEPs
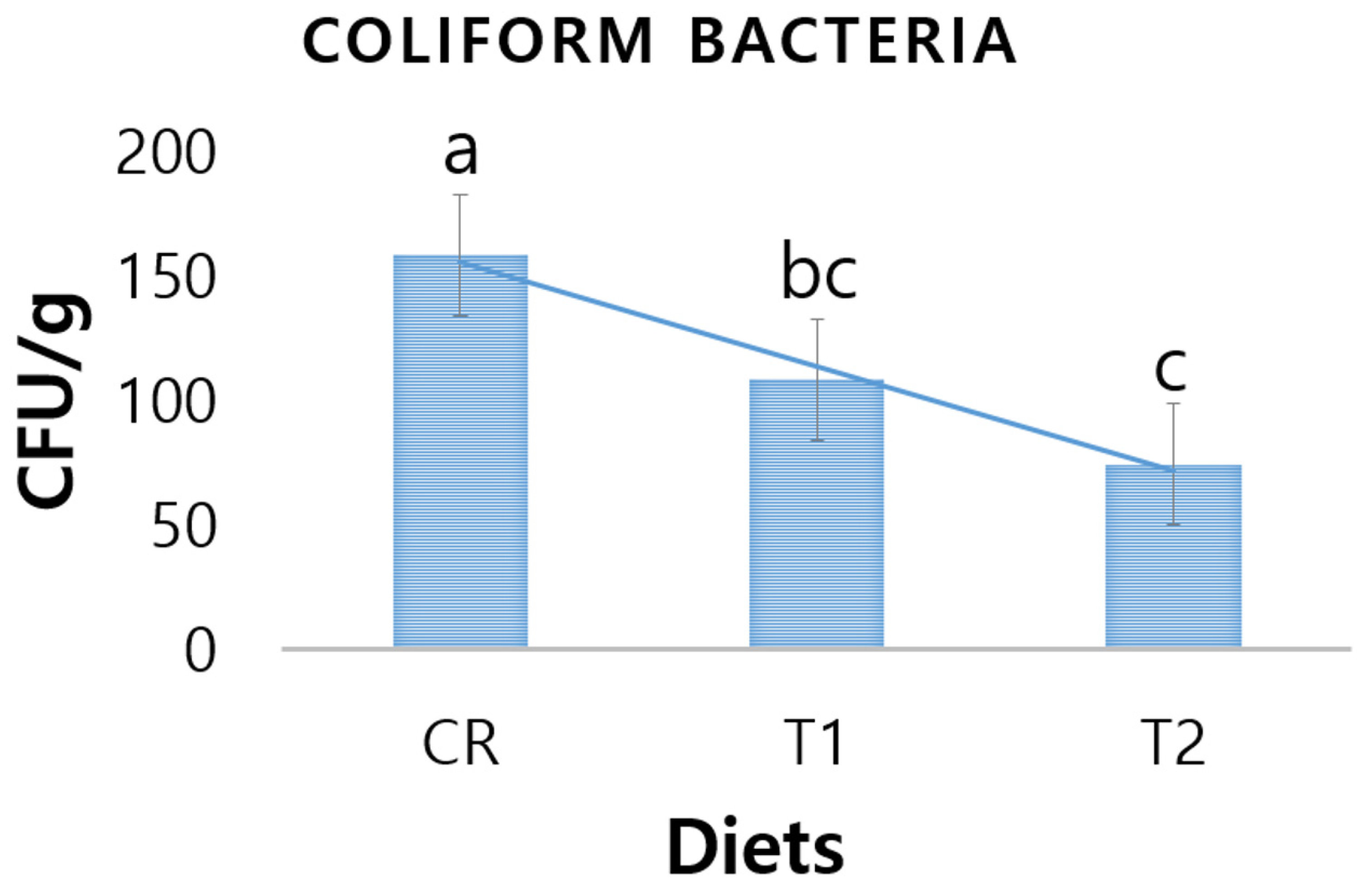
3.4. Fecal Coliform Bacteria
3.5. Fecal Ammonia and Hydrogen Peroxide
4. Discussion
4.1. Growth Performance of Piglets
4.2. Serum Biochemistry of Piglets
4.3. Serum Proteomics of Piglets
4.4. Fecal Pathogenic Bacteria of Piglets
4.5. Fecal Malodors of Piglets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.; Kim, N.Y.; Moniruzzaman, M.; Beyene, A.M.; Do, K.T.; Senthil, K.S.; Min, T. Curcumin and its modified formulations on inflammatory bowel disease (IBD); the sory so far and future outlook. Pharmaceutics 2021, 12, 447. [Google Scholar]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Int. Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. (In Copenh) [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Tzeng, C.W.; Lin, L.T.; Lin, C.C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef] [Green Version]
- Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S. Therapeutic application of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-Epoxide-Loaded SLNs: Evaluation of Drug Release, Antioxidant Activity, and Cytotoxicity in an HaCaT Cell Line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J. Agric. Food Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: An in vitro and in vivo study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Tang, J.; Li, M.; Ren, J.; Zheng, N.; Wu, L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Antican. Res. 2009, 29, 3867–3876. [Google Scholar]
- Klippstein, R.; Wang, J.T.W.; El-Gogary, R.I.; Bai, J.; Mustafa, F.; Rubio, N.; Bansal, S.; Al-Jamal, W.T.; Al-Jamal, K.T. Passively targeted curcumin-loaded PEGylated PLGA nanocapsules for colon cancer therapy in vivo. Small 2015, 11, 4704–4722. [Google Scholar] [CrossRef] [Green Version]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Verma, V.; Ryan, K.M.; Padrela, L. Production and isolation of Pharmaceutical drug nanoparticles. Int. J. Pharm. 2021, 603, 120708. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Porfire, A.S.; Tefas, L.R.; Boarescu, P.M.; Bolboaca, S.D.; Stanescu, I.C.; Bulboaca, A.C.; Dogaru, G. Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus. Molecules 2019, 24, 846. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Pan, W.; Wang, Y.; Lei, W.; Feng, B.; Du, C.; Wang, X. Enhanced efficacy of curcumin with phosphatidylserine-decorated nanoparticles in the treatment of hepatic fibrosis. Drug Del. 2018, 25, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef]
- Marcon, H.; Griss, L.G.; Molosse, V.L.; Cecere, B.G.O.; Alba, D.F.; Leal, K.W.; Galli, G.M.; Souza, C.F.; Baldissera, M.D.; Gundel, S.; et al. Dietary supplementation with curcumin-loaded nanocapsules in lambs: Nanotechnology as a new tool for nutrition. Anim. Nutr. 2021, 7, 521–529. [Google Scholar] [CrossRef]
- Jaguezeski, A.M.; Gündel, S.S.; Favarin, F.R.; Gündel, A.; Souza, C.F.; Baldissera, M.D.; Cazarotto, C.C.; Volpato, A.; Fortuoso, B.F.; Ourique, A.F. Low-dose curcumin-loaded Eudragit L-100-nanocapsules in the diet of dairy sheep increases antioxidant levels and reduces lipid peroxidation in milk. J. Food Eng. 2019, 43, e12942. [Google Scholar] [CrossRef]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharm. 2015, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Anim. Sci. 2014, 92, 3426–3440. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Ji, P.; Song, M.; Che, T.M.; Bravo, D.; Pettigrew, J.E.; Liu, Y. Dietary plant extracts modulate gene expression profiles in alveolar macrophages of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. Biotechnol. 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.M.; Kim, D.W.; Min, T.; Lee, S.J. Nanosphere Loaded with Curcumin Inhibits the Gastrointestinal Cell Death Signaling Pathway Induced by the Foodborne Pathogen Vibrio Vulnificus. Cells 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Min, T.; Lee, S.J. Nanospheres loaded with curcumin promote gut epithelial motility through F-actin-related migration signaling events. J. Nutr. Biochem. 2021, 88, 108555. [Google Scholar] [CrossRef]
- Kim, D.W.; Choi, C.-H.; Park, J.P.; Lee, S.-J. Nanospheres Loaded with Curcumin Improve the Bioactivity of Umbilical Cord Blood-Mesenchymal Stem Cells via c-Src Activation during the Skin Wound Healing Process. Cells 2020, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Kim, I.H. Evaluation of coated zinc oxide in young pigs challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 2020, 262, 114399. [Google Scholar] [CrossRef]
- Kim, J.A.; Vetrivel, P.; Kim, S.M.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Heo, J.D.; Lee, W.S.; Senthil, K.; Kim, G.S. Quantitative Proteomics Analysis for the Identification of Differential Protein Expression in Calf Muscles between Young and Old SD Rats Using Mass Spectrometry. ACS Omega 2021, 6, 7422–7433. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadiny, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of Dietary Supplementation of Biological Curcumin Nanoparticles on Growth and Carcass Traits, Antioxidant Status, Immunity and Caecal Microbiota of Japanese Quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef]
- Rahmani, M.; Golian, A.; Kermanshahi, H.; Bassami, M.R. Effects of curcumin and nanocurcumin on growth performance, blood gas indices and ascites mortalities of broiler chickens reared under normal and cold stress conditions. Ital. J. Anim. Sci. 2017, 16, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Marchiori, M.S.; Oliveira, R.C.; Souza, C.F.; Baldissera, M.D.; Ribeiro, Q.M.; Wagner, R.; Gundel, S.S.; Ourique, A.F.; Kirinus, J.K.; Stefani, L.M.; et al. Curcumin in the diet of quail in cold stress improves performance and egg quality. Anim. Feed Sci. Technol. 2019, 254, 144–157. [Google Scholar] [CrossRef]
- Mitruka, B.M.; Rawnsley, H.M. Clinical Biochemical and Hematological Reference Values in Normal Experimental Animals and Normal Humans; Masson Publishing: Masson, NY, USA, 1981. [Google Scholar]
- Amara, U.; Rittirsch, D.; Flier, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction Between the Coagulation and Complement System. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar]
- Boone, C.D.; Gill, S.; Habibzadegan, A.; McKenna, R. Carbonic Anhydrase: An Efficient Enzyme with Possible Global Implications. Int. J. Chem. Eng. 2013, 2013, 813931. [Google Scholar] [CrossRef] [Green Version]
- Collett, J.R.; Heck, R.W.; Zwoster, A.J. Dissolved carbonic anhydrase for enhancing post-combustion carbon dioxide hydration in aqueous ammonia. Energy Proc. 2011, 4, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabue, S.; Kerr, B.; Bearson, B.; Ziemer, C. Swine odor analyzed by odor panels and chemical techniques. J. Environ. Qual. 2011, 40, 1510–1520. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, Y.M.; Park, M.K.; Min, T.S.; Lee, S.J. Effects of anti-ecotoxicological curcumin nanospheres on feed efficiency and fecal odor in mice. J. Environ. Sci. Int. 2019, 28, 183–189. [Google Scholar] [CrossRef]

| Items | Dietary Treatments | p-Value | ||
|---|---|---|---|---|
| Control, C | T1 (0.5 mL CN/kg) | T2 (1.0 mL CN/kg) | ||
| FW 2 | 13.60 ± 0.04 b | 14.66 ± 0.76 ab | 15.44 ± 0.35 a | 0.0098 |
| WG 3 | 5.98 ± 0.55 b | 6.92 ± 0.46 ab | 7.68 ± 0.59 a | 0.0228 |
| ADG 4 | 0.285 ± 0.03 b | 0.329 ± 0.02 ab | 0.366 ± 0.03 a | 0.0223 |
| FI 5 | 9.76 ± 0.06 a | 10.07 ± 0.45 a | 10.65 ± 0.51 a | 0.0778 |
| ADFI 6 | 0.465 ± 0.00 a | 0.479 ± 0.02 a | 0.507 ± 0.02 a | 0.0787 |
| FE 7 | 61.28 ± 5.53 b | 68.65 ± 1.60 ab | 72.08 ± 2.18 a | 0.0251 |
| FCR 8 | 1.64 ± 0.14 a | 1.46 ± 0.03 ab | 1.38 ± 0.04 b | 0.0280 |
| Items | Dietary Treatments | p-Value | ||
|---|---|---|---|---|
| Control, C | T1 (0.5 mL CN/kg) | T2 (1.0 mL CN/kg) | ||
| GLU | 107.3 ± 0.6 a | 106.7 ± 0.6 a | 106.7 ± 0.6 a | 0.331 |
| CRT | 1.2± 0.1 b | 1.3 ± 0.1 b | 1.5± 0.1 a | 0.002 |
| BUN | 7.7± 0.6 b | 8.3 ± 0.6 ab | 9.3 ± 0.6 a | 0.033 |
| BUN:CRT | 6.4 ± 0.3 a | 6.2 ± 0.1 a | 6.2 ± 0.3 a | 0.448 |
| IP | 8.7 ± 0.1 c | 9.2 ± 0.1 b | 9.8 ± 0.1 a | 0.001 |
| Ca | 10.3 ± 0.1 c | 11.2 ± 0.1 b | 11.5 ± 0.2 a | 0.001 |
| T-Pro | 6.8 ± 0.1 b | 6.8 ± 0.1 b | 7.0 ± 0.1 a | 0.001 |
| ALB | 2.8 ± 0.1 c | 3.3 ± 0.2 b | 4.2 ± 0.1 a | 0.001 |
| GLB | 4.1 ± 0.1 a | 4.1 ± 0.1 a | 4.0 ± 0.1 a | 0.317 |
| ALB:GLB | 0.7 ± 0.1 a | 0.8 ± 0.1 a | 0.9 ± 0.1 a | 0.072 |
| ALT | 27.0 ± 1.0 b | 30.0 ± 2.0 ab | 33.0 ± 2.0 a | 0.015 |
| ALKP | 85.0 ± 1.0 b | 86.3 ± 1.0 b | 91.0 ± 1.0 a | 0.010 |
| GGT | 8.0 ± 1.0 a | 8.0 ± 1.0 a | 8.0 ± 1.0 a | 1.000 |
| TBIL | 0.4 ± 0.1 a | 0.3 ± 0.1 a | 0.4 ± 0.1 a | 0.422 |
| TCHOL | 145.7 ± 2.1 a | 133.7 ± 1.5 b | 124.7 ± 1.2 c | 0.001 |
| AMYL | 126.3 ± 1.5 a | 123.0 ± 1.0 a | 125.7 ± 1.5 a | 0.054 |
| LYPS | 1339.3 ± 17.8 a | 1335.3 ± 18.1 a | 1358.0 ± 7.0 a | 0.230 |
| Regulation | Acsession Number | Protein Name | Gene Symbol | Log2 (FC) 1 | Main Function | |
|---|---|---|---|---|---|---|
| T1 | T2 | |||||
| Up | F1SJT7 | Apolipoprotein A-IV | APOA4 | 0.92 | 1.96 | Lipid metabolism |
| F2Z5E2 | Antithrombin-III | SERPINC1 | 7.93 | 7.84 | Regulate blood coagulation | |
| Q9GLP2 | Vitamin K-dependent protein C | PROC | 3.17 | 5.35 | Regulate blood coagulation | |
| F1SMI8 | Complement C6 | C6 | 1.00 | 3.45 | Innate and adaptive immune response | |
| A0A4X1U519 | C1q domain-containing protein | ADIPOQ | 3.80 | 4.39 | Cellular response to drug | |
| O19062 | Pentaxin | CRP | 2.58 | 3.00 | Innate immune response | |
| F1RUQ0 | Immunoglobulin J chain | JCHAIN | 3.00 | 3.00 | Secretion of IgA and IgM into mucosa | |
| A0A286ZSJ7 | Complement C1q subcomponent subunit C | C1QC | 1.00 | 4.00 | Innate immune system | |
| A0A287AXN9 | IF rod domain-containing protein | KRT14 | 1.00 | 4.08 | Fibrous protein in cells of skin, hair, nail | |
| F1SS26 | Thrombospondin-1 | THBS1 | 4.00 | 1.00 | Mediates cell-to-cell and cell-to-matrix interactions | |
| F1SMJ6 | Complement component C9 | C9 | 0.28 | 1.44 | Innate immune system | |
| P48819 | Vitronectin | VTN | 0.74 | 1.29 | Inhibit cell membrane damage | |
| F1SN68 | Alpha-1-acid glycoprotein | ORM1 | −0.28 | 1.06 | Transport protein in blood stream | |
| F1SK70 | Vitamin K-dependent protein S | PROS1 | −0.58 | 2.50 | Prevent coagulation and stimulate fibrinolysis | |
| Down | F1SS24 | Fibronectin | FN1 | −0.14 | −1.93 | Blood coagulation, fibrin cloat formation |
| Q6VPV1 | Complement C5a anaphylatoxin | C5 | −0.54 | −1.36 | Smooth muscle contraction, basophil and mast cell degranulation | |
| I3LDS3 | Keratin 10 | KRT10 | −1.13 | −1.23 | Fibrous protein in cells of skin, hair, nail | |
| F1RXC2 | Carbonic anhydrase 2 isoform 1 | CA2 | 0.74 | −4.95 | Intracellular pH regulation in intestine | |
| Items | Dietary Treatments | p-Value | ||
|---|---|---|---|---|
| Control, C | T1 (0.5 mL CN/kg) | T2 (1.0 mL CN/kg) | ||
| Ammonia (ppm) | 6.33 ± 0.58 a | 1.33 ± 0.58 b | 1.67 ± 0.58 b | 0.0001 |
| Hydrogen sulfide (ppm) | 0.27 ± 0.06 a | 0.17 ± 0.06 a | 0.27 ± 0.06 a | 0.1250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniruzzaman, M.; Kim, H.; Shin, H.; Kim, H.; Kim, N.; Chin, S.; Karthikeyan, A.; Choi, H.; Kim, G.; Min, T. Evaluation of Dietary Curcumin Nanospheres in a Weaned Piglet Model. Antibiotics 2021, 10, 1280. https://doi.org/10.3390/antibiotics10111280
Moniruzzaman M, Kim H, Shin H, Kim H, Kim N, Chin S, Karthikeyan A, Choi H, Kim G, Min T. Evaluation of Dietary Curcumin Nanospheres in a Weaned Piglet Model. Antibiotics. 2021; 10(11):1280. https://doi.org/10.3390/antibiotics10111280
Chicago/Turabian StyleMoniruzzaman, Mohammad, Hunhwan Kim, Haewon Shin, Hyunsoo Kim, Nayoung Kim, Sungyeon Chin, Adhimoolam Karthikeyan, Hyojick Choi, Gonsup Kim, and Taesun Min. 2021. "Evaluation of Dietary Curcumin Nanospheres in a Weaned Piglet Model" Antibiotics 10, no. 11: 1280. https://doi.org/10.3390/antibiotics10111280
APA StyleMoniruzzaman, M., Kim, H., Shin, H., Kim, H., Kim, N., Chin, S., Karthikeyan, A., Choi, H., Kim, G., & Min, T. (2021). Evaluation of Dietary Curcumin Nanospheres in a Weaned Piglet Model. Antibiotics, 10(11), 1280. https://doi.org/10.3390/antibiotics10111280










