Assessing Reduction of Antibiotic Prescribing for Acute, Non-Complicated Infections in Primary Care in Germany: Multi-Step Outcome Evaluation in the Cluster-Randomized Trial ARena
Abstract
:1. Introduction
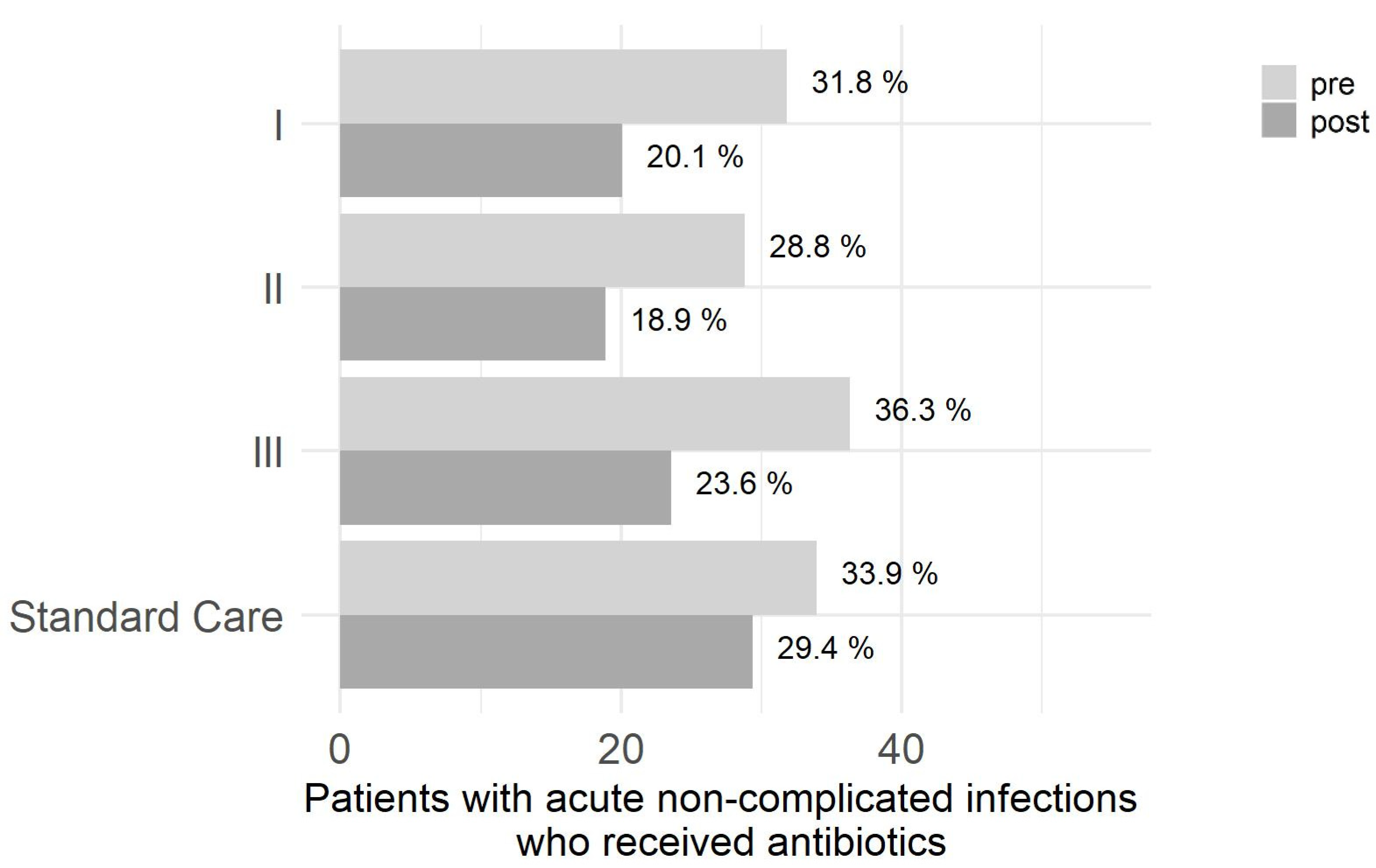
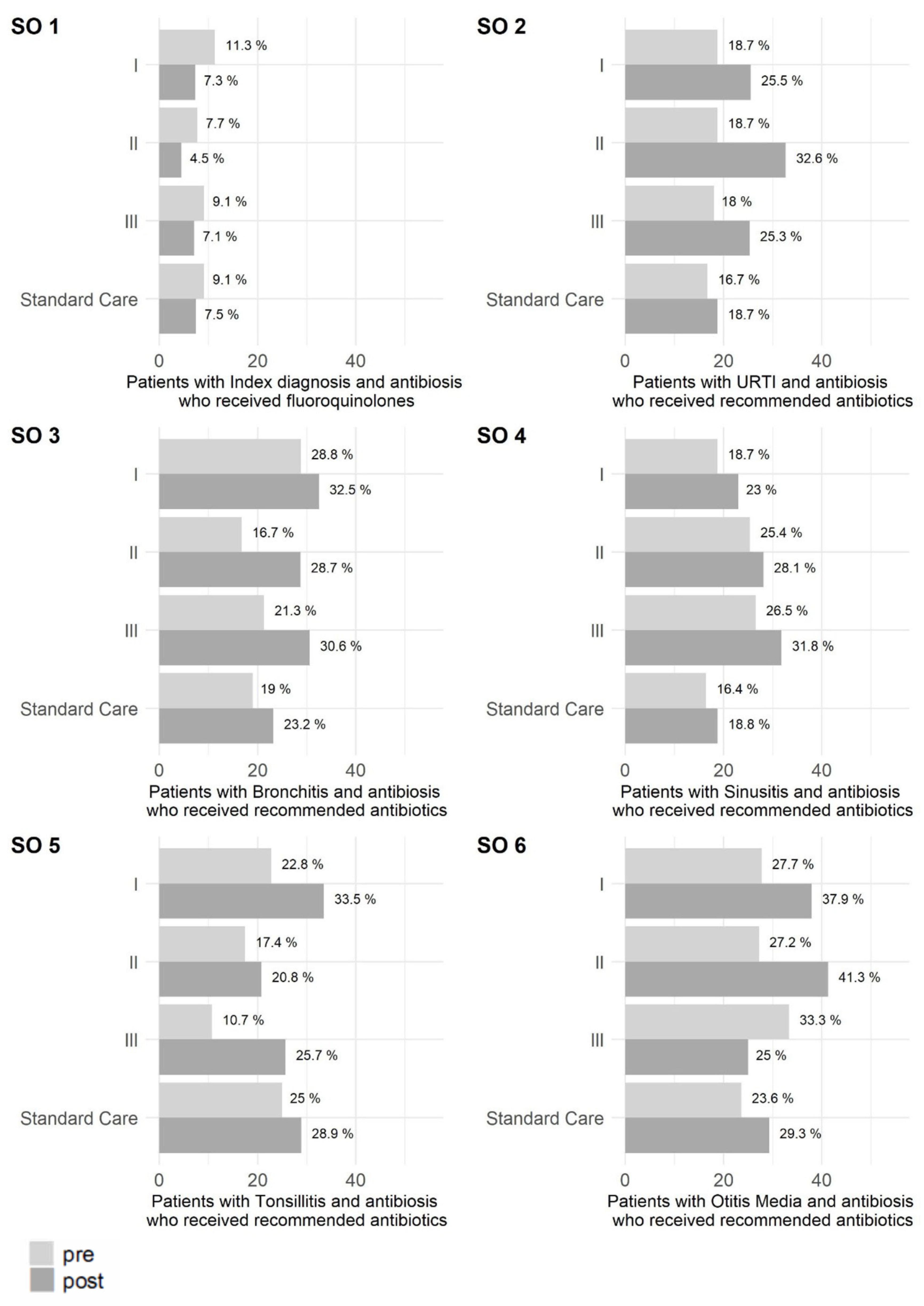
2. Results
2.1. Primary Outcome
2.2. Secondary Outcomes
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Measures
4.4. Data Analysis
- Step 1: Percentage of cases in intervention arms II and III are each lower post-intervention.
- Step 2: Percentage of cases in intervention arm I is lower post-intervention.
- Step 3: Compared to intervention arm I, the percentage is lower in intervention arms II and III each.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARTI | acute respiratory tract infections |
| AWMF | Association of the Scientific Medical Societies in Germany |
| CAP | community acquired pneumonia |
| CI | confidence interval |
| CCI | Charlson comorbidity Index |
| CDSS | Computerized Decision Support System |
| DDD | defined daily dose |
| DEGAM | German College of General Practitioners and Family Physicians |
| ESAC-Net | European Surveillance of Antimicrobial Consumption Network |
| GP | general practitioners |
| ICD | International Classification of Diseases |
| OR | odds ratio |
| PCNs | primary care networks |
| Q | quarter |
| QC | Quality circle |
| SO | Secondary outcome |
| URTI | upper respiratory tract infections |
References
- German Federal Ministry of Health. Bundesministerium für Gesundheit. DART 2020—Antibiotika-Resistenzen Bekämpfen Zum Wohl Von Mensch Und Tier; Bundesministerium für Gesundheit: Berlin, Germany, 2015. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/D/DART_2020/BMG_DART_2020_Bericht_dt.pdf (accessed on 27 June 2021).
- Federal Office of Consumer Protection and Food Safety P-E-GfCeV. Germap 2015: Antimicrobial Resistance and Consumption; Report on the Consumption of Antimicrobials and the Spread of Antimicrobial Resistance in Human and Veterinary Medicine in Germany; Antiinfectives Intelligence: Rheinbach, Germany, 2016.
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018. Stockholm 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf (accessed on 27 June 2021).
- European Centre for Disease Prevention Control. Antimicrobial Resistance Surveillance in Europe. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2015. Stockholm: ECDC. 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf (accessed on 27 July 2021).
- Kraus, E.M.; Pelzl, S.; Szecsenyi, J.; Laux, G. Antibiotic prescribing for acute lower respiratory tract infections (LRTI)—Guideline adherence in the German primary care setting: An analysis of routine data. PLoS ONE 2017, 12, e0174584. [Google Scholar] [CrossRef]
- Anthierens, S.; Tonkin-Crine, S.; Cals, J.W.; Coenen, S.; Yardley, L.; Brookes-Howell, L.; Fernandez-Vandellos, P.; Krawczyk, J.; Godycki-Cwirko, M.; Llor, C.; et al. Clinicians’ Views and Experiences of Interventions to Enhance the Quality of Antibiotic Prescribing for Acute Respiratory Tract Infections. J. Gen. Intern. Med. 2015, 30, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Jason, N. Effect of Behavioral Interventions on Inappropriate Antibiotic Prescribing Among Primary Care Practices: A Randomized Clinical Trial. JAMA 2016, 315, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012, 2012, CD000259. [Google Scholar] [CrossRef]
- Wensing, M.; Broge, B.; Riens, B.; Kaufmann-Kolle, P.; Akkermans, R.; Grol, R.; Szecsenyi, J. Quality circles to improve prescribing of primary care physicians. Three comparative studies. Pharmacoepidemiol. Drug Saf. 2009, 18, 763–769. [Google Scholar] [CrossRef]
- Köchling, A.; Löffler, C.; Reinsch, S.; Hornung, A.; Böhmer, F.; Altiner, A.; Chenot, J.-F. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: A systematic review. Implement. Sci. 2018, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Agentur deutscher Arztnetze (AdA). Ueber Netze. Was sind Arztnetze? Agentur deutscher Arztnetze. 2014. Available online: http://deutsche-aerztenetze.de/ueber_netze/was_sind_arztnetze.php (accessed on 16 June 2021).
- Cunningham, F.C.; Ranmuthugala, G.; Plumb, J.; Georgiou, A.; Westbrook, J.I.; Braithwaite, J. Health professional networks as a vector for improving healthcare quality and safety: A systematic review. BMJ Qual. Saf. 2012, 21, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, T.; Everett, C.; Griffiths, P.; Hudon, C.; Naccarella, L.; Laurant, M. Skill mix, roles and remuneration in the primary care workforce: Who are the healthcare professionals in the primary care teams across the world? Int. J. Nurs. Stud. 2015, 52, 727–743. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.A.; Blash, L.K. New Roles for Medical Assistants in Innovative Primary Care Practices. Health Serv. Res. 2017, 52 (Suppl. 1), 383–406. [Google Scholar] [CrossRef] [Green Version]
- Tonkin-Crine, S.K.; Tan, P.S.; van Hecke, O.; Wang, K.; Roberts, N.W.; McCullough, A.; Hansen, M.P.; Butler, C.C.; Del Mar, C.B. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: An overview of systematic reviews. Cochrane Database Syst. Rev. 2017, 9, CD012252. [Google Scholar] [CrossRef] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Rohrbasser, A.; Harris, J.; Mickan, S.; Tal, K.; Wong, G. Quality circles for quality improvement in primary health care: Their origins, spread, effectiveness and lacunae- A scoping review. PLoS ONE 2018, 13, e0202616. [Google Scholar] [CrossRef] [PubMed]
- Kamradt, M.; Kaufmann-Kolle, P.; Andres, E.; Brand, T.; Klingenberg, A.; Glassen, K.; Poß-Doering, R.; Uhlmann, L.; Hees, K.; Weber, D.; et al. Sustainable reduction of antibiotic-induced antimicrobial resistance (ARena) in German ambulatory care: Study protocol of a cluster randomised trial. Implement. Sci. 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poss-Doering, R.; Kühn, L.; Kamradt, M.; Stürmlinger, A.; Glassen, K.; Andres, E.; Kaufmann-Kolle, P.; Wambach, V.; Bader, L.; Szecsenyi, J.; et al. Fostering Appropriate Antibiotic Use in a Complex Intervention: Mixed-Methods Process Evaluation Alongside the Cluster-Randomized Trial ARena. Antibiotics 2020, 9, 878. [Google Scholar] [CrossRef]
- Poss-Doering, R.; Kamradt, M.; Stuermlinger, A.; Glassen, K.; Kaufmann-Kolle, P.; Andres, E.; Wensing, M. The complex phenomenon of dysrational antibiotics prescribing decisions in German primary healthcare: A qualitative interview study using dual process theory. Antimicrob. Resist. Infect. Control 2020, 9, 6. [Google Scholar] [CrossRef]
- Mann, D.; Hess, R.; McGinn, T.; Richardson, S.; Jones, S.; Palmisano, J.; Chokshi, S.K.; Mishuris, R.; McCullagh, L.; Park, L.; et al. Impact of Clinical Decision Support on Antibiotic Prescribing for Acute Respiratory Infections: A Cluster Randomized Implementation Trial. J. Gen. Intern. Med. 2020, 35 (Suppl. 2), 788–795. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.; Szecsenyi, J.; Garbe, K.; Hartmann, J.; Petruschke, I.; Schulz, M.; Sturm, H.; Altiner, A.; Bauer, A.; Bornemann, R.; et al. Rational Use of Antibiotics: Impulses for Primary Care (a Symposium Report). Z. für Allg. ZFA 2020, 96, 109–115. Available online: https://www.online-zfa.de/fileadmin/user_upload/Heftarchiv/ZFA/article/2020/03/6189C7EE2967480BA1C5928DB04D521A_andres_rationaler_antibiotika_einsatz.pdf (accessed on 21 September 2021).
- Valente, T.W.; Palinkas, L.A.; Czaja, S.; Chu, K.-H.; Brown, C.H. Social network analysis for program implementation. PLoS ONE 2015, 10, e0131712. [Google Scholar] [CrossRef] [Green Version]
- Poss-Doering, R.; Kamradt, M.; Glassen, K.; Andres, E.; Kaufmann-Kolle, P.; Wensing, M. Promoting rational antibiotic prescribing for non-complicated infections: Understanding social influence in primary care networks in Germany. BMC Fam. Pract. 2020, 21, 51. [Google Scholar] [CrossRef]
- Poss-Doering, R.; Kronsteiner, D.; Kamradt, M.; Andres, E.; Kaufmann-Kolle, P.; Wensing, M.; Szecsenyi, J. Antibiotic prescribing for acute, non-complicated infections in primary care in Germany: Baseline assessment in the cluster randomized trial ARena. BMC Infect. Dis. 2021, 21, 877. [Google Scholar] [CrossRef]
- Kamtsiuris, P.; Atzpodien, K.; Ellert, U.; Schlack, R.; Schlaud, M. Prävalenz von somatischen Erkrankungen bei Kindern und Jugendlichen in Deutschland. Bundesgesundheitsblatt Gesundh. Gesundh. 2007, 50, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobe, T.G.; Dörning, H.A.N.S.; Schwartz, F.W. Barmer Gek Arztreport; St. Augustin: Asgard. 2011. Available online: https://www.barmer.de/blob/36506/d5630a0f349e388b65fd28ad616b7257/data/arztreport-2011-pdf.pdf (accessed on 21 September 2021).
- Holm, N.H.; Rusan, M.; Ovesen, T. Acute otitis media and antibiotics—A systematic review. Dan. Med. J. 2020, 67, A04200272. [Google Scholar] [PubMed]
- Venekamp, R.P.; Mick, P.; Schilder, A.G.; Nunez, D.A. Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database Syst. Rev. 2018, 5, Cd012017. [Google Scholar] [CrossRef] [Green Version]
- Coker, T.R.; Chan, L.S.; Newberry, S.J.; Limbos, M.A.; Suttorp, M.J.; Shekelle, P.G.; Takata, G.S. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: A systematic review. JAMA 2010, 304, 2161–2169. [Google Scholar] [CrossRef] [Green Version]
- McBride, J.A.; Eickhoff, J.; Wald, E.R. Impact of COVID-19 Quarantine and School Cancelation on Other Common Infectious Diseases. Pediatr. Infect. Dis. J. 2020, 39, e449–e452. [Google Scholar] [CrossRef]
- Britten, N. Patients’ expectations of consultations. BMJ 2004, 328, 416–417. [Google Scholar] [CrossRef]
- Mangione-Smith, R.; McGlynn, E.A.; Elliott, M.N.; Krogstad, P.; Brook, R.H. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics 1999, 103 Pt 1, 711–718. [Google Scholar] [CrossRef]
- Venekamp, R.P.; Sanders, S.L.; Glasziou, P.P.; Del Mar, C.B.; Rovers, M.M. Antibiotics for acute otitis media in children. Cochrane Database Syst. Rev. 2015, 2015, Cd000219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbaznejad, L.; Talaei, E.; Hosseinzadeh, F.; Masoumi, B.; Rezai, S.; Rezai, M.S. Comparing Watchful Waiting Approach vs. Antibiotic Therapy in Children with Nonsevere Acute Otitis Media: A Randomized Clinical Trial. Int. J. Pediatr. 2021, 2021, 1–8. [Google Scholar]
- Mather, M.W.; Drinnan, M.; Perry, J.D.; Powell, S.; Wilson, J.A.; Powell, J. A systematic review and meta-analysis of antimicrobial resistance in paediatric acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Holstiege, J.; Schulz, M.; Akmatov, M.; Kern, W.; Steffen, A.; Bätzing, J. The decline in outpatient antibiotic use—An analysis of nationwide prescription data from 2010 to 2018. Dtsch. Ärzteblatt 2020, 117, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Association of the Scientific Medical Societies in Germany (AWMF). Aktuelle Leitlinien (Current guidelines) Online: AWMF. Available online: https://www.awmf.org/leitlinien/aktuelle-leitlinien.html (accessed on 21 September 2021).
- German College of General Practitioners and Family Physicians (DEGAM). Leitlinien der DEGAM. Available online: https://www.degam.de/degam-leitlinien-379.html (accessed on 21 September 2021).
- De Sutter, A.I.; De Meyere, M.J.; De Maeseneer, J.M.; Peersman, W.P. Antibiotic prescribing in acute infections of the nose or sinuses: A matter of personal habit? Fam. Pract. 2001, 18, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Hueber, S.; Kuehlein, T.; Gerlach, R.; Tauscher, M.; Schedlbauer, A. “What they see is what you get”: Prescribing antibiotics for respiratory tract infections in primary care: Do high prescribers diagnose differently? An analysis of German routine data. PLoS ONE 2017, 12, e0188521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient antibiotic use in Europe (1997-2009). J. Antimicrob. Chemother. 2011, 66 (Suppl. 6), vi3–vi12. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Westfall, P.H.; Krishen, A. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J. Stat. Plan. Inference 2001, 99, 25–40. [Google Scholar] [CrossRef]
| Intervention (Cases) | Matched Standard Care (No Intervention) | ||||
|---|---|---|---|---|---|
| Arm I | Arm II | Arm III | |||
| (n = 10,143) | (n = 6730) | (n = 5076) | (n = 25,144) | ||
| Age, n (%) | <18 | 578 (5.7) | 757 (11.2) | 143 (2.8) | 4210 (16.7) |
| 18–65 | 7869 (77.6) | 4684 (69.6) | 3910 (77.0) | 18,960 (75.4) | |
| >65 | 1696 (16.7) | 1289 (19.2) | 1023 (20.2) | 1974 (7.9) | |
| Gender, n (%) | Male | 4405 (43.4) | 2900 (43.1) | 2074 (40.9) | 11,891 (47.3) |
| Nationality, n (%) | German | 9115 (89.9) | 5425 (80.6) | 4144 (81.6) | 18,681 (74.3) |
| CCI, n (%) | 0 | 5734 (56.5) | 3858 (57.3) | 2604 (51.3) | 17,412 (69.2) |
| 1, 2 | 2926 (28.8) | 1945 (28.9) | 1609 (31.7) | 6259 (24.9) | |
| 3, 4 | 781 (7.7) | 453 (6.7) | 492 (9.7) | 830 (3.3) | |
| ≥5 | 702 (6.9) | 474 (7.0) | 371 (7.3) | 643 (2.6) | |
| Index diseases, n (%) | Bronchitis | 2442 (24.1) | 1569 (23.3) | 1457 (28.7) | 5796 (23.1) |
| URTI | 7620 (75.1) | 4916 (73.0) | 3719 (73.3) | 17,663 (70.2) | |
| Sinusitis | 962 (9.5) | 867 (12.9) | 700 (13.8) | 1642 (6.5) | |
| Tonsillitis | 507 (5.0) | 335 (5.0) | 220 (4.3) | 1654 (6.6) | |
| Otitis Media | 474 (4.7) | 357 (5.3) | 263 (5.2) | 1809 (7.2) | |
| OR | 95% CI of OR | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Intervention arm I | post vs. pre | 0.523 | 0.485 | 0.563 | <0.001 |
| Female vs. male | 1.274 | 1.179 | 1.376 | <0.001 | |
| Age < 18 vs. 18–65 | 0.675 | 0.559 | 0.815 | <0.001 | |
| Age > 65 vs. 18–65 | 1.324 | 1.193 | 1.469 | <0.001 | |
| Intervention arm II | post vs. pre | 0.547 | 0.493 | 0.607 | <0.001 |
| Female vs. male | 1.371 | 1.227 | 1.532 | <0.001 | |
| Age < 18 vs. 18–65 | 0.64 | 0.412 | 0.994 | 0.047 | |
| Age > 65 vs. 18–65 | 1.167 | 1.008 | 1.351 | 0.038 | |
| Intervention arm III | post vs. pre | 0.519 | 0.467 | 0.576 | <0.001 |
| Female vs. male | 1.296 | 1.164 | 1.443 | <0.001 | |
| Age < 18 vs. 18–65 | 0.676 | 0.494 | 0.925 | 0.014 | |
| Age > 65 vs. 18–65 | 1.479 | 1.299 | 1.684 | <0.001 | |
| Comparison between intervention arms | Intervention arm II vs. I | 0.863 | 0.658 | 1.130 | 0.284 |
| Intervention arm III vs. I | 1.019 | 0.781 | 1.331 | 0.888 | |
| Intervention arm III vs. II | 1.182 | 0.895 | 1.561 | 0.239 | |
| post vs. pre | 0.561 | 0.535 | 0.589 | <0.001 | |
| Female vs. male | 1.274 | 1.214 | 1.338 | <0.001 | |
| Age < 18 vs. 18–65 | 0.683 | 0.595 | 0.784 | <0.001 | |
| Age > 65 vs. 18–65 | 1.255 | 1.179 | 1.337 | <0.001 | |
| OR | 95% CI of OR | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Comparison to standard care | Intervention arm I vs. standard care | 0.596 | 0.572 | 0.621 | <0.001 |
| Intervention arm II vs. standard care | 0.661 | 0.629 | 0.695 | <0.001 | |
| Intervention arm III vs. standard care | 0.726 | 0.689 | 0.764 | <0.001 | |
| post vs. pre | 0.699 | 0.679 | 0.721 | <0.001 | |
| Female vs. male | 1.198 | 1.162 | 1.234 | <0.001 | |
| Age < 18 vs. 18–65 | 0.54 | 0.503 | 0.579 | <0.001 | |
| Age > 65 vs. 18–65 | 0.836 | 0.778 | 0.899 | <0.001 | |
| CCI 1 and 2 vs. 0 | 1.584 | 1.531 | 1.638 | <0.001 | |
| CCI 3 and 4 vs. 0 | 1.603 | 1.498 | 1.716 | <0.001 | |
| CCI ≥ 5 vs. 0 | 1.503 | 1.395 | 1.62 | <0.001 | |
| Northern Europe vs. German | 0.916 | 0.740 | 1.128 | 0.417 | |
| Eastern Europe, Turkey, Arabic states vs. German | 0.978 | 0.935 | 1.022 | 0.321 | |
| Other vs. German | 0.818 | 0.719 | 0.929 | 0.002 | |
| Southern Europe vs. German | 1.087 | 0.994 | 1.189 | 0.066 | |
| urban vs. rural | 0.736 | 0.712 | 0.761 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poss-Doering, R.; Kronsteiner, D.; Kamradt, M.; Kaufmann-Kolle, P.; Andres, E.; Wambach, V.; Bleek, J.; Wensing, M.; ARena-Study Group; Szecsenyi, J. Assessing Reduction of Antibiotic Prescribing for Acute, Non-Complicated Infections in Primary Care in Germany: Multi-Step Outcome Evaluation in the Cluster-Randomized Trial ARena. Antibiotics 2021, 10, 1151. https://doi.org/10.3390/antibiotics10101151
Poss-Doering R, Kronsteiner D, Kamradt M, Kaufmann-Kolle P, Andres E, Wambach V, Bleek J, Wensing M, ARena-Study Group, Szecsenyi J. Assessing Reduction of Antibiotic Prescribing for Acute, Non-Complicated Infections in Primary Care in Germany: Multi-Step Outcome Evaluation in the Cluster-Randomized Trial ARena. Antibiotics. 2021; 10(10):1151. https://doi.org/10.3390/antibiotics10101151
Chicago/Turabian StylePoss-Doering, Regina, Dorothea Kronsteiner, Martina Kamradt, Petra Kaufmann-Kolle, Edith Andres, Veit Wambach, Julian Bleek, Michel Wensing, ARena-Study Group, and Joachim Szecsenyi. 2021. "Assessing Reduction of Antibiotic Prescribing for Acute, Non-Complicated Infections in Primary Care in Germany: Multi-Step Outcome Evaluation in the Cluster-Randomized Trial ARena" Antibiotics 10, no. 10: 1151. https://doi.org/10.3390/antibiotics10101151
APA StylePoss-Doering, R., Kronsteiner, D., Kamradt, M., Kaufmann-Kolle, P., Andres, E., Wambach, V., Bleek, J., Wensing, M., ARena-Study Group, & Szecsenyi, J. (2021). Assessing Reduction of Antibiotic Prescribing for Acute, Non-Complicated Infections in Primary Care in Germany: Multi-Step Outcome Evaluation in the Cluster-Randomized Trial ARena. Antibiotics, 10(10), 1151. https://doi.org/10.3390/antibiotics10101151






