Rapid Profiling of Soybean Aromatic Compounds Using Electronic Nose
Abstract
1. Introduction
2. Current Analytical Approaches in Volatile Compound Measurements, Especially in Seeds
3. Materials and Methods
3.1. Plant Materials
3.2. Electronic Nose
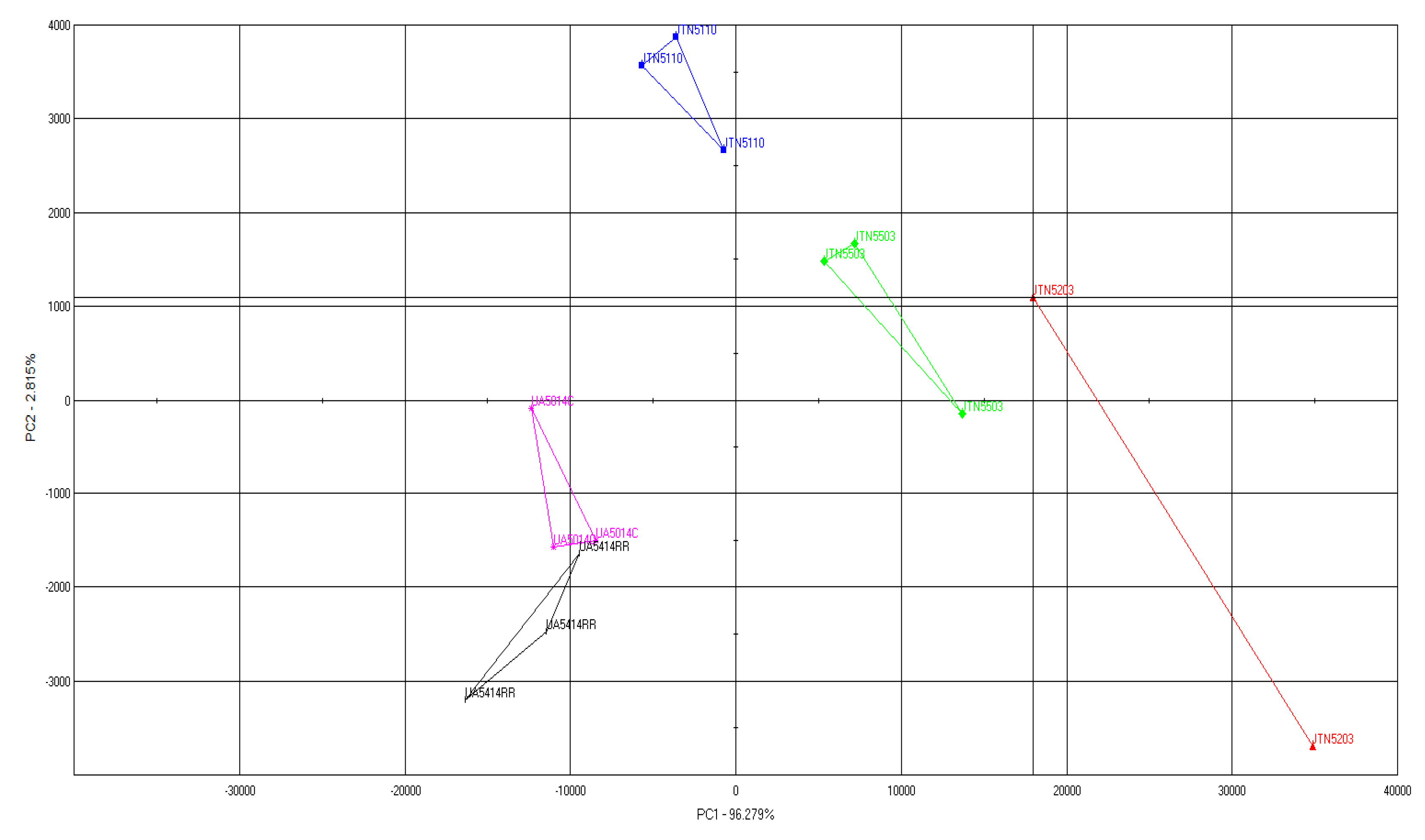
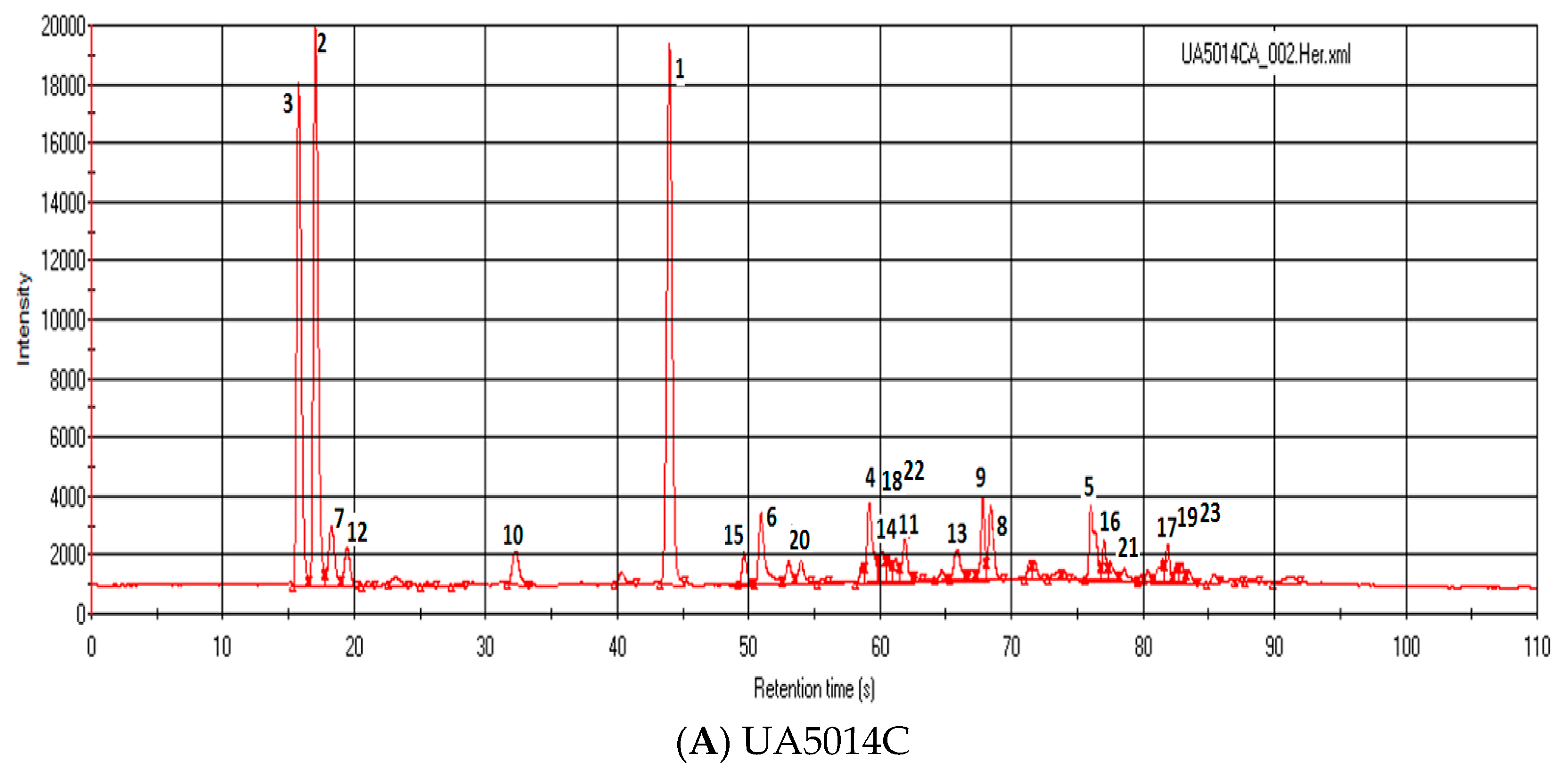
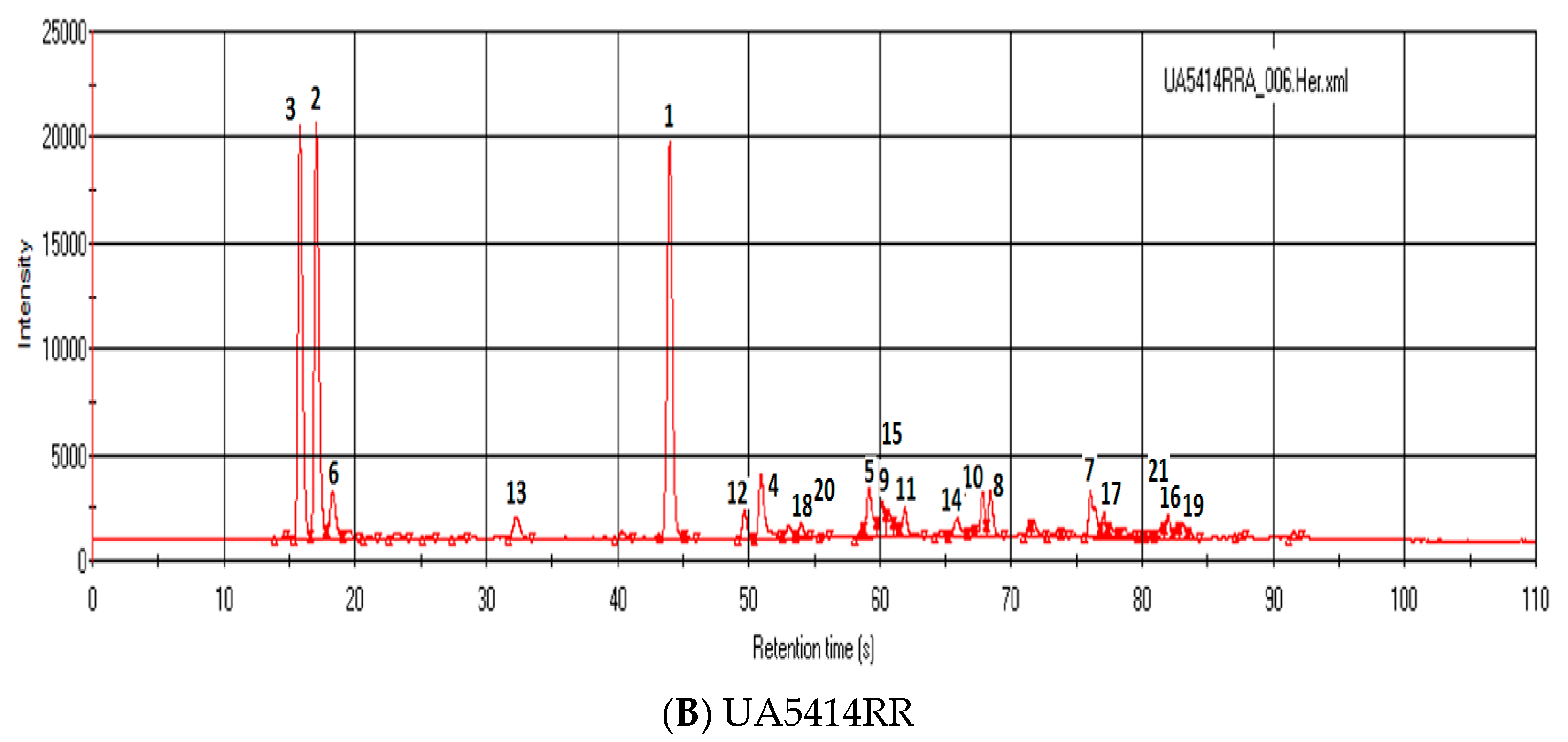
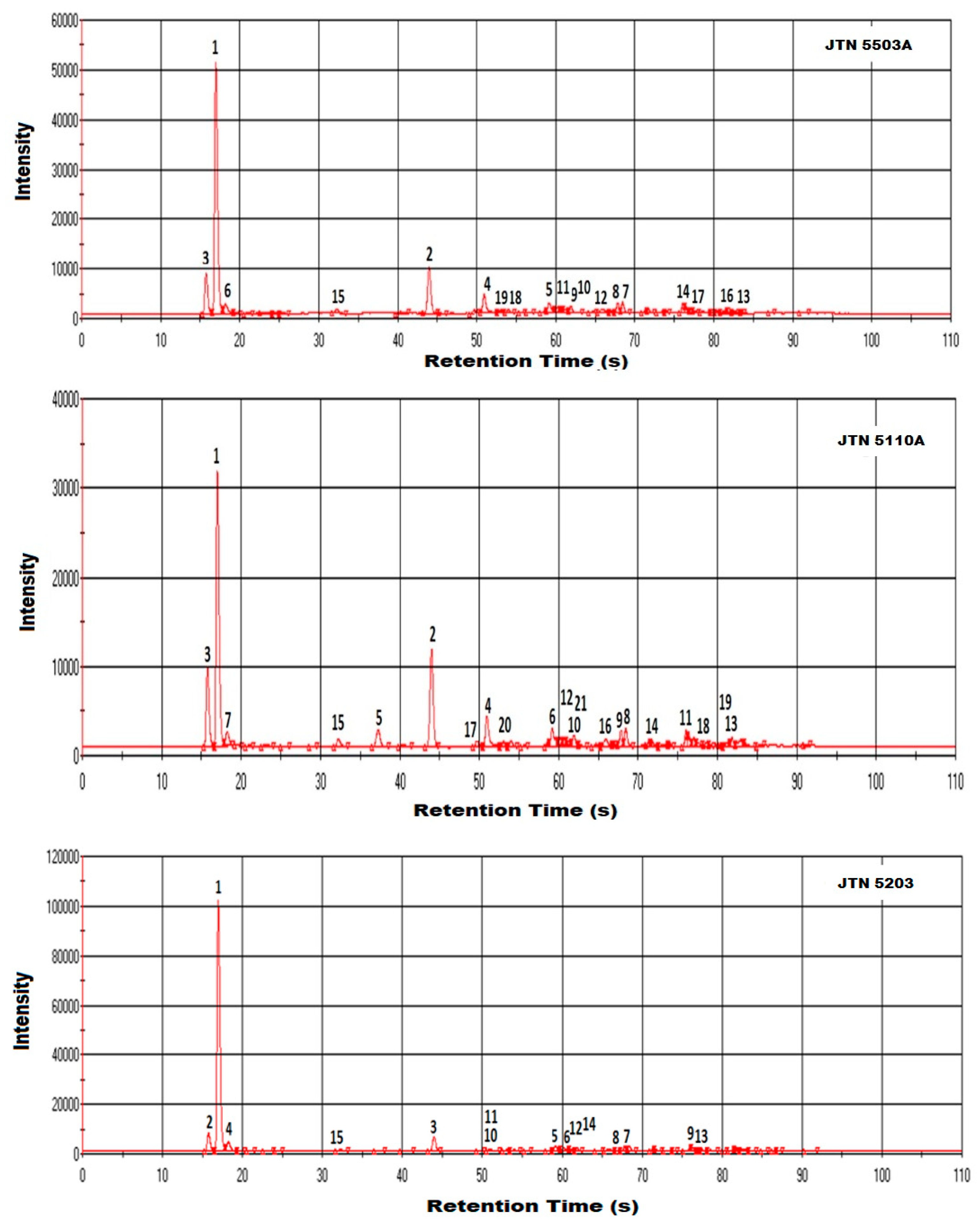
3.3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lv, Y.-C.; Song, H.-L.; Li, X.; Wu, L.; Guo, S.-T. Influence of Blanching and Grinding Process with Hot Water on Beany and Non-Beany Flavor in Soymilk. J. Food Sci. 2011, 76, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Lenis, J.M.; Gillman, J.D.; Lee, J.D.; Shannon, J.G.; Bilyeu, K.D. Soybean seed lipoxygenase genes: Molecular characterization and development of molecular marker assays. Theor. Appl. Genet. 2010, 120, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, R.; Hu, Y.; Xu, B. Flavor profiles of soymilk processed with four different processing technologies and 26 soybean cultivars grown in China. Int. J. Food Prop. 2017, 20, S2887–S2898. [Google Scholar] [CrossRef]
- Kato, H.; Doi, Y.; Tsugita, T.; Kosai, K.; Kamiya, T.; Kurata, T. Changes in volatile flavour components of soybeans during roasting. Food Chem. 1981, 7, 87–94. [Google Scholar] [CrossRef]
- Arai, S.; Noguchi, M.; Kaji, M.; Kato, H.; Fujimaki, M. n-Hexanal and Some Volatile Alcohols. Agric. Biol. Chem. 1970, 34, 1420–1423. [Google Scholar]
- Fujimaki, M.; Arai, S.; Kirigaya, N.; Sakurai, Y. Studies on Flavor Components in Soybean. Agric. Biol. Chem. 1965, 29, 855–863. [Google Scholar]
- Ram, M.S.; Seitz, L.M.; Rengarajan, R. Use of an Autosampler for Dynamic Headspace Extraction of Volatile Compounds from Grains and Effect of Added Water on the Extraction. J. Agric. Food Chem. 1999, 47, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, R.; de Lumen, B.O.; Habu, T.; Flath, R.A.; Richard Mon, T.; Teranishi, R. Comparison of Headspace Volatiles from Winged Beans and Soybeans. J. Agric. Food Chem. 1984, 32, 1011–1015. [Google Scholar] [CrossRef]
- Seitz, L.M.; Sauer, D.B. Cereal Foods World; AACC International: St. Paul, MN, USA, 1992. [Google Scholar]
- Boué, S.M.; Shih, B.Y.; Carter-Wientjes, C.H.; Cleveland, T.E. Identification of Volatile Compounds in Soybean at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2003, 51, 4873–4876. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Studies on the Key Aroma Compounds in Soy Milk Made from Three Different Soybean Cultivars. J. Agric. Food Chem. 2011, 59, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Advances in electronic-nose technologies developed for biomedical applications. Sensors (Basel) 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Arelli, P.R.; Young, L.D.; Mengistu, A. Registration of High Yielding and Multiple Disease-Resistant Soybean Germplasm JTN-5503. Crop Sci. 2006, 46, 2723. [Google Scholar] [CrossRef]
- Arelli, P.R.; Young, L.D.; Mengistu, A.; Gillen, A.M.; Fritz, L. Abstract: Newly Developed Conventional Soybean JTN-5110 Has Resistance to Multiple Pathogens. In Proceedings of the ASA, CSSA and SSSA International Annual Meetings, Long Beach, CA, USA, 2–5 November 2014. [Google Scholar]
- Arelli, P.R.; Pantalone, V.R.; Allen, F.L.; Mengistu, A.; Fritz, L.A. Registration of JTN-5203 Soybean Germplasm with Resistance to Multiple Cyst Nematode Populations. J. Plant Regist. 2014, 9, 108. [Google Scholar] [CrossRef]
- Chen, P.; Orazaly, M.; Wu, C.; Manjarrez-Sandoval, P.; Florez-Palacios, L.; Rupe, J.C.; Dombek, D.G.; Kirkpatrick, T.; Robbins, R.T. Registration of ‘UA 5014C’ Soybean. J. Plant Regist. 2016, 10, 119. [Google Scholar] [CrossRef]
- Chen, P. Soybean Cultivar UA 5414RR. U.S. Patent No. 9,326,478, 3 May 2016. [Google Scholar]


| Breeding Material | Plant Introduction # | Parental Lines | MG | Reference |
|---|---|---|---|---|
| JTN5503 | PI 641938 | Fowler × Manokin | V (5.5) | Arelli et al. [13] |
| JTN5110 | PI 678369 | J98-32 (Manokin × Fowler.) × Anand | V (5) | Arelli et al. [14] |
| JTN5203 | PI 664903 | Caviness × Anand | V (5) | Arelli et al. [15] |
| UA 5014C | PI 675648 | Ozark × Anand | V (5) | Chen et al. [16] |
| UA 5414RR | NA | R96-3427 × 98,601 | V (5.4) | Pengyin et al. [17] |
| Peak No | Volatile Compounds | Retention Time (s) | Kovat’s Index | |||
|---|---|---|---|---|---|---|
| Name | Surface Percent | Category/Total Percent | Sensory Descriptors | |||
| 19 | Pentanoic Acid | 0.91 ± 0.07 | Acids 1.73 | Beefy, cheese, pungent, sour, sweaty | 81.47 | 1366 |
| 21 | Butanoic Acid | 0.82 ± 0.06 | 77.49 | 1281 | ||
| 3 | 2-Propanol | 16.45 ± 1.26 | Alcohols 19.15 | Alcoholic, ethereal | 15.78 | 505 |
| 12 | 2-Methyl-1-Propanol | 1.54 ± 0.08 | Alcoholic, bitter, chemical, glue | 19.43 | 599 | |
| 15 | 3-Heptanol | 1.16 ± 0.13 | Green, herbaceous | 49.68 | 881 | |
| 2 | 2-Methyl Propanal | 18.21 ± 0.71 | Aldehydes 32.35 | Burnt, fruity, green malty, pungent, spicy, toasted | 17.03 | 538 |
| 4 | Benzaldehyde | 3.67 ± 0.33 | Almond, burnt sugar, fruity, woody | 59.17 | 971 | |
| 5 | P-Anisaldehyde | 3.39 ± 0.13 | Anise, minty, sweet | 76.02 | 1252 | |
| 7 | Butanal | 2.61 ± 0.08 | Chocolate, green, malty, pungent | 18.26 | 569 | |
| 8 | N-Nonanal | 2.44 ± 0.11 | Chlorine, citrus, fatty, floral, fruity, gaseous, gravy, green, lavender | 68.42 | 1110 | |
| 14 | Benzaldehyde | 1.28 ± 0.06 | Almond, burnt sugar, fruity, woody | 60.19 | 984 | |
| 22 | 2-Decenal | 0.75 ± 0.01 | Fatty, orange | 60.67 | 990 | |
| 1 | Ethyl-2-methyl Butyrate | 22.72 ± 1.92 | Esters 28.42 | Apple, blackberry, fruity, green, strawberry, sweet | 43.96 | 854 |
| 9 | Ethyl Heptanoate | 2.29 ± 0.08 | Grape like | 67.81 | 1099 | |
| 10 | Ethyl Butyrate | 1.71 ± 0.04 | Acetone, banana, bubblegum, caramelized, fruity | 32.26 | 799 | |
| 11 | Hexyl Acetate | 1.66 ± 0.04 | Acidulous, citrus, fruity, green, herbaceous, sweet wine, tobacco, rubber, spicy | 61.90 | 1007 | |
| 23 | Ethyl Hexanoate | 0.04 ± 0.01 | Anise, apple, fruity, strawberry, sweet, winegum | 82.96 | 1399 | |
| 6 | 2-Heptanone | 3.22 ± 0.10 | Ketones 7.72 | Cheese, cured ham, fruity, gaseous, gravy, nutty, soapy | 50.93 | 887 |
| 13 | Acetophenone | 1.51 ± 0.11 | Almond, cheese, floral, musty, sweet | 65.87 | 1069 | |
| 16 | Carvone | 1.09 ± 0.02 | Minty, warm, herbaceous | 77.04 | 1272 | |
| 17 | Delta Nonalactone | 1.05 ± 0.05 | Coconut | 81.86 | 1375 | |
| 20 | Gamma Nonalactone | 0.85 ± 0.09 | Coconut, fruity, peach, woody | 53.03 | 897 | |
| 18 | Trimethyl Pyrazine | 0.95 ± 0.08 | Pyrazines 0.95 | Cocoa, earthy, musty, nutty, peanut, potato, roasted nut | 61.20 | 997 |
| SUM | 90.26 ± 0.08 | |||||
| Peak No. | Volatile Compounds | Retention Time (s) | Kovat’s Index | |||
|---|---|---|---|---|---|---|
| Name | Surface Percent | Category/Total Percent | Sensory Descriptors | |||
| 12 | 3-Heptanol | 1.49 ± 0.05 | Alcohol 2.29 | Green, herbaceous | 49.68 | 881 |
| 20 | 1-Heptanol | 0.80 ± 0.04 | Green, herbaceous | 53.05 | 897 | |
| 2 | 2-Methyl Propanal | 19.42 ± 1.59 | Aldehydes 27.31 | Burnt, fruity, green malty, pungent, spicy, toasted | 17.05 | 538 |
| 5 | Benzaldehyde | 3.15 ± 0.23 | Almond, burnt sugar, fruity, woody | 59.16 | 970 | |
| 7 | p-Anisaldehyde | 2.78 ± 0.10 | Anise, minty, sweet | 76.05 | 1252 | |
| 9 | Phenylmethanal | 1.96 ± 0.12 | Almond, burnt sugar, fruity, woody | 60.20 | 984 | |
| 1 | Ethyl-2-Methyl Butyrate | 24.07 ± 4.36 | Esters 47.19 | Apple, blackberry, fruity, green, strawberry, sweet | 43.98 | 855 |
| 3 | Ethyl 2-Methylbutanoate | 16.01 ± 3.44 | Apple, blackberry, fruity, green, strawberry, sweet | 15.80 | 506 | |
| 8 | Ethyl Heptanoate | 2.11 ± 0.10 | Grape like | 68.43 | 1110 | |
| 10 | Ethyl Enanthate | 1.94 ± 0.10 | Acidic, fruity | 67.82 | 1099 | |
| 11 | Hexyl Acetate | 1.61 ± 0.12 | Acidulous, citrus, fruity, green, herbaceous, sweet wine, tobacco, rubber, spicy | 61.92 | 1007 | |
| 13 | Ethyl Butyrate | 1.45 ± 0.08 | Acetone, banana, bubblegum, caramelized, fruity | 32.30 | 799 | |
| 4 | 2-Heptanone | 4.30 ± 0.30 | Ketones 12.07 | Cheese, cured ham, fruity, gaseous, gravy, nutty, soapy | 50.95 | 887 |
| 6 | Butane-2,3-Dione | 2.83 ± 0.12 | Butter, caramelized, creamy, fruity, pineapple, spirit | 18.28 | 570 | |
| 14 | Acetophenone | 1.35 ± 0.03 | Almond, cheese, floral, musty, sweet | 65.92 | 1069 | |
| 16 | Delta Nonalactone | 1.04 ± 0.09 | Coconut | 81.96 | 1377 | |
| 17 | Carvone | 0.96 ± 0.01 | Minty, warm, herbaceous | 77.08 | 1273 | |
| 19 | Delta Nonalactone | 0.86 ± 0.03 | Coconut | 83.08 | 1401 | |
| 21 | γ-Nonalactone | 0.73 ± 0.01 | Coconut, fruity, peach, woody | 81.57 | 1368 | |
| 15 | ß-Pinene | 1.21 ± 0.06 | Monoterpens 1.21 | Terpenic | 60.68 | 990 |
| 18 | 2,5-Dimethyl Pyrazine | 0.89 ± 0.02 | Pyrazines 0.89 | Chocolate, cocoa, medicinal, roast beef, roasted nut, woody | 54.03 | 905 |
| SUM | 90.94 | |||||
| Peak No. | Volatile Compounds | Retention Time (s) | Kovat’s Index | |||
|---|---|---|---|---|---|---|
| Name | Surface Percent | Category/Total Percent | Sensory Descriptors | |||
| 13 | Methyl Eugenol | 1.15 ± 0.07 | Alcohol 1.9 | Clove, spicy. | 83.00 | 1400 |
| 19 | 3-Heptanol | 0.75 ± 0.04 | Green, herbaceous | 53.04 | 897 | |
| 5 | Benzaldehyde | 2.51 ± 0.11 | Aldehyde 9.3 | Almond, burnt sugar, fruity, woody | 59.15 | 970 |
| 6 | Butanal | 2.43 ± 0.14 | Chocolate, green, malty, pungent | 18.21 | 568 | |
| 7 | N-Nonanal | 1.86 ± 0.16 | Chlorine, citrus, fatty, floral, fruity, gaseous, gravy, green, lavender | 68.44 | 1110 | |
| 10 | P-Anisaldehyde | 1.28 ± 0.08 | Anise, minty, sweet | 76.06 | 1253 | |
| 11 | Benzaldehyde | 1.22 ± 0.07 | Almond, burnt sugar, fruity, woody | 60.19 | 984 | |
| 1 | Ethyl Formate | 48.29± 3.85 | Ester 70.21 | Smell of rum, Ethereal, pungent | 16.98 | 536 |
| 2 | Ethyl-2-Methyl Butyrate | 10.12± 1.14 | Apple, blackberry, fruity, green, strawberry, sweet | 43.97 | 855 | |
| 3 | Methyl Formate | 7.76 ± 0.58 | Ethereal, pungent | 15.75 | 505 | |
| 8 | Ethyl Heptanoate | 1.60 ± 0.12 | Grape like | 67.83 | 1099 | |
| 9 | Hexyl Acetate | 1.35 ± 0.06 | Acidulous, citrus, fruity, green, herbaceous, sweet wine, tobacco, rubber, spicy | 61.91 | 1007 | |
| 15 | Ethyl Butyrate | 1.09 ± 0.06 | Acetone, banana, bubblegum, caramelized, fruity | 32.26 | 799 | |
| 4 | 2-Heptanone | 4.38 ± 0.63 | Ketone 8.41 | Cheese, cured ham, fruity, gaseous, gravy, nutty, soapy | 50.93 | 887 |
| 12 | Acetophenone | 1.19 ± 0.05 | Almond, cheese, floral, musty, sweet | 65.91 | 1069 | |
| 14 | (+)-Carvone | 1.13 ± 0.06 | Caraway, minty, peppermint | 76.38 | 1259 | |
| 16 | Gamma Nonalactone | 0.87 ± 0.03 | Coconut, fruity, peach, woody | 81.88 | 1375 | |
| 17 | (−)-Carvone | 0.84 ± 0.02 | Caraway, minty, peppermint | 77.09 | 1273 | |
| 18 | 2,5-Dimethyl Pyrazine | 0.74 ± 0.09 | Pyrazines 0.74 | Chocolate, cocoa, medicinal, roast beef, roasted nut, woody | 53.99 | 904 |
| SUM | ||||||
| Peak No | Volatile Compounds | Retention Time (s) | Kovat’s Index | |||
|---|---|---|---|---|---|---|
| Name | Surface Percentage | Category/Total Percent | Sensory Descriptors | |||
| 20 | Pentanoic Acid | 0.83 ± 0.05 | Acids 0.83 | Beefy, cheese, pungent, sour, sweaty | 53.99 | 904 |
| 17 | 3-Heptanol | 0.95 ± 0.03 | Alcohol 2.29 | Green, herbaceous | 46.69 | 881 |
| 13 | Methyl Eugenol | 1.34 ± 0.07 | Clove, spicy | 82.95 | 1398 | |
| 18 | 2-Decenal | 0.92 ± 0.05 | Aldehyde 19.5 | Fatty, orange | 77.04 | 1272 |
| 6 | Benzaldehyde | 2.94 ± 0.19 | Almond, burnt sugar, fruity, woody | 59.17 | 971 | |
| 12 | Phenylmethanal | 1.39 ± 0.08 | Burnt sugar, fruity, woody | 60.19 | 984 | |
| 7 | Butanal | 2.45 ± 0.14 | Chocolate, green, malty, pungent | 18.25 | 569 | |
| 11 | P-Anisaldehyde | 1.45 ± 0.09 | Anise, minty, sweet | 76.02 | 1252 | |
| 3 | Propanal | 10.35 ± 0.33 | Ethereal, plastic, pungent, solvent | 15.76 | 505 | |
| 15 | Ethyl Butyrate | 1.28 ± 0.06 | Ester 18.5 | Acetone, banana, bubblegum, caramelized, fruity | 32.29 | 799 |
| 16 | Mthyl Butyrate | 1.25 ± 0.10 | Banana, bubblegum, caramelized, fruity | 65.92 | 1069 | |
| 2 | Ethyl-2-Methyl Butyrate | 14.39 ± 1.63 | Apple, blackberry, fruity, green, strawberry, sweet | 43.98 | 855 | |
| 10 | Hexyl Acetate | 1.58 ± 0.09 | Acidulous, citrus, fruity, green, herbaceous, sweet wine, tobacco, rubber, spicy | 61.92 | 1007 | |
| 5 | Furfural | 3.12 ± 0.40 | Furans 3.12 | Almond, bread, sweet | 37.26 | 823 |
| 14 | (-)-Carvone | 1.30 ±0.06 | Ketone 10.86 | Caraway, minty, peppermint | 76.35 | 1258 |
| 4 | 2-Heptanone | 4.80 ± 0.36 | Cheese, cured ham, fruity, gaseous, gravy, nutty, soapy | 50.95 | 887 | |
| 19 | Delta Nonalactone | 0.88 ± 0.06 | Coconut | 81.84 | 1374 | |
| 8 | Ethyl Heptanoate | 2.09 ± 0.12 | Grape like | 68.43 | 1110 | |
| 9 | Ethyl enanthate | 1.79 ± 0.12 | Pleasant, floral | 97.84 | 1099 | |
| 21 | Trimethyl Pyrazine | 0.80 ± 0.03 | Pyrazine 0.8 | Cocoa, earthy, musty, nutty, peanut, potato, roasted nut | 60.67 | 990 |
| 1 | Dimethyl Sulphide | 34.20 ± 3.31 | Sulfur 34.2 | Cabbage, fruity, gaseous, gasoline, moldy, vegetable soup | 17.01 | 537 |
| SUM | 90.09 ± 0.15 | |||||
| Peak No. | Volatile Compounds | Surface Percentage | Category | Sensory Descriptors | Retention Time (s) | Kovat’s Index |
|---|---|---|---|---|---|---|
| 15 | Butanoic Acid | 0.82 ± 0.02 | Acids 0.82 | Butter, cheese, rancid, sweaty | 65.87 | 1069 |
| 2 | 2-Propanol | 5.84 ± 0.63 | Alcohol 7.93 | Alcoholic, ethereal | 15.57 | 505 |
| 10 | 3-Heptanol | 1.04 ± 0.16 | 3-Heptanol | 75.96 | 1251 | |
| 13 | Methyl Eugenol | 1.05 ± 0.02 | Clove, spicy | 76.29 | 1257 | |
| 5 | Benzaldehyde | 2.13 ± 0.41 | Aldehyde 7.28 | Almond, burnt sugar, fruity, woody | 18.23 | 568 |
| 6 | Phenylmethanal | 1.46 ± 0.15 | Burnt sugar, fruity, woody | 59.12 | 970 | |
| 7 | N-Nonanal | 1.37 ± 0.10 | Chlorine, citrus, fatty, floral, fruity, gaseous, gravy, green, lavender | 60.16 | 983 | |
| 8 | Nonanaldehyde | 1.21 ± 0.15 | Chlorine, citrus, fatty, floral, fruity, gaseous, gravy, green, lavender | 68.38 | 1109 | |
| 9 | p-Anisaldehyde | 1.11 ± 0.22 | Anise, minty, sweet | 67.77 | 1098 | |
| 3 | Ethyl-2-Methyl Butyrate | 5.26 ± 0.70 | Ester 7.24 | Apple, blackberry, fruity, green, strawberry, sweet | 48.81 | 889 |
| 11 | 2,3-Hexen-1-Ol, Acetate | 1.01 ± 0.13 | Banana, fruity, green, sweet, sharp | 50.95 | 887 | |
| 12 | Phenyl Ethyl Acetate | 0.97 ± 0.16 | Phenyl Ethyl Acetate | 61.86 | 1006 | |
| 4 | Butane-2,3-Dione | 3.52 ± 0.37 | Ketone 4.5 | Butter, caramelized, creamy, fruity, pineapple, spirit | 43.97 | 855 |
| 14 | Acetophenone | 0.98±0.01 | Almond, cheese, floral, musty, sweet | 82.86 | 1397 | |
| 1 | Dimethyl Sulphide | 64.14 ± 3.91 | Sulfur 64.14 | Cabbage, fruity, gaseous, gasoline, moldy, vegetable soup | 16.97 | 536 |
| Sum | 91.92 ± 0.18 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, R.; Taheri, A.; Khandekar, D.; Millas, R. Rapid Profiling of Soybean Aromatic Compounds Using Electronic Nose. Biosensors 2019, 9, 66. https://doi.org/10.3390/bios9020066
Ravi R, Taheri A, Khandekar D, Millas R. Rapid Profiling of Soybean Aromatic Compounds Using Electronic Nose. Biosensors. 2019; 9(2):66. https://doi.org/10.3390/bios9020066
Chicago/Turabian StyleRavi, Ramasamy, Ali Taheri, Durga Khandekar, and Reneth Millas. 2019. "Rapid Profiling of Soybean Aromatic Compounds Using Electronic Nose" Biosensors 9, no. 2: 66. https://doi.org/10.3390/bios9020066
APA StyleRavi, R., Taheri, A., Khandekar, D., & Millas, R. (2019). Rapid Profiling of Soybean Aromatic Compounds Using Electronic Nose. Biosensors, 9(2), 66. https://doi.org/10.3390/bios9020066





