Fluoroquinolone Can Be an Effective Treatment Option for Acute Pyelonephritis When the Minimum Inhibitory Concentration of Levofloxacin for the Causative Escherichia coli Is ≤16 mg/L
Abstract
1. Introduction
2. Material and Methods
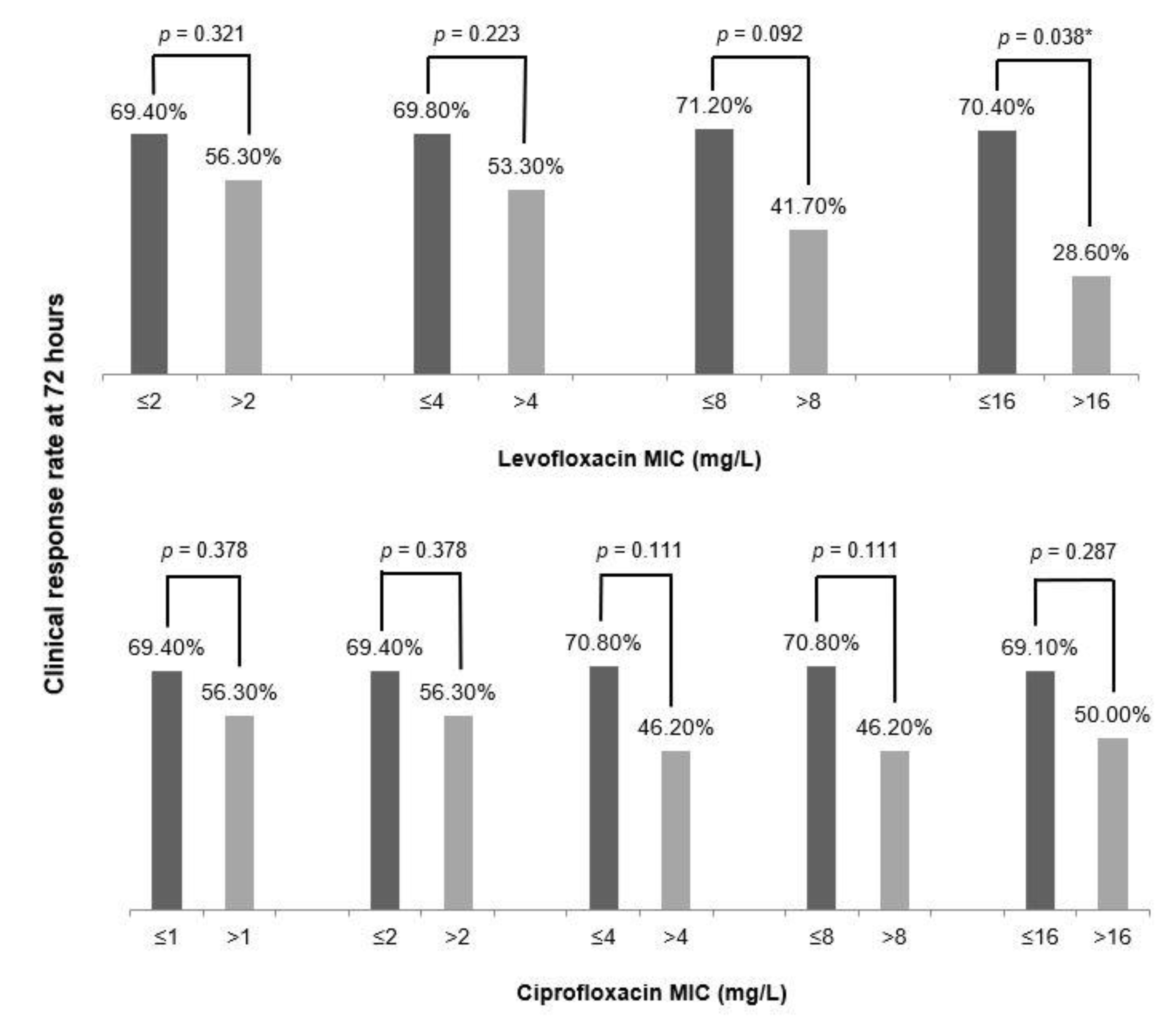
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czaja, C.A.; Scholes, D.; Hooton, T.M.; Stamm, W.E. Population-based epidemiologic analysis of acute pyelonephritis. Clin. Infect. Dis. 2007, 45, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.I.; Kim, J.; Park, D.W.; Kim, B.N.; Ha, U.S.; Lee, S.J.; Yeo, J.K.; Min, S.K.; Lee, H.; Wie, S.H. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect. Chemother. 2018, 50, 67–100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Wagenlehner, C.; Redman, R.; Weidner, W.; Naber, K.G. Urinary Bactericidal Activity of Doripenem versus That of Levofloxacin in Patients with Complicated Urinary Tract Infections or Pyelonephritis. Antimicrob. Agents Chemother. 2009, 53, 1567–1573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.S.; Kim, Y.; Chung, D.R. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J. Infect. 2011, 62, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, J.; Wie, S.H.; Cho, Y.K.; Lim, S.K.; Shin, S.Y.; Yeom, J.S.; Lee, J.S.; Kweon, K.T.; Lee, H.; et al. Fluoroquinolone resistance in uncomplicated acute pyelonephritis: Epidemiology and clinical impact. Microb. Drug Resist. 2012, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kolho, E.; Huotari, K.; Tarkka, E.; Valtonen, V. Incidence and Risk Factors for Nosocomial Infections Caused by Fluoroquinolone-Resistant Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Ko, W.C.; Hsueh, P.R. The role of fluoroquinolones in the management of urinary tract infections in areas with high rates of fluoroquinolone-resistant uropathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Wie, S.H.; Ki, M.; Kim, J.; Cho, Y.K.; Lim, S.K.; Lee, J.S.; Kwon, K.T.; Lee, H.; Cheong, H.J.; Park, D.W.; et al. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: A prospective multicentre study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, O721–O729. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.; Seo, M.R.; Wie, S.H.; Cho, Y.K.; Lim, S.K.; Lee, J.S.; Kwon, K.T.; Lee, H.; Cheong, H.J.; et al. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection 2013, 41, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 15th Informational Supplement, CLSI Document M100-S15; CLSI: Wayne, PA, USA, 2007. [Google Scholar]
- Karlowsky, J.A.; Adam, H.J.; Desjardins, M.; Lagace-Wiens, P.R.S.; Hoban, D.J.; Zhanel, G.G.; Zhanel, G.G.; Hoban, D.J.; Adam, H.J.; Karlowsky, J.A.; et al. Changes in fluoroquinolone resistance over 5 years (CANWARD 2007-11) in bacterial pathogens isolated in Canadian hospitals. J. Antimicrob. Chemother. 2013, 68, i39–i46. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Master, R.N.; Karlowsky, J.A.; Bordon, J.M. In VitroAntimicrobial Resistance of Urinary Escherichia coli Isolates among U.S. Outpatients from 2000 to 2010. Antimicrob. Agents Chemother. 2012, 56, 2181–2183. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Marco, F.; Soriano, A.; Almela, M.; Martínez, J.; Munoz, A.; Mensa, J. Analysis of 4758 Escherichia coli bacteraemia episodes: Predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J. Antimicrob. Chemother. 2009, 63, 568–574. [Google Scholar] [CrossRef]
- Klausner, H.A.; Brown, P.; Peterson, J.; Kaul, S.; Khashab, M.; Fisher, A.C.; Kahn, J.B. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr. Med. Res. Opin. 2007, 23, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Mehrotra, R.; Tang, A.W. Does in vitro fluoroquinolone resistance predict clinical failure in urinary tract infections? Int. J. Antimicrob. Agents 2007, 29, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G. Which fluoroquinolones are suitable for the treatment of urinary tract infections? Int. J. Antimicrob. Agents 2001, 17, 331–341. [Google Scholar] [CrossRef]
- Stein, G.E.; Schooley, S.L.; Nicolau, D.P. Urinary bactericidal activity of single doses (250, 500, 750 and 1000mg) of levofloxacin against fluoroquinolone-resistant strains of Escherichia coli. Int. J. Antimicrob. Agents 2008, 32, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 30th Informational Supplement, CLSI Document M100-S15; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints: Bacteria, Version 10.0; EUCAST: Växjö, Sweden, 2020. [Google Scholar]
| Total = 78 | ||
|---|---|---|
| Demographic data | ||
| Age, years, mean ± SD | 58.9 ± 16.5 | |
| Past history (%) | ||
| History of antibiotic use within 1 year | 22/61 (36.1) | |
| History of urinary tract infection | 18/61 (29.5) | |
| History of admission within 1 year | 18/69 (26.1) | |
| Co-morbidity condition (%) | ||
| Charlson comorbidity index ≥ 2 | 15 (19.2) | |
| Diabetes mellitus | 19 (24.4) | |
| Cerebrovascular disorder | 4 (5.1) | |
| Congestive heart failure | 4 (5.1) | |
| Chronic pulmonary disease | 2 (2.6) | |
| Chronic liver disease | 7 (9.0) | |
| Clinical features (%) | ||
| Flank pain | 23 (29.5) | |
| Lower urinary tract infection symptoms a | 50 (64.1) | |
| Costovertebral angle tenderness | 47 (60.3) | |
| Pitt bacteremia score ≥ 1 b | 34 (43.6) | |
| Laboratory findings at presentation (%) | ||
| C-reactive protein > 20 mg/dL | 43 (55.1) | |
| White blood cells ≥ 20,000/mm3 | 10 (12.8) | |
| Hematuria (≥5–9 red blood cells/high-power field) | 50 (64.1) | |
| Azotemia c | 15 (19.2) | |
| ESBL positivity | 5 (6.4) | |
| FQ resistance | 33 (17.7) | |
| Antibiotic change during hospitalization period (%) | 31 (39.7) | |
| Clinical outcomes | ||
| Clinical response after 72 h (%) | 52 (66.7) | |
| Overall mortality (%) | 2 (2.6) | |
| Overall relapse (%) | 3 (3.8) | |
| Hospitalization duration, days (IQR) | 7 (5-9) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, B.; Wie, S.H.; Kim, J.; Ki, M.; Cho, Y.K.; Lim, S.K.; Lee, J.S.; Kwon, K.T.; Lee, H.; et al. Fluoroquinolone Can Be an Effective Treatment Option for Acute Pyelonephritis When the Minimum Inhibitory Concentration of Levofloxacin for the Causative Escherichia coli Is ≤16 mg/L. Antibiotics 2021, 10, 37. https://doi.org/10.3390/antibiotics10010037
Kim Y, Kim B, Wie SH, Kim J, Ki M, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, et al. Fluoroquinolone Can Be an Effective Treatment Option for Acute Pyelonephritis When the Minimum Inhibitory Concentration of Levofloxacin for the Causative Escherichia coli Is ≤16 mg/L. Antibiotics. 2021; 10(1):37. https://doi.org/10.3390/antibiotics10010037
Chicago/Turabian StyleKim, Yeonjae, Bongyoung Kim, Seong Heon Wie, Jieun Kim, Moran Ki, Yong Kyun Cho, Seung Kwan Lim, Jin Seo Lee, Ki Tae Kwon, Hyuck Lee, and et al. 2021. "Fluoroquinolone Can Be an Effective Treatment Option for Acute Pyelonephritis When the Minimum Inhibitory Concentration of Levofloxacin for the Causative Escherichia coli Is ≤16 mg/L" Antibiotics 10, no. 1: 37. https://doi.org/10.3390/antibiotics10010037
APA StyleKim, Y., Kim, B., Wie, S. H., Kim, J., Ki, M., Cho, Y. K., Lim, S. K., Lee, J. S., Kwon, K. T., Lee, H., Cheong, H. J., Park, D. W., Ryu, S. Y., Chung, M. H., & Pai, H. (2021). Fluoroquinolone Can Be an Effective Treatment Option for Acute Pyelonephritis When the Minimum Inhibitory Concentration of Levofloxacin for the Causative Escherichia coli Is ≤16 mg/L. Antibiotics, 10(1), 37. https://doi.org/10.3390/antibiotics10010037





