Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrodeposition of AuNPs
2.3. Electrochemical Techniques
2.4. Atomic Force Microscope Imaging
3. Results and Discussions
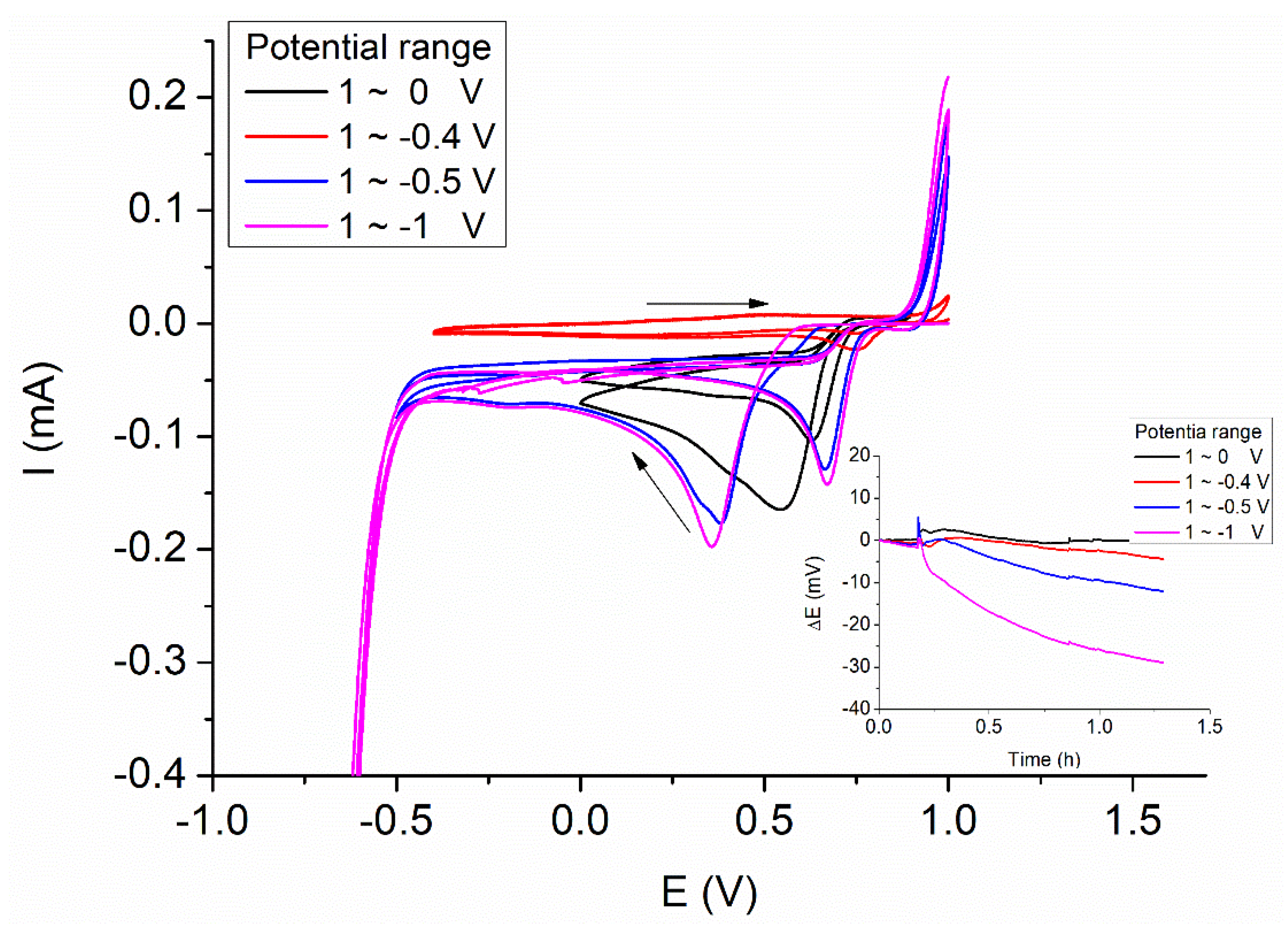
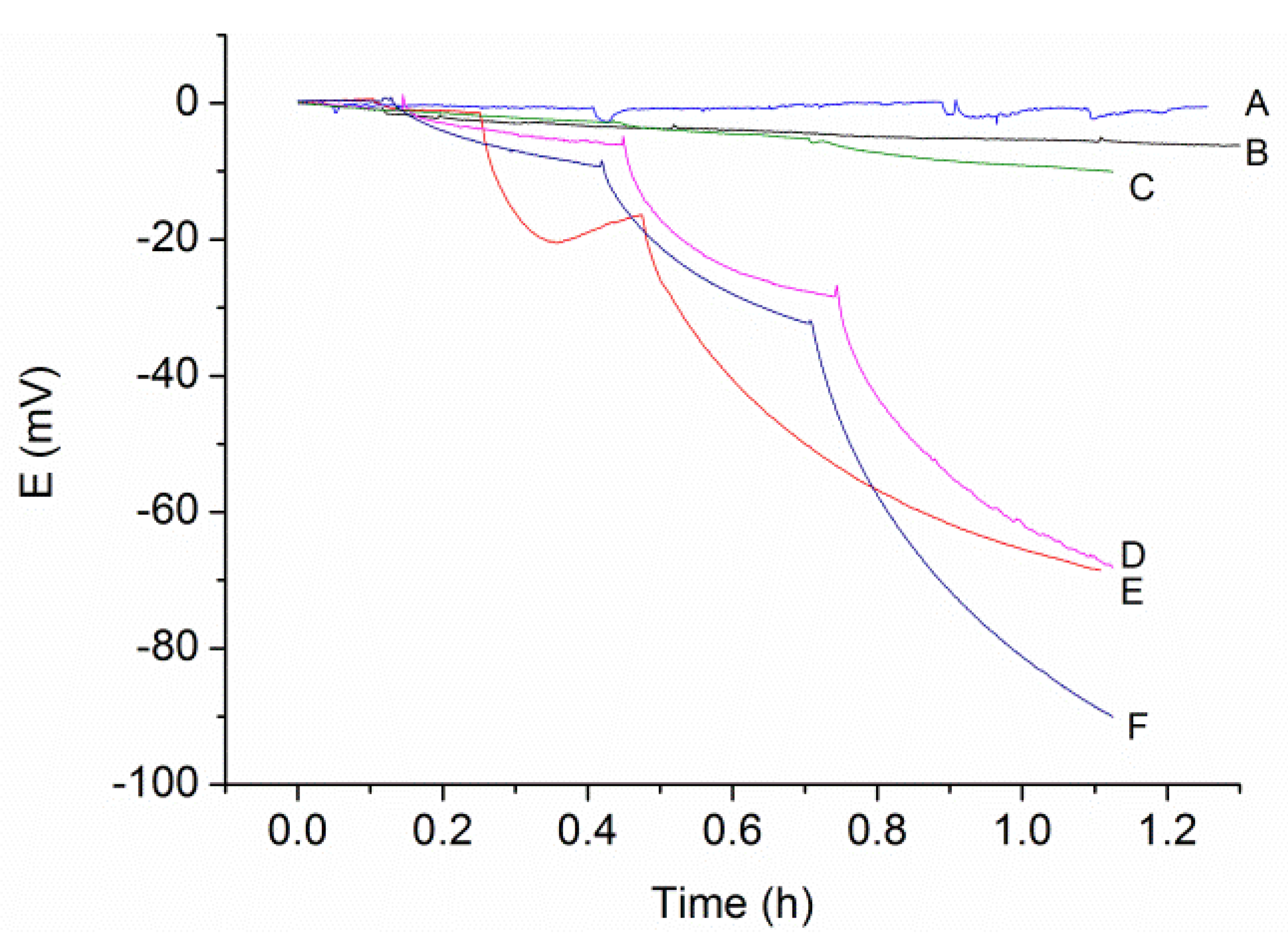
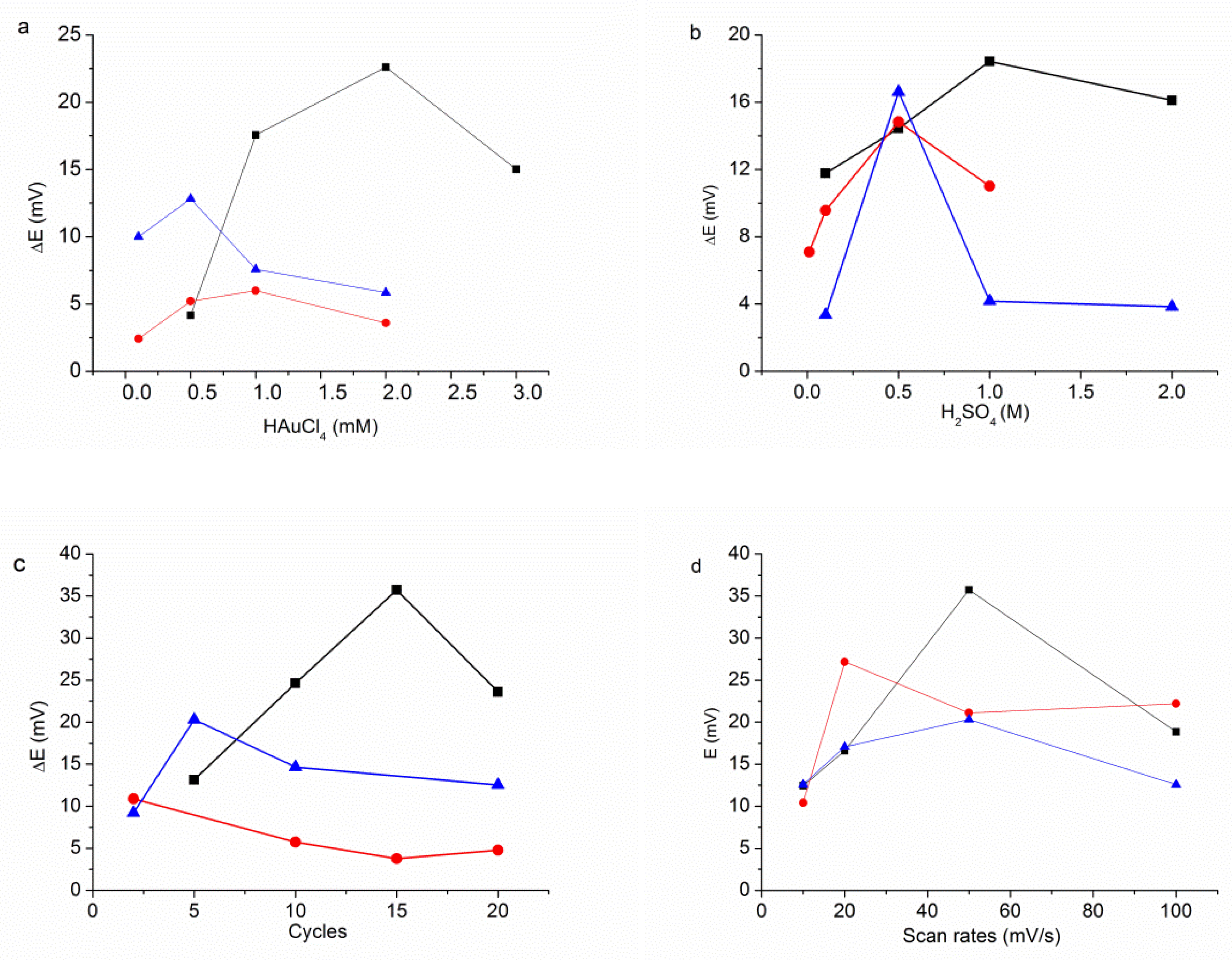
3.1. Electrodeposition Process
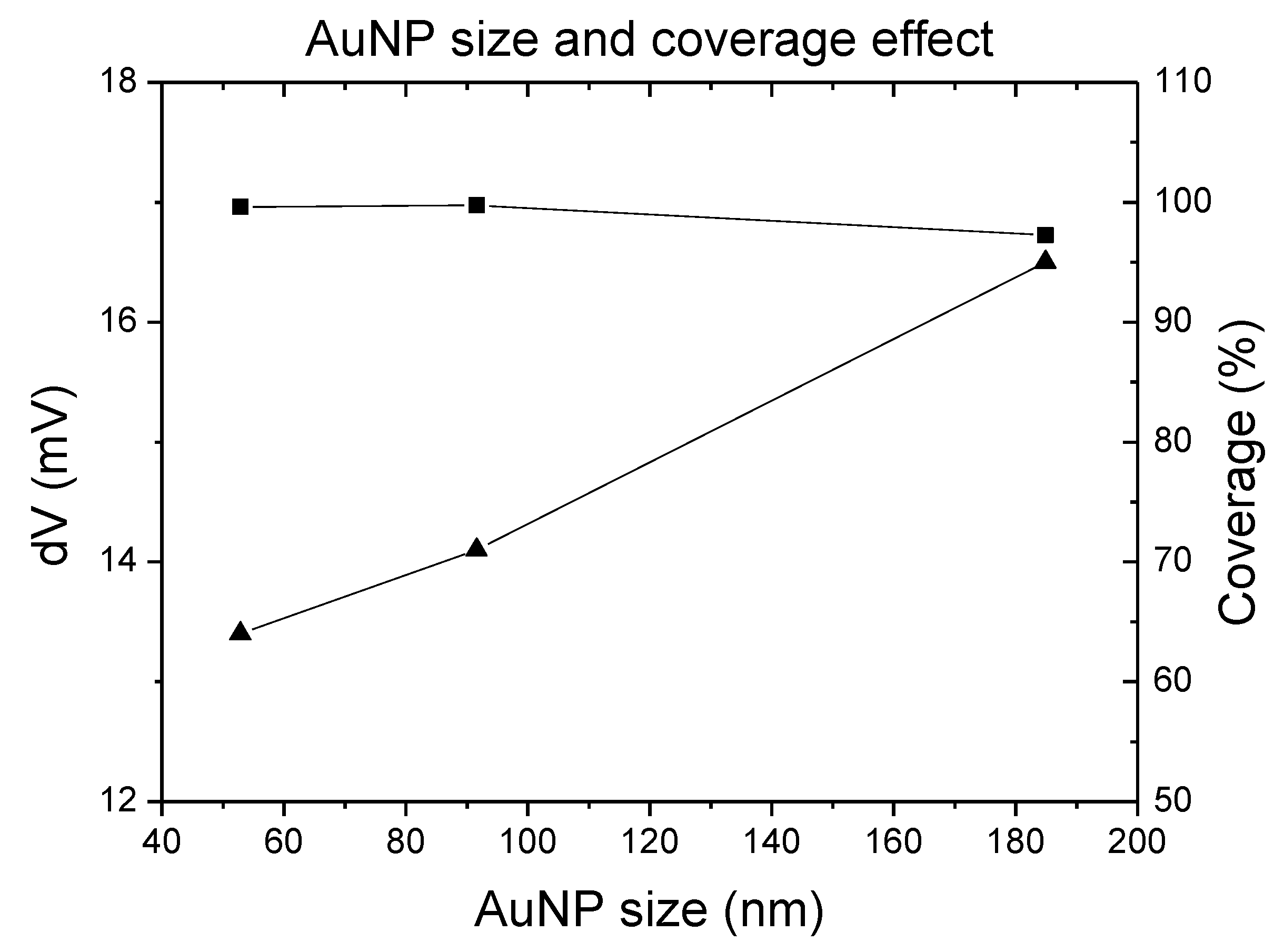
3.2. The Modification of AuNPs Electrode
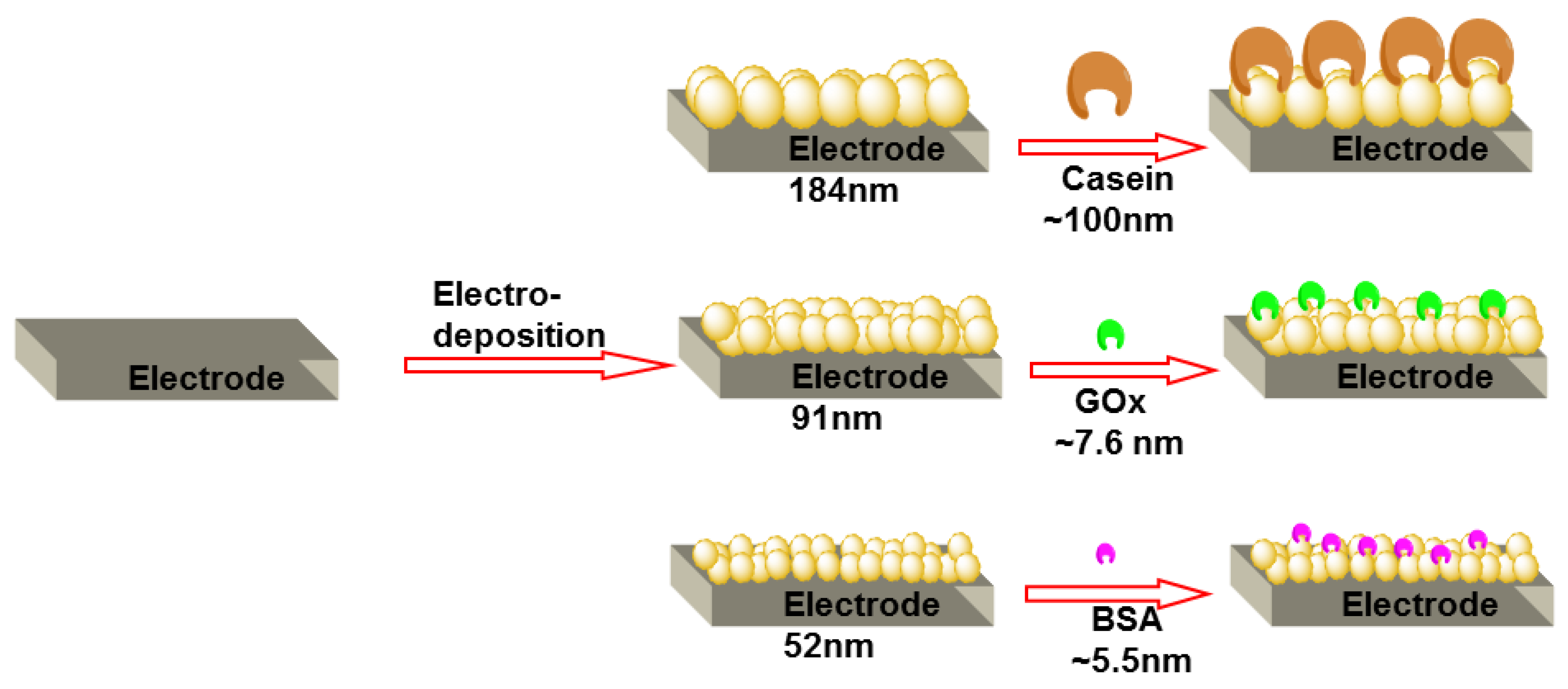
3.3. Deposition of Proteins on AuNP Covered Electrode Surface
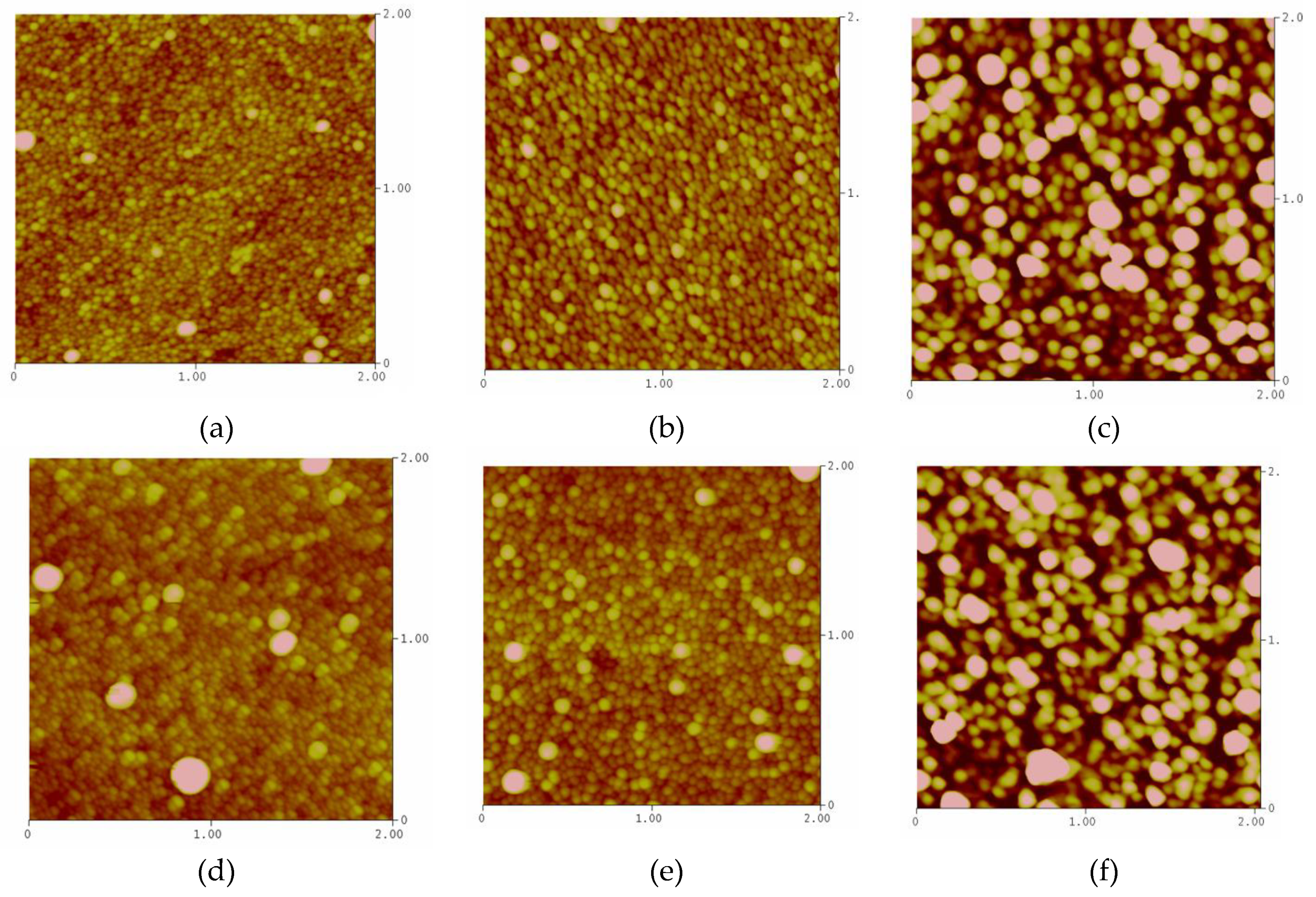
3.4. AFM Results and a Comparative Model
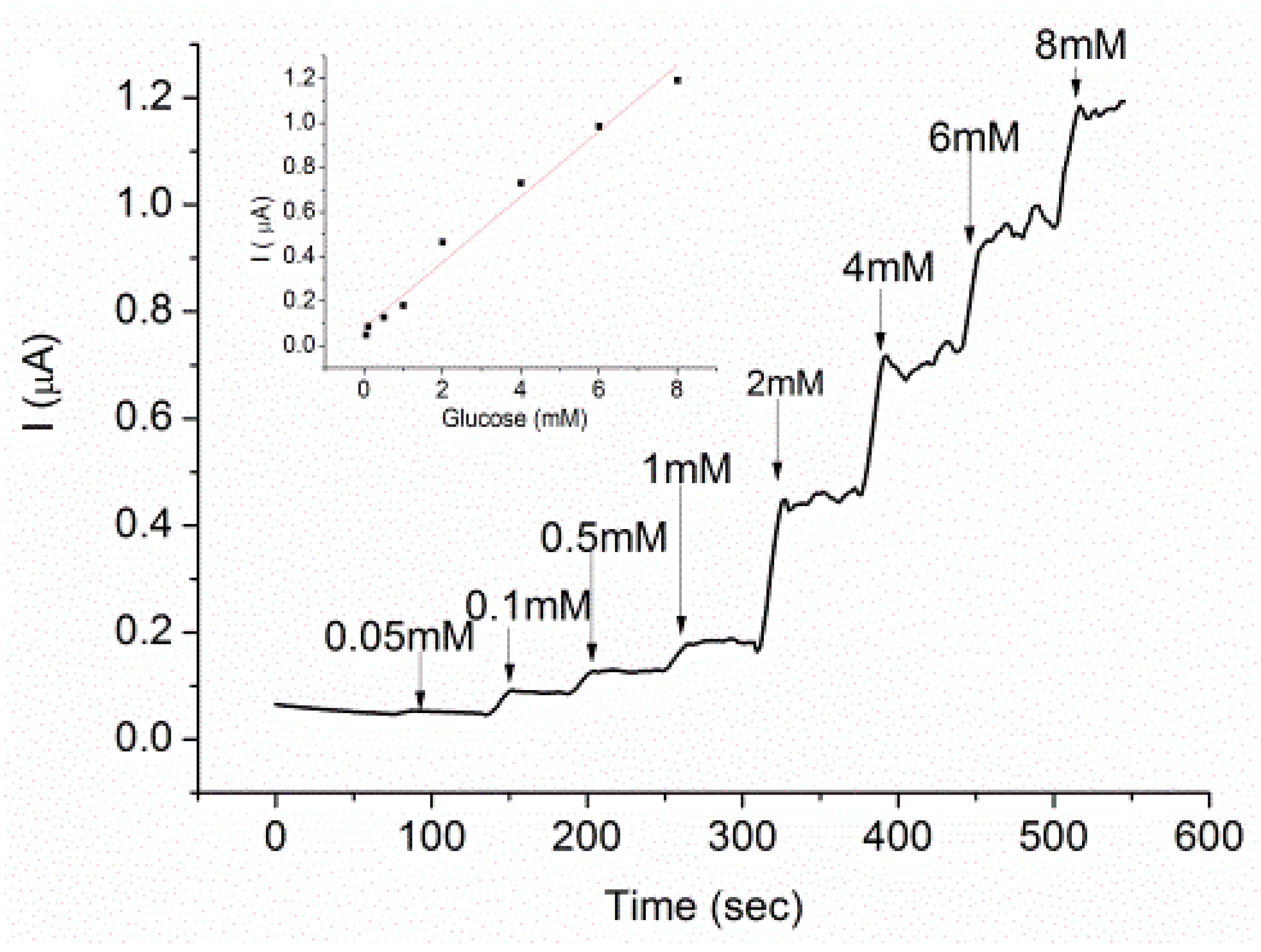
3.5. Glucose Characterization on GOx-Modified AuNP-Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays—A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2011, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Sokolov, J.; Rigas, B.; Levon, K.; Rafailovich, M. A potentiometric protein sensor built with surface molecular imprinting method. Biosens. Bioelectron. 2008, 24, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Jain, V.; Yi, J.; Mueller, S.; Sokolov, J.; Liu, Z.; Levon, K.; Rigas, B.; Rafailovich, M.H. Potentiometric sensors based on surface molecular imprinting: Detection of cancer biomarkers and viruses. Sens. Actuators B Chem. 2010, 146, 381–387. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Chang, C.C.; Liu, Y.; Yang, Z.; Guo, Y.; Wang, Y.; Galanakis, D.K.; Levon, K.; Rafailovich, M. Design of a molecular imprinting biosensor with multi-scale roughness for detection across a broad spectrum of biomolecules. Analyst 2016, 141, 5607–5617. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta. 2007, 599, 7–15. [Google Scholar] [CrossRef]
- Schöning, M.J.; Poghossian, A. Bio FEDs (field-effect devices): State-of-the-art and new directions. Electroanalysis 2006, 18, 1893–1900. [Google Scholar] [CrossRef]
- Zhang, Q.; Kaisti, M.; Prabhu, A.; Yu, Y.; Song, Y.A.; Rafailovich, M.H.; Rahman, A.; Levon, K. Polyaniline-functionalized ion-sensitive floating-gate FETs for the on-chip monitoring of peroxidase-catalyzed redox reactions. Electrochim. Acta 2018, 261, 256–264. [Google Scholar] [CrossRef]
- Palmeri, J.; Manghi, M.; Destainville, N. Thermal denaturation of fluctuating finite DNA chains: The role of bending rigidity in bubble nucleation. Phys. Rev. E 2008, 77, 011913. [Google Scholar] [CrossRef]
- Zhao, B.; Sai, T.; Rahman, A.; Levon, K. Floating-Gate Ion Sensitive Field-Effect Transistor for Chemical and Biological Sensing. MRS Online Proc. Libr. Arch. 2004, A7.8. [Google Scholar] [CrossRef]
- Zhang, Q.; Majumdar, H.S.; Kaisti, M.; Prabhu, A.; Ivaska, A.; Österbacka, R.; Rahman, A.; Levon, K. Surface functionalization of ion-sensitive floating-gate field-effect transistors with organic electronics. IEEE Trans. Electron Devices 2015, 62, 1291–1298. [Google Scholar] [CrossRef]
- Kaisti, M.; Zhang, Q.; Prabhu, A.; Lehmusvuori, A.; Rahman, A.; Levon, K. An Ion-Sensitive Floating Gate FET Model: Operating Principles and Electrofluidic Gating. IEEE Trans. Electron Devices 2015, 62, 2628–2635. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle Arrays on Surfaces for Electronic, Optical, and Sensor Applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Le Breton, J.P.; Alozy, E.; Boutin, J.Y.; Duval, A.; Gary, S.; Gontier, D.; Jasmin, S.; Naudy, M.; Reverdin, C.; Rosch, R.; et al. Laser Integration Line target diagnostics first results. Rev. Sci. Instrum. 2006, 77, 10F530. [Google Scholar] [CrossRef]
- El-Deab, M.S.; Sotomura, T.; Ohsaka, T. Size and Crystallographic Orientation Controls of Gold Nanoparticles Electrodeposited on GC Electrodes. J. Electrochem. Soc. 2005, 152, C1–C6. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Toth, I.; Monteiro, M.J.; Minchin, R.F. Molecular Interaction of Poly(acrylic acid) Gold Nanoparticles with Human Fibrinogen. ACS Nano 2012, 6, 8962–8969. [Google Scholar] [CrossRef]
- Grabar, K.C.; Smith, P.C.; Musick, M.D.; Davis, J.A.; Walter, D.G.; Jackson, M.A.; Guthrie, A.P.; Natan, M.J. Kinetic control of interparticle spacing in Au colloid-based surfaces: Rational nanometer-scale architecture. J. Am. Chem. Soc. 1996, 118, 1148–1153. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Arai, M.; Yu, K.; Tatsuma, T. Electrodeposition of gold nanoparticles on ITO: Control of morphology and plasmon resonance-based absorption and scattering. J. Electroanal. Chem. 2009, 628, 7–15. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based amplified transduction of biomolecular interactions. Small 2005, 1, 1036–1043. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Chen, X.; Li, Y.; Wu, L.; Zhang, J.; Tao, C.A. Construction of a Biosensor Based on a Combination of Cytochrome c, Graphene, and Gold Nanoparticles. Sensors 2019, 19, 40. [Google Scholar] [CrossRef]
- Gagner, J.E.; Lopez, M.D.; Dordick, J.S.; Siegel, R.W. Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 2011, 32, 7241–7252. [Google Scholar] [CrossRef]
- Libertino, S.; Aiello, V.; Scandurra, A.; Renis, M.; Sinatra, F. Immobilization of the enzyme glucose oxidase on both bulk and porous SiO2 surfaces. Sensors 2008, 8, 5637–5648. [Google Scholar] [CrossRef]
- Horne, D.S. Casein interactions: Casting light on the black boxes, the structure in dairy products. Int. Dairy J. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Di, J.; Tu, Y. Electrodeposition of large size gold nanoparticles on indium tin oxide glass and application as refractive index sensor. Electrochem. Commun. 2009, 11, 1034–1037. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Zhang, Y.; Chen, Z.; Cao, X.; Di, J.; Yang, J. Electrocatalytic behavior and amperometric detection of morphine on ITO electrode modified with directly electrodeposited gold nanoparticles. Electroanalysis 2009, 21, 939–943. [Google Scholar] [CrossRef]
- Wu, S.H.; Chiang, H.C.; Chen, K.C.; Hsu, C.C.; Tsiang, R.C.C. Morphological and electroluminescent effects of Au nanoparticles on light-emitting polymers. Nanotechnology 2007, 18, 345702. [Google Scholar] [CrossRef]
- Zhang, Q.; Prabhu, A.; San, A.; Al-Sharab, J.F.; Levon, K. A polyaniline based ultrasensitive potentiometric immunosensor for cardiac troponin complex detection. Biosens. Bioelectron. 2015, 72, 100–106. [Google Scholar] [CrossRef]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Development of an amperometric-based glucose biosensor to measure the glucose content of fruit. PLoS ONE 2015, 10, e0111859. [Google Scholar] [CrossRef]
- Moulton, S.E.; Barisci, J.N.; Bath, A.; Stella, R.; Wallace, G.G. Investigation of protein adsorption and electrochemical behavior at a gold electrode. J. Colloid Interface Sci. 2003, 261, 312–319. [Google Scholar] [CrossRef]
- El Roustom, B.; Fóti, G.; Comninellis, C. Preparation of gold nanoparticles by heat treatment of sputter deposited gold on boron-doped diamond film electrode. Electrochem. Commun. 2005, 7, 398–405. [Google Scholar] [CrossRef]
- Karnik, R.; Castelino, K.; Majumdar, A. Field-effect control of protein transport in a nanofluidic transistor circuit. Appl. Phys. Lett. 2006, 88, 123114. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Etesami, M.; Karoonian, F.S.; Mohamed, N. Electrochemical Deposition of Gold Nanoparticles on Pencil Graphite by Fast Scan Cyclic Voltammetry. J. Chin. Chem. Soc. 2011, 58, 688–693. [Google Scholar] [CrossRef]
- Chiang, H.C.; Levicky, R. Electrochemical Analysis of Ultrathin Polythiolsiloxane Films for Surface Biomodification. Int. J. Electrochem. 2018. [Google Scholar] [CrossRef]
- He, L.; Ren, X.; Gao, Q.; Zhao, X.; Yao, B.; Chao, Y. The connected-component labeling problem: A review of state-of-the-art algorithms. Pattern Recog. 2017, 70, 25–43. [Google Scholar] [CrossRef]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef]
- Jordan, B.J.; Hong, R.; Gider, B.; Hill, J.; Emrick, T.; Rotello, V.M. Stabilization of α-chymotrypsin at air-water interface through surface binding to gold nanoparticle scaffolds. Soft Matter 2006, 2, 558–560. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, H.-C.; Wang, Y.; Zhang, Q.; Levon, K. Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor. Biosensors 2019, 9, 50. https://doi.org/10.3390/bios9020050
Chiang H-C, Wang Y, Zhang Q, Levon K. Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor. Biosensors. 2019; 9(2):50. https://doi.org/10.3390/bios9020050
Chicago/Turabian StyleChiang, Hao-Chun, Yanyan Wang, Qi Zhang, and Kalle Levon. 2019. "Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor" Biosensors 9, no. 2: 50. https://doi.org/10.3390/bios9020050
APA StyleChiang, H.-C., Wang, Y., Zhang, Q., & Levon, K. (2019). Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor. Biosensors, 9(2), 50. https://doi.org/10.3390/bios9020050





