Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Characterization of Col/haNPs
2.2. Antimicrobial Capacity of Col/haNPs
2.2.1. Minimum Inhibitory Concentration (MIC) of Col/haNPs
2.2.2. Effect of Col/haNPs on Bacterial Growth
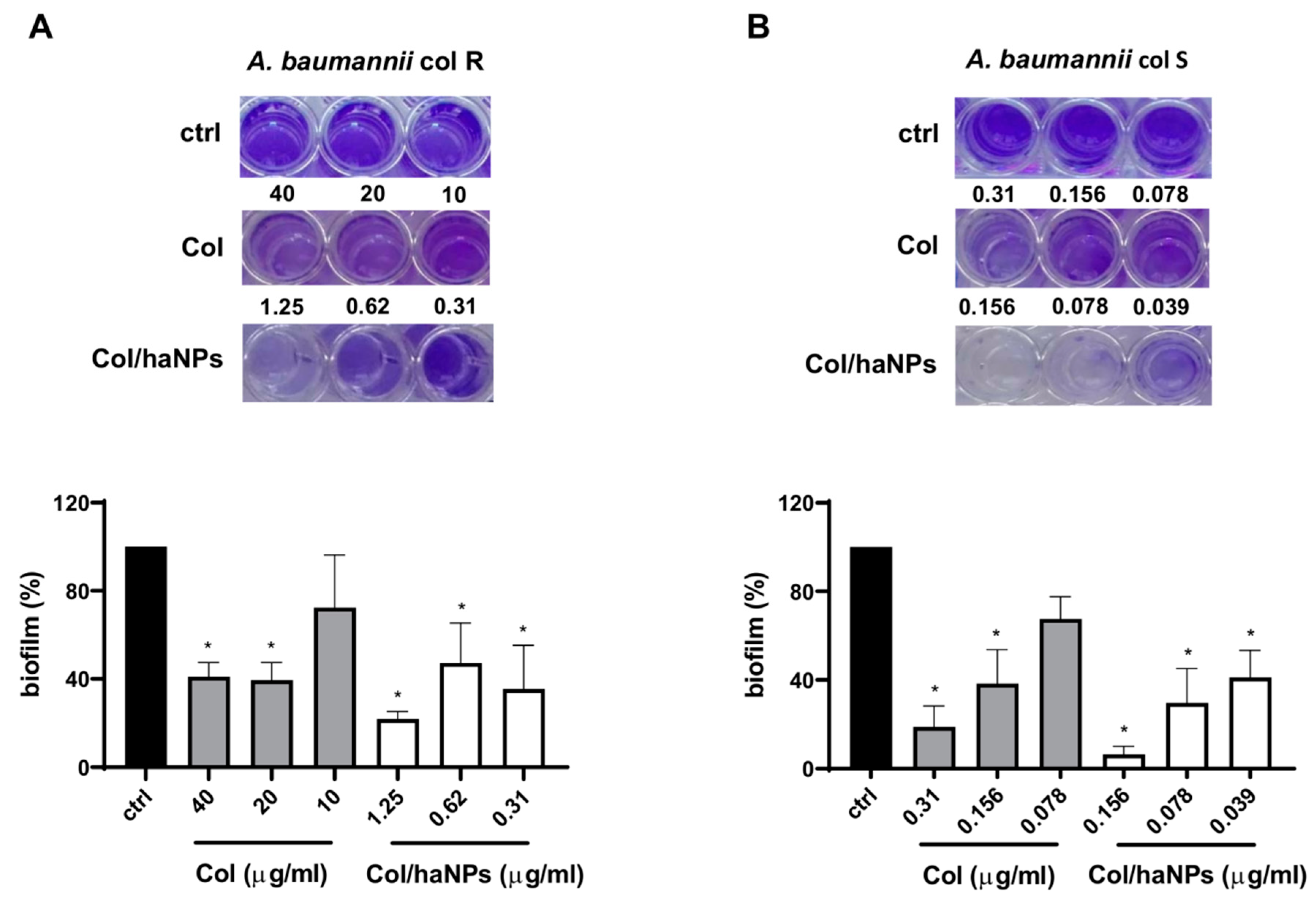
2.2.3. Antibiofilm Effect
2.3. Cytocompatibility of Col/haNPs
2.3.1. In Vitro Hemolytic Activity
2.3.2. In Vitro Cytotoxicity Effect
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Chitosan-Coated Albumin Nanoparticles for the Delivery of Col
4.1.1. Preparation of Blank and Colistin-Loaded Chitosan-Coated Albumin Nanoparticles
4.1.2. Characterization of Col-Loaded Chitosan-Coated Albumin Nanoparticles
4.1.3. HPLC Quantitative Determination of Colistin
4.1.4. Determination of Encapsulation Efficiency
4.1.5. In Vitro Release Studies
4.1.6. In Vitro Stability Studies
4.1.7. Hemolytic Activity Determination
4.1.8. Cytotoxicity Assay
4.2. Microbiological Experiments
4.2.1. Microorganisms
4.2.2. Susceptibility Testing
4.2.3. Assessment of Biofilm Formation
4.2.4. Microbial Growth Kinetics Assay
4.2.5. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Ahmed, M.A.E.-G.E.-S.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Yahav, D.; Farbman, L.; Leibovici, L.; Paul, M. Colistin: New Lessons on an Old Antibiotic. Clin. Microbiol. Infect. 2012, 18, 18–29. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Kafil, H.S. Colistin, Mechanisms and Prevalence of Resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G.; et al. Vancomycin-Loaded Nanobubbles: A New Platform for Controlled Antibiotic Delivery against Methicillin-Resistant Staphylococcus Aureus Infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid Lipid Nanoparticles as Promising Tool for Intraocular Tobramycin Delivery: Pharmacokinetic Studies on Rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; El-Mokhtar, M.A.; Abdelkader, O.; Hamad, M.A.; Elsabahy, M.; El-Gazayerly, O.N. Ultrahigh Antibacterial Efficacy of Meropenem-Loaded Chitosan Nanoparticles in a Septic Animal Model. Carbohydr. Polym. 2017, 174, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.; Kobarfard, F.; Mahboubi, A.; Vatanara, A.; Mortazavi, S.A. Preparation of an Optimized Ciprofloxacin-Loaded Chitosan Nanomicelle with Enhanced Antibacterial Activity. Drug Dev. Ind. Pharm. 2018, 44, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Freudenthal, O.; Quilès, F.; Francius, G.; Wojszko, K.; Gorczyca, M.; Korchowiec, B.; Rogalska, E. Nanoscale Investigation of the Interaction of Colistin with Model Phospholipid Membranes by Langmuir Technique, and Combined Infrared and Force Spectroscopies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.F.; Abou-Shleib, H.M.; Khalil, A.M.; El-Guink, N.M.; El-Nakeeb, M.A. Membrane Permeabilization of Colistin toward Pan-Drug Resistant Gram-Negative Isolates. Braz. J. Microbiol. 2016, 47, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; Angelis, G.D.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID Guidelines for the Management of the Infection Control Measures to Reduce Transmission of Multidrug-Resistant Gram-Negative Bacteria in Hospitalized Patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-Based Drug Delivery Systems for Control of Microbial Biofilms: A Review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Chopra, S.; Torres-Ortiz, M.; Hokama, L.; Madrid, P.; Tanga, M.; Mortelmans, K.; Kodukula, K.; Galande, A.K. Repurposing FDA-Approved Drugs to Combat Drug-Resistant Acinetobacter Baumannii. J. Antimicrob. Chemother. 2010, 65, 2598–2601. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Huang, T.-H.; Yang, S.-C.; Chen, C.-C.; Fang, J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Prankerd, R.J.; Boyd, B.J. Interaction of Colistin and Colistin Methanesulfonate with Liposomes: Colloidal Aspects and Implications for Formulation. J. Pharm. Sci. 2012, 101, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Sans-Serramitjana, E.; Fusté, E.; Martínez-Garriga, B.; Merlos, A.; Pastor, M.; Pedraz, J.L.; Esquisabel, A.; Bachiller, D.; Vinuesa, T.; Viñas, M. Killing Effect of Nanoencapsulated Colistin Sulfate on Pseudomonas Aeruginosa from Cystic Fibrosis Patients. J. Cyst. Fibros. 2016, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Kuo, S.-C.; Yao, B.-Y.; Fang, Z.-S.; Lee, Y.-T.; Chang, Y.-C.; Chen, T.-L.; Hu, C.-M.J. Colistin Nanoparticle Assembly by Coacervate Complexation with Polyanionic Peptides for Treating Drug-Resistant Gram-Negative Bacteria. Acta Biomater. 2018, 82, 133–142. [Google Scholar] [CrossRef]
- d’Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming Barriers in Pseudomonas Aeruginosa Lung Infections: Engineered Nanoparticles for Local Delivery of a Cationic Antimicrobial Peptide. Colloids Surf. B Biointerfaces 2015, 135, 717–725. [Google Scholar] [CrossRef]
- Bayat, F.; Karimi, A.R. Design of Photodynamic Chitosan Hydrogels Bearing Phthalocyanine-Colistin Conjugate as an Antibacterial Agent. Int. J. Biol. Macromol. 2019, 129, 927–935. [Google Scholar] [CrossRef]
- Yasar, H.; Ho, D.-K.; De Rossi, C.; Herrmann, J.; Gordon, S.; Loretz, B.; Lehr, C.-M. Starch-Chitosan Polyplexes: A Versatile Carrier System for Anti-Infectives and Gene Delivery. Polymers 2018, 10, 252. [Google Scholar] [CrossRef]
- Ran, H.-H.; Cheng, X.; Gao, G.; Sun, W.; Jiang, Y.-W.; Zhang, X.; Jia, H.-R.; Qiao, Y.; Wu, F.-G. Colistin-Loaded Polydopamine Nanospheres Uniformly Decorated with Silver Nanodots: A Nanohybrid Platform with Improved Antibacterial and Antibiofilm Performance. ACS Appl. Bio Mater. 2020, 3, 2438–2448. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Bessone, F.; Dianzani, C.; Argenziano, M.; Cangemi, L.; Spagnolo, R.; Maione, F.; Giraudo, E.; Cavalli, R. Albumin Nanoformulations as an Innovative Solution to Overcome Doxorubicin Chemoresistance. Cancer Drug Resist. 2020, 3. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Chen, T.; Wang, X.; Zhang, H.; Chen, Q. Insight into the Interaction between Chitosan and Bovine Serum Albumin. Carbohydr. Polym. 2017, 176, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, H.; Xiong, S.; Tian, T.; Liu, T.; Sun, Y. Chitosan-Stablized Bovine Serum Albumin Nanoparticles Having Ability to Control the Release of NELL-1 Protein. Int. J. Biol. Macromol. 2018, 109, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.A.; Wakabayashi, R.; Goto, M.; Kamiya, N. Self-Assembled Reduced Albumin and Glycol Chitosan Nanoparticles for Paclitaxel Delivery. Langmuir 2019, 35, 2610–2618. [Google Scholar] [CrossRef]
- Obuobi, S.; Wang, Y.; Khara, J.S.; Riegger, A.; Kuan, S.L.; Ee, P.L.R. Antimicrobial and Anti-Biofilm Activities of Surface Engineered Polycationic Albumin Nanoparticles with Reduced Hemolytic Activity. Macromol. Biosci. 2018, 18, 1800196. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Chien, C.-S.; Wang, C.-C.; Shih, C.-J. Antibacterial Activity of BSA-Capped Gold Nanoclusters against Methicillin-Resistant Staphylococcus Aureus (MRSA) and Vancomycin-Intermediate Staphylococcus Aureus (VISA). Available online: https://www.hindawi.com/journals/jnm/2019/4101293/ (accessed on 28 October 2020).
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Loyola-Rodríguez, J.P.; Niño-Martínez, N.; Ruiz, F.; Zavala-Alonso, N.V.; Lara, R.H.; Reyes-López, S.Y. Bovine Serum Albumin and Chitosan Coated Silver Nanoparticles and Its Antimicrobial Activity against Oral and Nonoral Bacteria. Available online: https://www.hindawi.com/journals/jnm/2015/420853/ (accessed on 4 December 2020).
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Mushtaq, S.; Khan, J.A.; Rabbani, F.; Latif, U.; Arfan, M.; Yameen, M.A. Biocompatible Biodegradable Polymeric Antibacterial Nanoparticles for Enhancing the Effects of a Third-Generation Cephalosporin against Resistant Bacteria. J. Med. Microbiol. 2017, 66, 318–327. [Google Scholar] [CrossRef]
- Mazzaccaro, D.; Ticozzi, R.; D’Alessandro, S.; Delbue, S.; Nano, G.; Costa, E.; Argenziano, M.; Cavalli, R.; Prato, M.; Basilico, N. Effect of Antibiotic-Loaded Chitosan Nanodroplets on Enterococci Isolated from Chronic Ulcers of the Lower Limbs. Future Microbiol. 2020, 15, 1227–1236. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Naparstek, L.; Carmeli, Y.; Navon-Venezia, S.; Banin, E. Biofilm Formation and Susceptibility to Gentamicin and Colistin of Extremely Drug-Resistant KPC-Producing Klebsiella Pneumoniae. J. Antimicrob. Chemother. 2014, 69, 1027–1034. [Google Scholar] [CrossRef]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm Formation by Acinetobacter Baumannii Strains Isolated from Urinary Tract Infection and Urinary Catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Unno, Y.; Ubagai, T.; Ono, Y. Sub-Minimum Inhibitory Concentrations of Colistin and Polymyxin B Promote Acinetobacter Baumannii Biofilm Formation. PLoS ONE 2018, 13, e0194556. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.; Moreno-Sastre, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Asensio, V.J.; Pozo, Á.D.; Gainza, E.; Pedraz, J.L. Sodium Colistimethate Loaded Lipid Nanocarriers for the Treatment of Pseudomonas Aeruginosa Infections Associated with Cystic Fibrosis. Int. J. Pharm. 2014, 477, 485–494. [Google Scholar] [CrossRef]
- Casciaro, B.; Lin, Q.; Afonin, S.; Loffredo, M.R.; de Turris, V.; Middel, V.; Ulrich, A.S.; Di, Y.P.; Mangoni, M.L. Inhibition of Pseudomonas Aeruginosa Biofilm Formation and Expression of Virulence Genes by Selective Epimerization in the Peptide Esculentin-1a(1-21)NH2. FEBS J. 2019, 286, 3874–3891. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-Induced Biofilm Formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Yella, J.K.; Yaddanapudi, S.; Wang, Y.; Jegga, A.G. Changing Trends in Computational Drug Repositioning. Pharmaceuticals 2018, 11, 57. [Google Scholar] [CrossRef]
- England, C.G.; Miller, M.C.; Kuttan, A.; Trent, J.O.; Frieboes, H.B. Release Kinetics of Paclitaxel and Cisplatin from Two and Three Layered Gold Nanoparticles. Eur. J. Pharm. Biopharm. 2015, 92, 120–129. [Google Scholar] [CrossRef]



| Sample | Average Diameter ± SD (nm) | PDI | Zeta Potential ± SD (mV) | pH |
|---|---|---|---|---|
| blank haNPs | 174.7 ± 2.1 | 0.20 | 29.76 ± 1.5 | 7.52 |
| Col/haNPs | 176.1 ± 0.9 | 0.12 | 28.05 ± 2.1 | 7.46 |
| Kinetics Model | Col/haNPs | |
|---|---|---|
| K | R2 | |
| Zero-order | 0.9279 | 0.8708 |
| First-order | 0.0047 | 0.8982 |
| Simplified Higuchi | 5.2381 | 0.9774 |
| Korsmeyer-Peppas | 0.7189 | 0.9734 |
| Antibiotics | MDR Clinical Strains | ||||||
|---|---|---|---|---|---|---|---|
| A. baumannii ATCC 19606 | A. baumannii Col S | A. baumannii Col R1 | A. baumannii Col R2 | A. baumannii Col R3 | K. pneumoniae KPC 1 | K. pneumoniae KPC 2 | |
| PIP/TAZO | NT | NT | NT | NT | NT | >16 (R) | >16 (R) |
| MEM | 1 (S) | >8 (R) | >8 (R) | >8 (R) | 8 (S) | >8 (R) | >8 (R) |
| IMP | ≤1 (S) | >8 (R) | >8 (R) | >8 (R) | >8 (R) | >8 (R) | >8 (R) |
| ERT | NT | NT | NT | NT | NT | >1 (R) | >1 (R) |
| CAZ | NT | NT | NT | NT | NT | >8 (R) | >8 (R) |
| CTX | NT | NT | NT | NT | NT | >16 (R) | >16 (R) |
| CEFE | NT | NT | NT | NT | NT | >8 (R) | >8 (R) |
| GENTA | >4 (R) | >4 (R) | >4 (R) | >4 (R) | >4 (R) | 4 (R) | 4 (R) |
| AMIKA | NT | NT | >16 (R) | NT | NT | >16 (R) | >16 (R) |
| FOSFO | NT | NT | NT | NT | NT | >32 (R) | >32 (R) |
| SXT | >4/76 (R) | >4/76 (R) | >4/76 (R) | >4/76 (R) | >4/76 (R) | >4/76 (R) | ≤2/38 (S) |
| CIPRO | 1(S) | >1 (R) | >1 (R) | >1 (R) | >1 (R) | >1 (R) | NT |
| LEVO | ≤0.5 (S) | >1 (R) | >1 (R) | >1 (R) | >1 (R) | >1 (R) | >1 (R) |
| CAZ/AVI | NT | NT | NT | NT | NT | ≤2 (S) | ≤2 (S) |
| COL | ≤2 (S) | ≤2 (S) | >4 (R) | >4 (R) | >4 (R) | >4 (R) | >4 (R) |
| Pathogenic Bacteria | Col MIC (μg/mL) | Col/haNPs MIC (μg/mL) |
|---|---|---|
| Acinetobacter baumannii ATCC19606 | 0.31 | 0.156 |
| Acinetobacter baumannii Col S | 0.31 | 0.156 |
| Acinetobacter baumannii Col R 1 | >40 | 1.25 |
| Acinetobacter baumannii Col R 2 | >40 | 1.25 |
| Acinetobacter baumannii Col R 3 | >40 | 2.5 |
| Klebsiella pneumoniae KPC 1 | 20 | 2.5 |
| Klebsiella pneumoniae KPC 2 | 40 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutera, S.; Argenziano, M.; Sparti, R.; Bessone, F.; Bianco, G.; Bastiancich, C.; Castagnoli, C.; Stella, M.; Musso, T.; Cavalli, R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics 2021, 10, 57. https://doi.org/10.3390/antibiotics10010057
Scutera S, Argenziano M, Sparti R, Bessone F, Bianco G, Bastiancich C, Castagnoli C, Stella M, Musso T, Cavalli R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics. 2021; 10(1):57. https://doi.org/10.3390/antibiotics10010057
Chicago/Turabian StyleScutera, Sara, Monica Argenziano, Rosaria Sparti, Federica Bessone, Gabriele Bianco, Chiara Bastiancich, Carlotta Castagnoli, Maurizio Stella, Tiziana Musso, and Roberta Cavalli. 2021. "Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles" Antibiotics 10, no. 1: 57. https://doi.org/10.3390/antibiotics10010057
APA StyleScutera, S., Argenziano, M., Sparti, R., Bessone, F., Bianco, G., Bastiancich, C., Castagnoli, C., Stella, M., Musso, T., & Cavalli, R. (2021). Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics, 10(1), 57. https://doi.org/10.3390/antibiotics10010057








