Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Biosensor Fabrication
2.3. Sample Preparation
2.4. Measurements
3. Results and Discussion
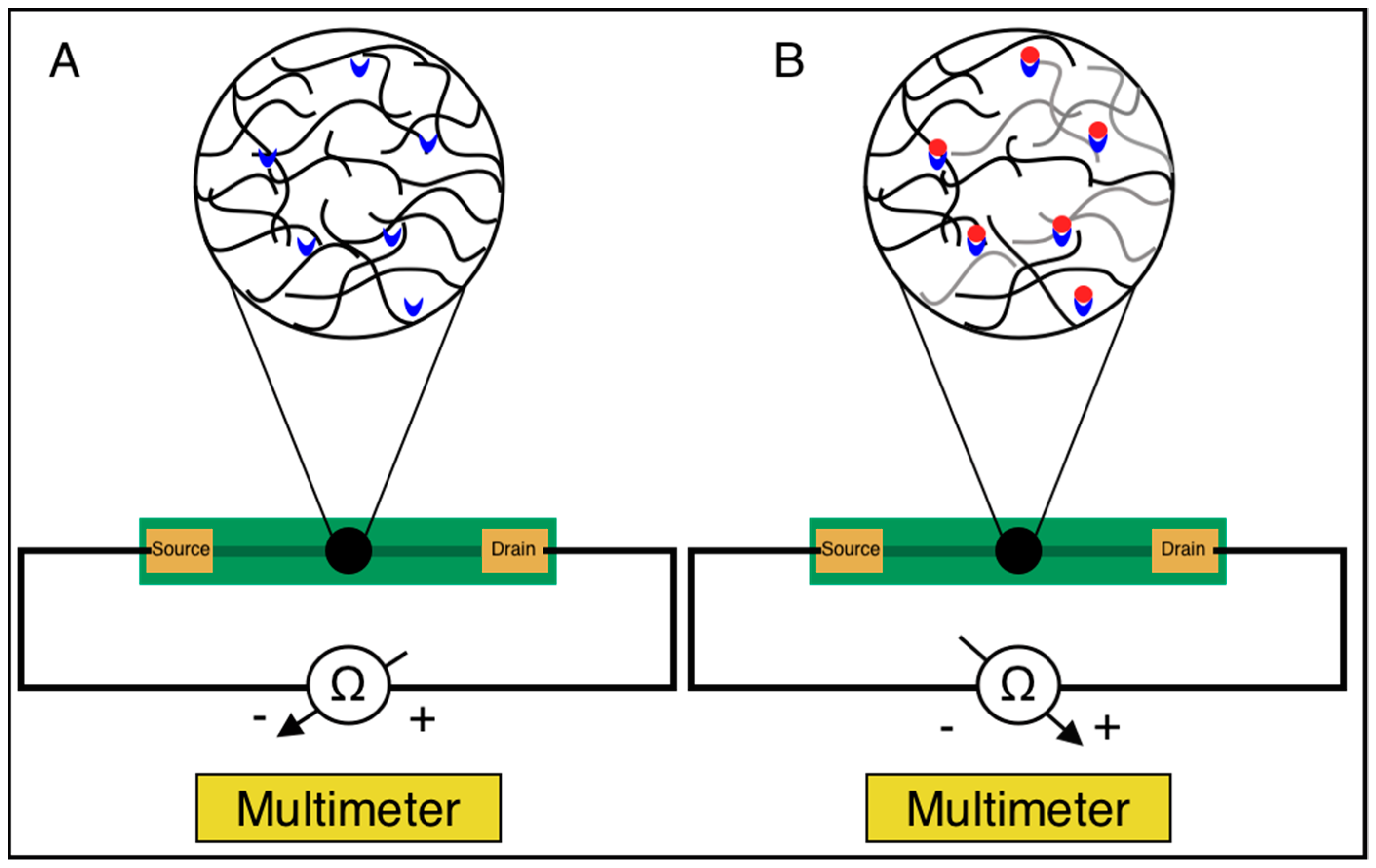
3.1. Biosensor Design
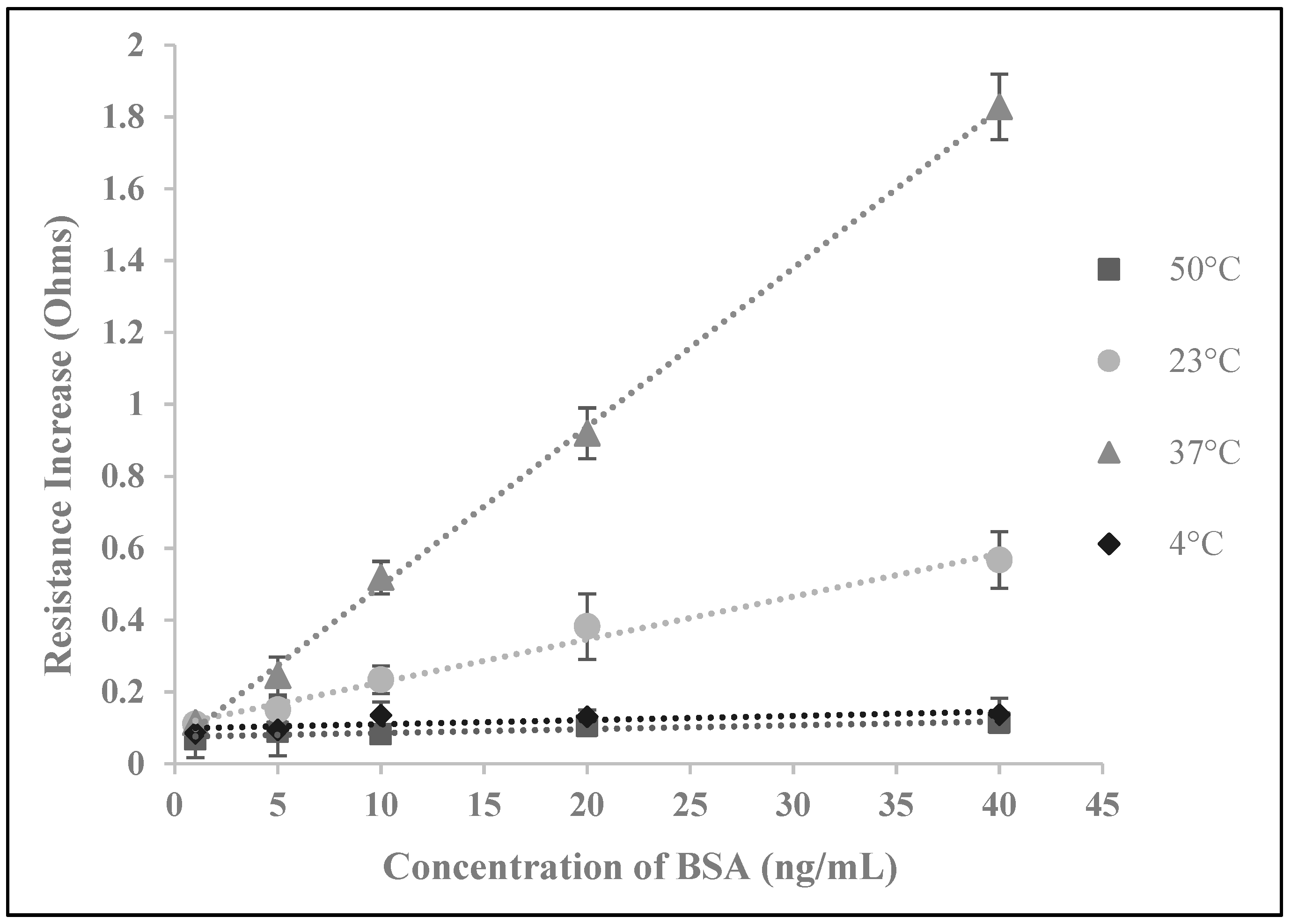
3.2. Biosensor Testing with BSA
3.3. Temperature Optimization
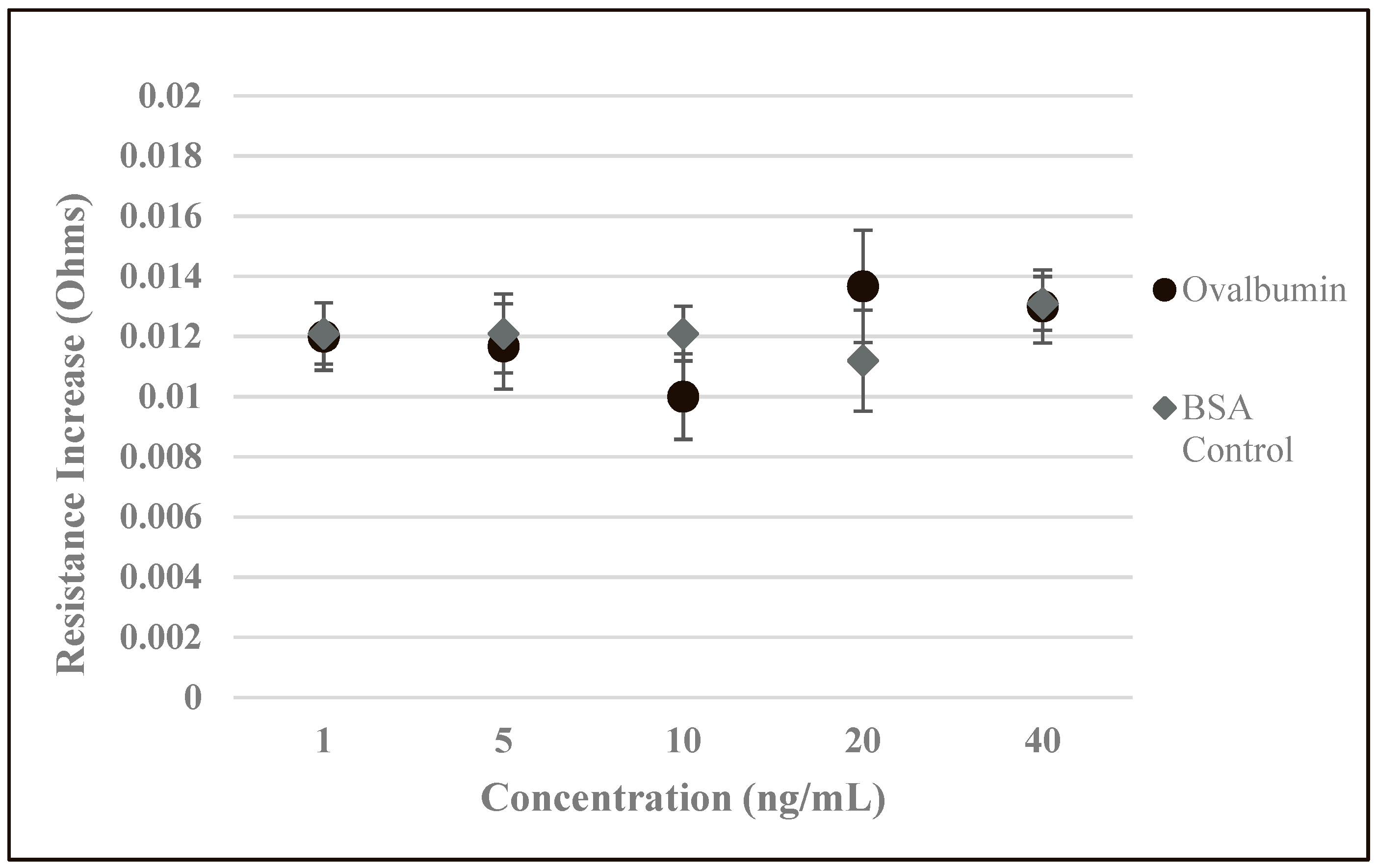
3.4. Analysis of Biosensor Performance and Controlled Experiments
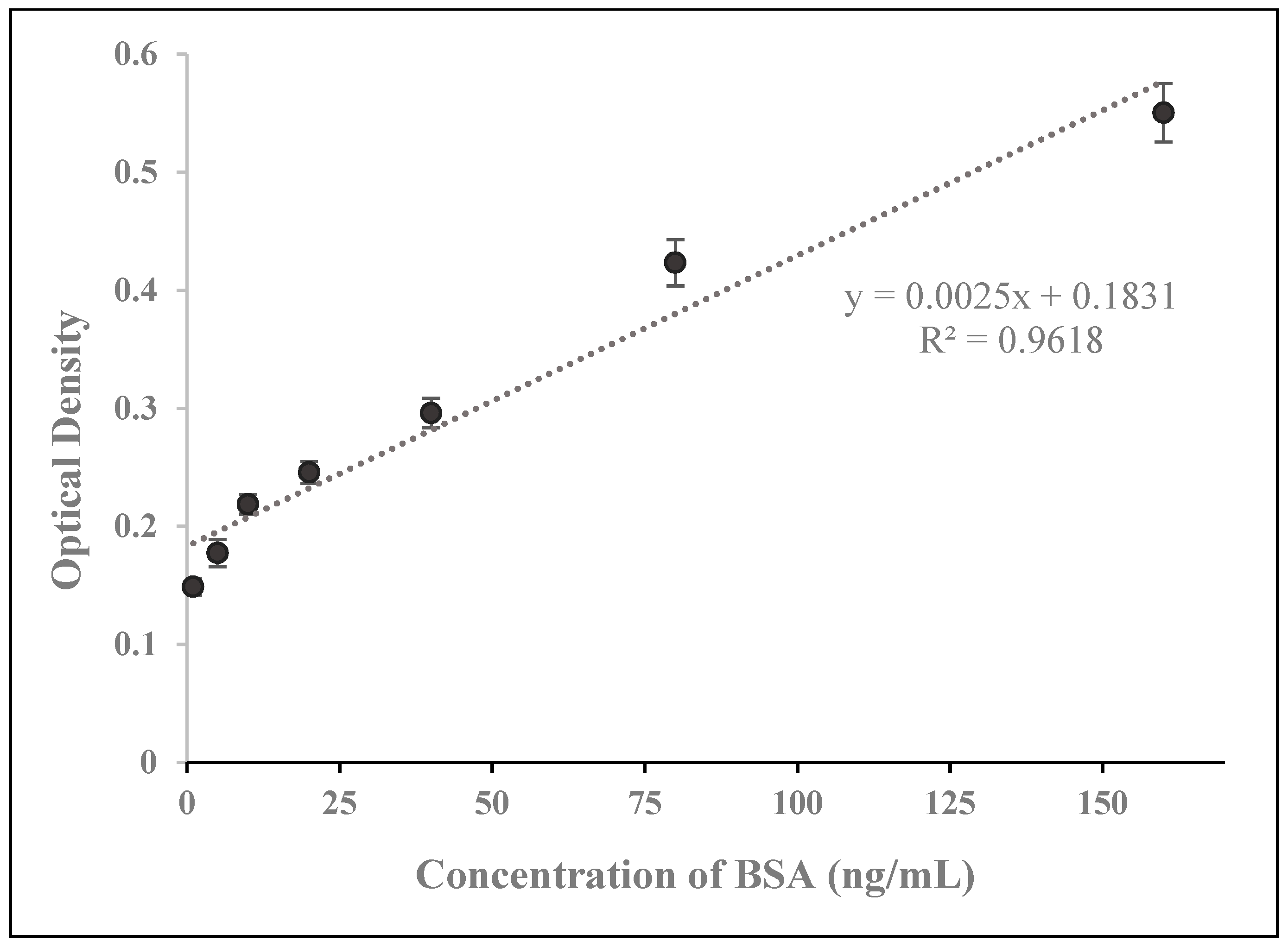
3.5. Analysis and Comparison with ELISA and Recently Developed Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unger-Saldana, K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J. Clin. Oncol. 2014, 5, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Jakesz, R. Breast cancer issues in developing countries: An overview of the Breast Health Global Initiative. World J. Surg. 2008, 32, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.A.; Westcombe, A.M.; Love, S.B.; Littlejohns, P.; Ramirez, A.J. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999, 353, 1119–1126. [Google Scholar] [CrossRef]
- Rivera-Franco, M.M.; Rodriquez, E.L. Delays in breast cancer detection and treatment in developing countries. Breast Cancer: Basic Clin. Res. 2018, 12. [Google Scholar] [CrossRef]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-based sensors: Emerging themes and applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.; Cheung, K.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef]
- Yamada, K.; Kim, C.; Kim, J.; Chung, J.; Lee, H.G.; Jun, S. Single walled carbon nanotube-based junction biosensor for detection of Escherichia coli. PloS ONE 2014, 9, e105767. [Google Scholar] [CrossRef]
- Vestergaard, M.; Kerman, K.; Eiichi, T. An overview of label-free electrochemical protein sensors. Sensors 2007, 7, 3442–3458. [Google Scholar] [CrossRef]
- Kerman, K.; Kobayashi, M.; Tamiya, E. Recent trends in electrochemical DNA biosensor technology. Meas. Sci. Technol. 2004, 15, R1. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Vestergaard, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. Electrochemical genosensor based on peptide nucleic acid-mediated PCR and asymmetric PCR techniques: Electrostatic interactions with a metal cation. Anal. Chem. 2006, 8, 2182–2189. [Google Scholar] [CrossRef]
- Bini, A.; Minunni, M.; Tombelli, S.; Centi, S.; Mascini, M. Analytical performance of aptamer-based sensing for thrombin detection. Anal. Chem. 2007, 79, 3016–3019. [Google Scholar] [CrossRef] [PubMed]
- Evgeni, E.; Cosnier, S.; Marks, R. Biosensors based on combined optical and electrochemical transduction for molecular diagnostics. Expert Rev. Mol. Diagn. 2011, 11, 533–546. [Google Scholar]
- Sin, M.L.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.; Alvarez, S.D.; Choi, W.; Sailor, M. A stable, Label-free optical interferometric biosensor based on TIO2 nanotube arrays. ACS Nano 2010, 4, 2070–2076. [Google Scholar] [CrossRef]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef]
- Chammem, H.; Hafaid, I.; Meilhac, O.; Menaa, F.; Mora, L.; Abdelghani, A. Surface plasmon resonance for C-reactive protein detection in human plasma. J. Biomater. Nanobiotechnol. 2014, 5, 153–158. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.P.; Han, S.J.; Sim, S.J. Aptamer biosensor for label-free detection of human immunoglobulin E based on surface plasmon resonance. Sens. Actuators B. Chem. 2009, 139, 471–475. [Google Scholar] [CrossRef]
- Endo, T.; Kerman, K.; Nagatani, N.; Hiepa, H.M.; Kim, D.; Yonezawa, Y.; Nakano, K.; Tamiya, E. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structures nanoparticle layer nanochip. Anal. Chem. 2006, 78, 6464–6475. [Google Scholar] [CrossRef]
- Nascimento, N.M.; Juste-Dolz, A.; Grau-Garcia, E.; Roman-Ivorra, J.A.; Puchades, R.; Maquieira, A.; Morais, S.; Gimenez-Romero, D. Label-free piezoelectric biosensor for prognosis and diagnosis of Systemic Lupus Erythematosus. Biosens. Bioelectron. 2017, 15, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, K.H.; Hwang, K.S.; Park, J.; Ahn, S.; Kim, T.S. Label free novel electrical detection using micromachined PZT monolithic thin film cantilever for the detection of C-reactive protein. Biosens. Bioelectron. 2004, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hwang, K.S.; Park, J.; Yoon, K.H.; Yoon, D.S.; Kim, T.S. Immunoassay of prostate-specific antigen (PSA) using resonant frequency shift of piezoelectric nanomechanical microcantilever. Biosens. Bioelectron. 2005, 20, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, J.A.; Shi, W.Y.; Adams, G.P.; Shih, W. Label-free Her2 detection and dissociation constant assessment in diluted human serum using a longitudinal extension mode of a piezoelectric microcantilever sensor. Sens. Actuators B. Chem. 2011, 160, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Hwang, S.; Kwak, J.; Park, S.; Lee, S.S.; Lee, K. An electrochemical impedance biosensor with aptamer-modified pyrolyzed carbon electrode for label-free protein detection. Sens. Actuators B. Chem. 2008, 129, 372–379. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Wei, H.; Wang, Y.; Wang, E. Multifunctional label-free electrochemical biosensor based on an integrated aptamer. Anal. Chem. 2008, 80, 5110–5117. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Xu, S.; Gan, T.; Huang, K.; Liu, Y. A sensitive and label-free electrochemical impedance biosensor for protein detection based on terminal protection of small molecule-linked DNA. Sens. Actuators B. Chem. 2014, 194, 478–483. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Santschi, C.; Martin, O.J.F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors 2018, 8, 34. [Google Scholar] [CrossRef]
- Harrad, E.; Bourais, I.; Mohammadi, H.; Amine, A. Recent advances in electrochemical biosensors based on enzyme inhibition for clinical and pharmaceutical applications. Sensors 2018, 18, 164. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Tilmaciu, C.M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Bruck, H.A.; Yang, M.; Kostov, Y.; Rasooly, A. Electrical percolation-based biosensors. Methods 2013, 63, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.C. Electronic and transport properties of nanotubes. Rev. Mod. Phys. 2007, 79, 677. [Google Scholar] [CrossRef]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chou, Y.C. Electrical-conductivity fluctuations near the percolation threshold. Phys. Rev. Lett. 1985, 54, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Balasubramania, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef]
- Kuang, Z.; Huang, R.; Yang, Z.; Lv, Z.; Chen, X.; Xu, F.; Yi, Y.; Wu, J.; Huang, R. Quantitative screening of serum protein biomarkers by reverse phase protein arrays. Oncotarget 2018, 99, 32624–32641. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, J.; Lambeta, M.; White, P.; Byagowi, A. Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection. Biosensors 2019, 9, 144. https://doi.org/10.3390/bios9040144
Janssen J, Lambeta M, White P, Byagowi A. Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection. Biosensors. 2019; 9(4):144. https://doi.org/10.3390/bios9040144
Chicago/Turabian StyleJanssen, Jesslyn, Mike Lambeta, Paul White, and Ahmad Byagowi. 2019. "Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection" Biosensors 9, no. 4: 144. https://doi.org/10.3390/bios9040144
APA StyleJanssen, J., Lambeta, M., White, P., & Byagowi, A. (2019). Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection. Biosensors, 9(4), 144. https://doi.org/10.3390/bios9040144



