Plasma from Patients with Rheumatoid Arthritis Reduces Nitric Oxide Synthesis and Induces Reactive Oxygen Species in A Cell-Based Biosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Sample Collection
2.3. ECV-304 Cell Culture
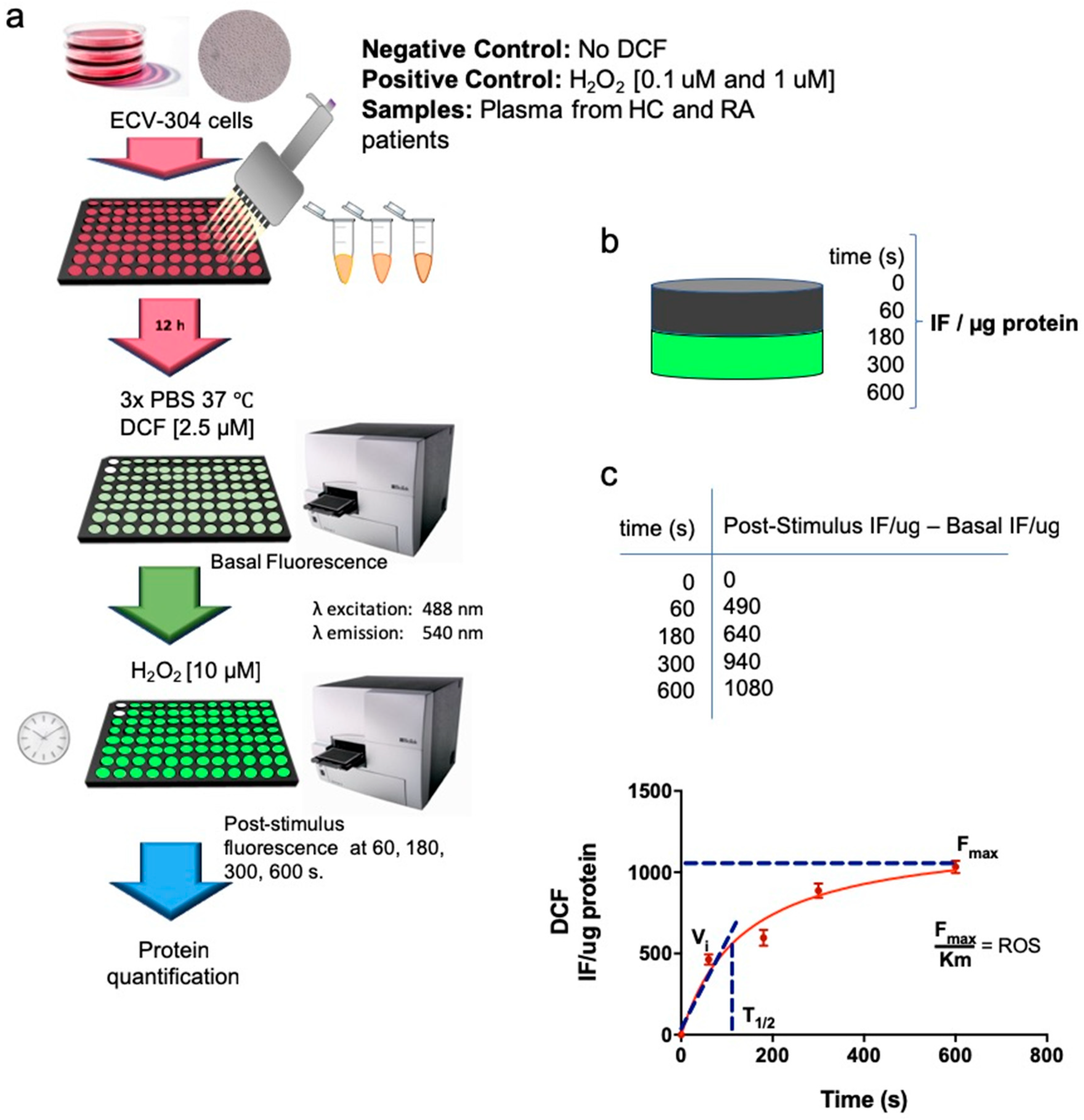
2.4. Intracellular ROS Production
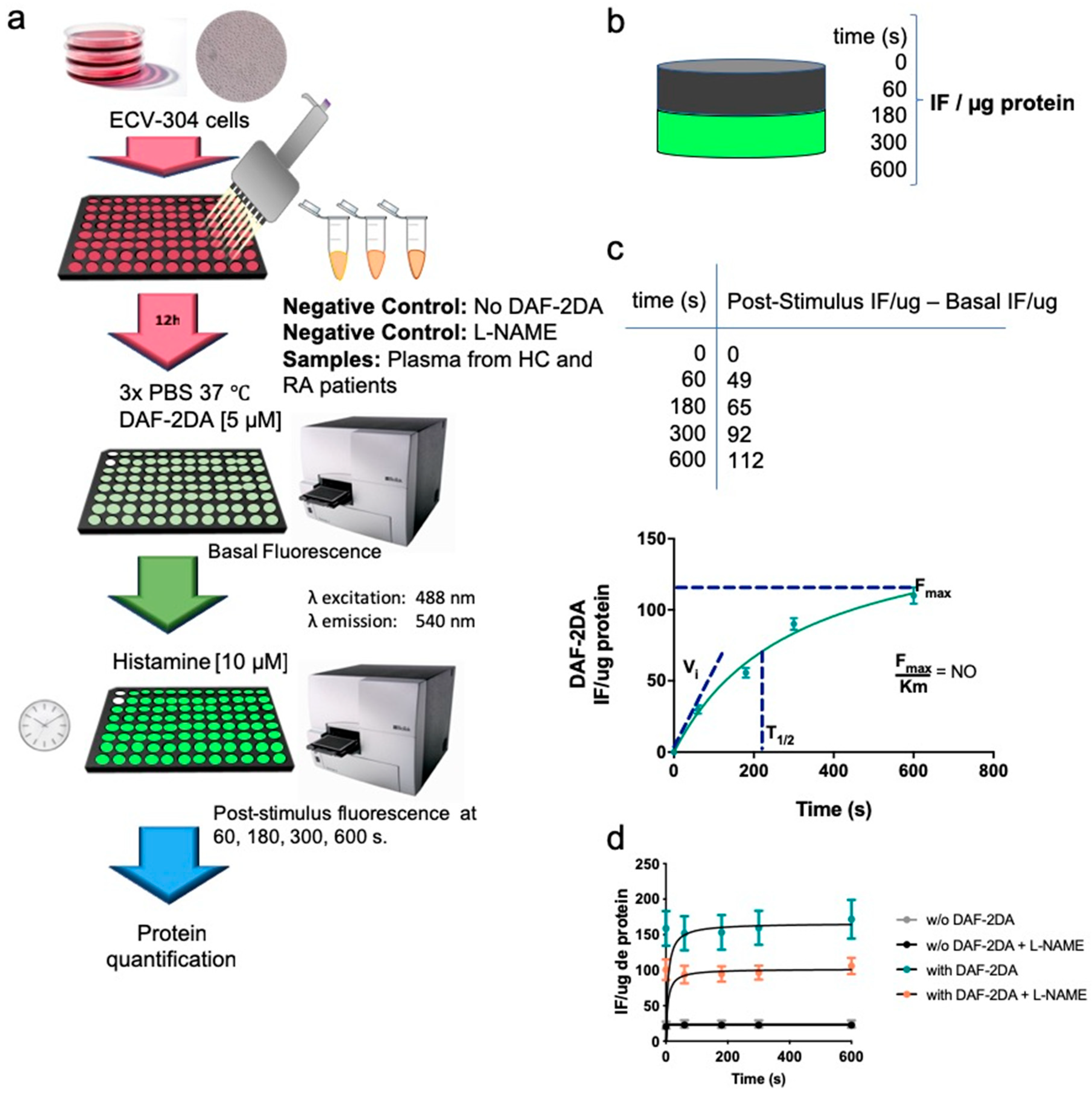
2.5. Intracellular Synthesis of NO
2.6. IL-6, sVCAM-1 and hs-CRP Levels in Plasma
2.7. Statistical Analysis
3. Results
3.1. RA Patients and Healthy Control Exhibit Similar Traditional CV Risk Factors
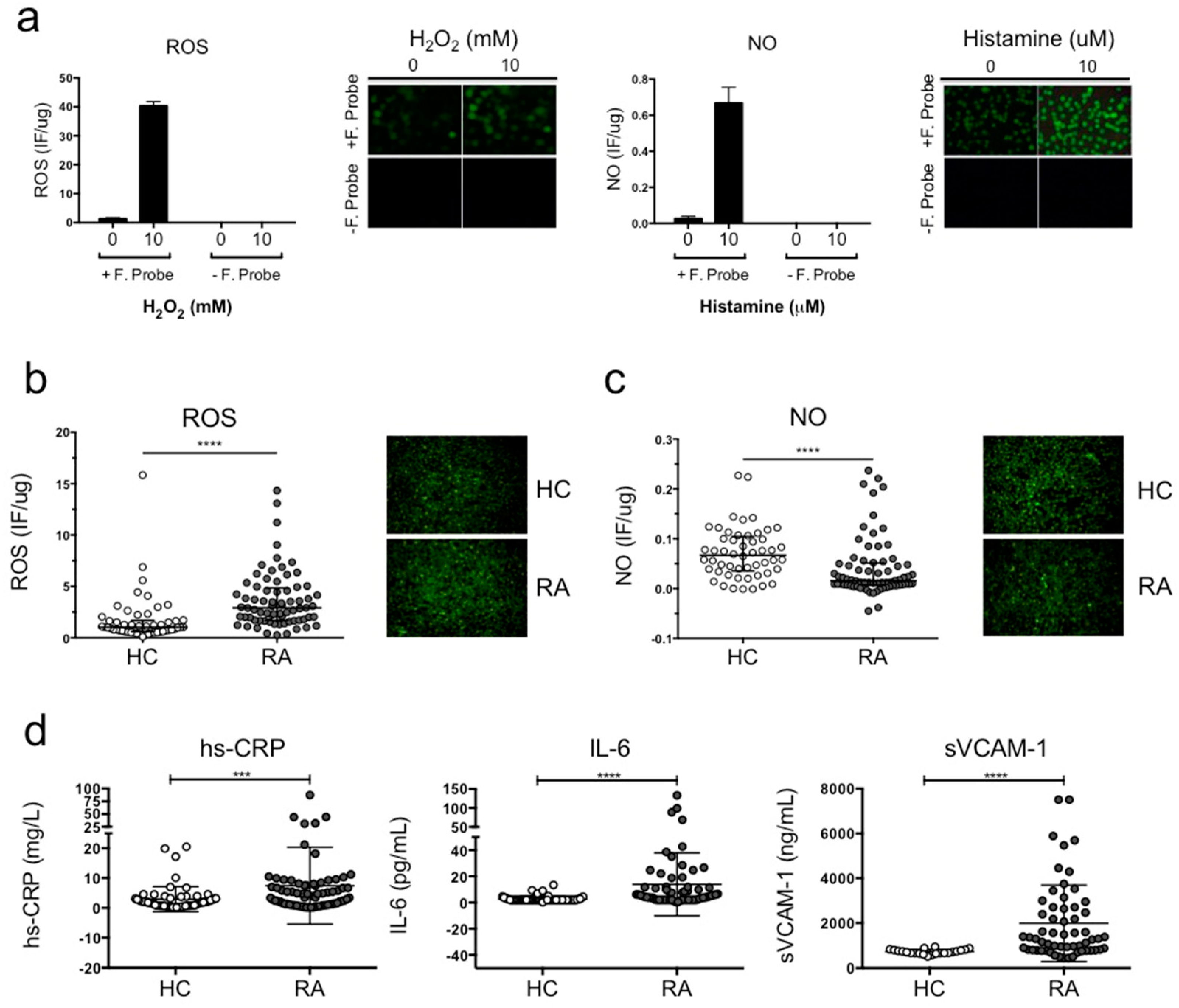
3.2. ECV-304 Cells Incubated with Plasma from RA Patients Exhibited Reduced NO Synthesis and Increased ROS Production Compared To Healthy Volunteers
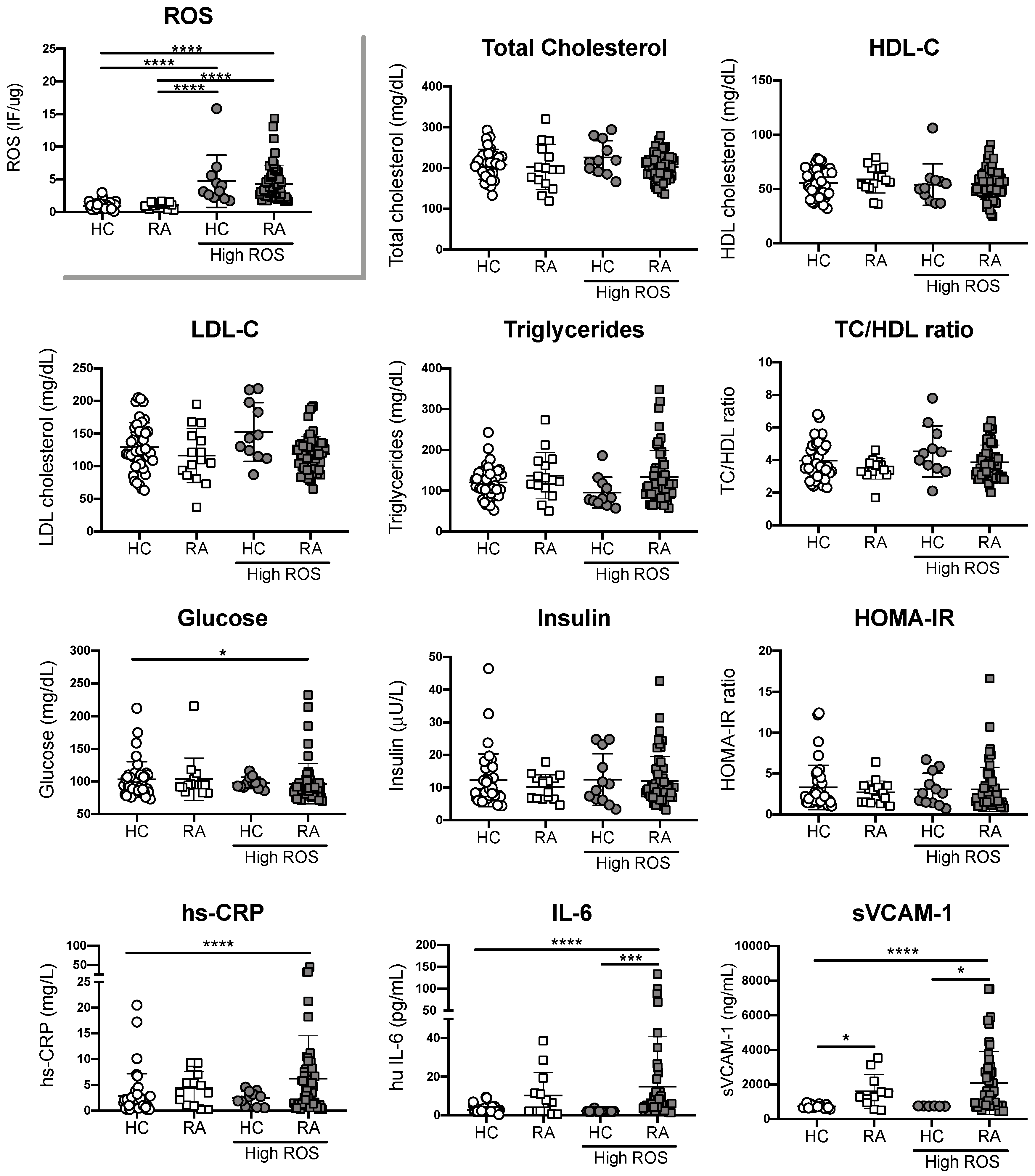
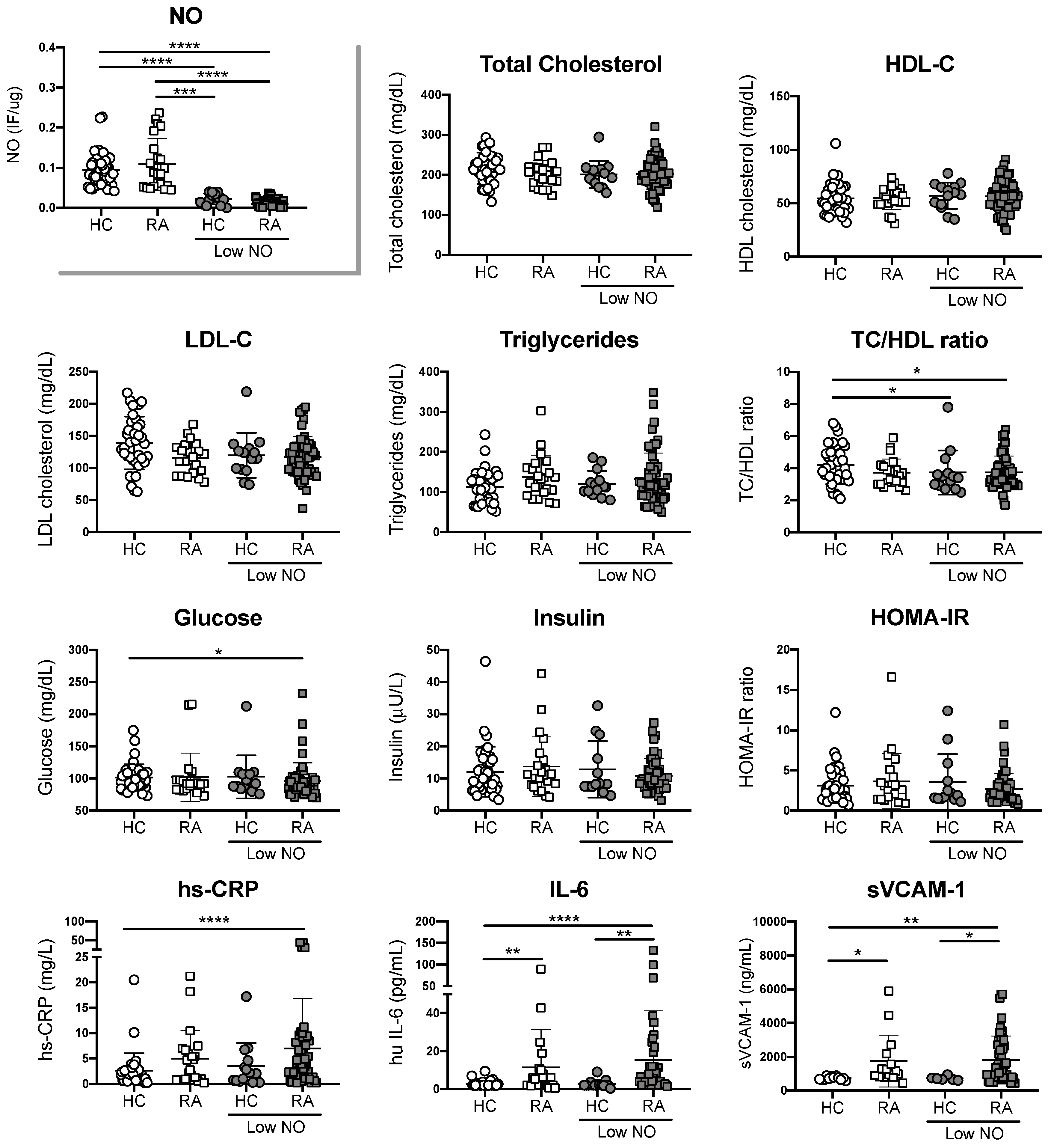
3.3. Endothelial Cell Oxidative Stress in RA Patients is Not Associated with Traditional CV Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- McInnes, I.B.; O’Dell, J.R. State-of-the-art: Rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Goodson, N.J.; Katz, J.N.; Weinblatt, M.E.; Avorn, J.; Setoguchi, S.; Canning, C.; Schneeweiss, S. Patterns of cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Farragher, T.M.; Goodson, N.J.; Naseem, H.; Silman, A.J.; Thomson, W.; Symmons, D.; Barton, A. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum. 2008, 58, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.E.H.; Smith, A.; Esdaile, J.M.; Barnabe, C.; Martin, L.O.; Faris, P.; Hazlewood, G.; Noormohamed, R.; Alvarez, N.; Mancini, G.B.J.; et al. Best practices for cardiovascular disease prevention in rheumatoid arthritis: A systematic review of guideline recommendations and quality indicators. Arthritis Care Res. 2015, 67, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.L.; van Halm, V.P.; Voskuyl, A.E.; Smulders, Y.M.; Boers, M.; Lems, W.F.; Visser, M.; Stehouwer, C.D.A.; Dekker, J.M.; Nijpels, G.; et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009, 61, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Freundlich, B.; Straus, W.L. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J. Rheumatol. 2003, 30, 36–40. [Google Scholar] [PubMed]
- Södergren, A.; Stegmayr, B.; Lundberg, V.; Ohman, M.-L.; Wållberg-Jonsson, S. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 263–266. [Google Scholar] [CrossRef] [PubMed]
- van den Oever, I.A.M.; van Sijl, A.M.; Nurmohamed, M.T. Management of cardiovascular risk in patients with rheumatoid arthritis: Evidence and expert opinion. Ther. Adv. Musculoskelet. Dis. 2013, 5, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.J.; Douglas, K.M.J.; Saratzis, A.N.; Kitas, G.D. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev. Mol. Med. 2005, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Del Rincón, I.D.; Williams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Chung, C.P.; Giles, J.T.; Petri, M.; Szklo, M.; Post, W.; Blumenthal, R.S.; Gelber, A.C.; Ouyang, P.; Jenny, N.S.; Bathon, J.M. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: Comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin. Arthritis Rheum. 2012, 41, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Maradit Kremers, H.; Crowson, C.S.; Ballman, K.V.; Roger, V.L.; Jacobsen, S.J.; O’Fallon, W.M.; Gabriel, S.E. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann. Rheum. Dis. 2008, 67, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Profumo, E.; Di Franco, M.; Buttari, B.; Masella, R.; Filesi, C.; Tosti, M.E.; Scrivo, R.; Scarno, A.; Spadaro, A.; Saso, L.; et al. Biomarkers of subclinical atherosclerosis in patients with autoimmune disorders. Mediat. Inflamm. 2012, 2012, 503942. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Kitas, G.D.; Carroll, D.; Veldhuijzen van Zanten, J.J.C.S. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: A cross-sectional and longitudinal study. Arthritis Res. Ther. 2012, 14, R117. [Google Scholar] [CrossRef] [PubMed]
- Finckh, A.; Courvoisier, D.S.; Pagano, S.; Bas, S.; Chevallier-Ruggeri, P.; Hochstrasser, D.; Roux-Lombard, P.; Gabay, C.; Vuilleumier, N. Evaluation of cardiovascular risk in patients with rheumatoid arthritis: Do cardiovascular biomarkers offer added predictive ability over established clinical risk scores? Arthritis Care Res. 2012, 64, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Södergren, A.; Karp, K.; Boman, K.; Eriksson, C.; Lundström, E.; Smedby, T.; Söderlund, L.; Rantapää-Dahlqvist, S.; Wållberg-Jonsson, S. Atherosclerosis in early rheumatoid arthritis: Very early endothelial activation and rapid progression of intima media thickness. Arthritis Res. Ther. 2010, 12, R158. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, A.N.; Voulgari, P.V.; Argyropoulou, M.I.; Alamanos, Y.; Elisaf, M.; Tselepis, A.D.; Drosos, A.A. Early treatment reduces the cardiovascular risk factors in newly diagnosed rheumatoid arthritis patients. Semin. Arthritis Rheum. 2008, 38, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Dimitroulas, T.; Veldhuijzen van Zanten, J.J.C.S.; Smith, J.P.; Metsios, G.S.; Nightingale, P.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Lack of association between asymmetric dimethylarginine and in vivo microvascular and macrovascular endothelial function in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, 388–396. [Google Scholar] [PubMed]
- Tanasescu, C.; Jurcut, C.; Jurcut, R.; Ginghina, C. Vascular disease in rheumatoid arthritis: From subclinical lesions to cardiovascular risk. Eur. J. Intern. Med. 2009, 20, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Gómez-Rosso, L.; Meroño, T.; Salomon, C.; Durán-Sandoval, D.; Giunta, G.; Grant, C.; Calvo, C.; Lamperti, L.; Brites, F.; et al. High LDL levels are associated with increased lipoprotein-associated phospholipase A(2) activity on nitric oxide synthesis and reactive oxygen species formation in human endothelial cells. Clin. Biochem. 2011, 44, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Knepler, J.L.; Taher, L.N.; Gupta, M.P.; Patterson, C.; Pavalko, F.; Ober, M.D.; Hart, C.M. Peroxynitrite causes endothelial cell monolayer barrier dysfunction. Am. J. Physiol. Cell Physiol. 2001, 281, C1064–C1075. [Google Scholar] [CrossRef] [PubMed]
- Mojiminiyi, O.A.; Abdella, N.A. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin. Chem. Lab. Med. 2010, 48, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of inflammatory cytokines in rheumatoid arthritis patients: Correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef] [PubMed]
- Kwaśny-Krochin, B.; Głuszko, P.; Undas, A. Plasma asymmetric dimethylarginine in active rheumatoid arthritis: Links with oxidative stress and inflammation. Pol. Arch. Med. Wewn. 2012, 122, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Dimitroulas, T.; Hodson, J.; Smith, J.P.; Douglas, K.M.; Kitas, G.D. Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: A 6 year follow-up study. Rheumatology 2015, 54, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial injury in rheumatoid arthritis: A crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Zinellu, A.; Sotgia, S.; Carru, C.; Piga, M.; Erre, G.L. Protective Effects of Methotrexate against Proatherosclerotic Cytokines: A Review of the Evidence. Mediat. Inflamm. 2017, 2017, 9632846. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.; Syngle, A.; Krishan, P. Predictors of endothelial dysfunction and atherosclerosis in rheumatoid arthritis in Indian population. Indian Heart J. 2017, 69, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hjeltnes, G.; Hollan, I.; Førre, Ø.; Wiik, A.; Mikkelsen, K.; Agewall, S. Anti-CCP and RF IgM: Predictors of impaired endothelial function in rheumatoid arthritis patients. Scand. J. Rheumatol. 2011, 40, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Totoson, P.; Maguin-Gaté, K.; Nappey, M.; Wendling, D.; Demougeot, C. Endothelial Dysfunction in Rheumatoid Arthritis: Mechanistic Insights and Correlation with Circulating Markers of Systemic Inflammation. PLoS ONE 2016, 11, e0146744. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Selmi, C.; Angelini, C.; Meroni, P.L. Evaluation of Endothelial Function by Flow-Mediated Dilation: A Comprehensive Review in Rheumatic Disease. Arch. Immunol. Ther. Exp. 2017, 65, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Hamza, A.-B. Reactive oxygen and nitrogen species in patients with rheumatoid arthritis as potential biomarkers for disease activity and the role of antioxidants. Free Radic. Biol. Med. 2016, 97, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Mondal, C.; Haldar, P.K.; Khandelwal, B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: Clinical efficacy of dietary antioxidants. Inflammopharmacology 2017, 25, 595–607. [Google Scholar] [CrossRef] [PubMed]
| RA Patients (n = 73) | |
|---|---|
| Sex female | 66 (90%) |
| Disease duration (years) | 14.5 ± 9.7 |
| VAS (mm) | 52.5 ± 24.7 |
| Global assessment (mm) | 46.5 ± 23.9 |
| Tender joints | 3 ± 3.9 |
| Swollen joints | 2.7 ± 3.4 |
| DAS28 | 3.7 ± 1.2 |
| HAQ | 1.3 ± 0.9 |
| ESR (mm/h) | 22 ± 15.6 |
| Anti-CCP (UA/mL) † | 48.8 ± 90.5 |
| RF (mg/dL) ‡ | 241.6 ± 449.3 |
| DMARDs Methotrexate Leflunomide Sulfasalazine Hydroxychloroquine | 64 (88%) 21 (29%) 4 (6%) 1 (1%) |
| Prednisolone | 72 (99%) |
| RA Patients (n = 73) | Control Subjects (n = 52) | P† | |
|---|---|---|---|
| Sex female Age(years) | 66/73 58 (50−67) | 42/52 57 (48−62) | 0.200 |
| Weight (kg) | 69 (59−75) | 71.7 (61.0−79.7) | 0.149 |
| Height (m) ‡ | 1.6 (1.5−1.6) | 1.6 (1.5−1.6) | 0.179 |
| BMI (kg/m2) | 27.6 (24.0−30.8) | 28.6 (25.0−30.7) | 0.499 |
| Waist circumference (cm) | 100 (90−106) | 95 (82−104) | 0.027 |
| SBP (mm Hg) ‡ | 125 (120−140) | 120 (110−120) | <0.001 |
| DBP (mm Hg) | 80 (70−90) | 77.5 (70−80) | 0.007 |
| Total cholesterol (mg/dL) | 200 (181−221) | 212 (185−230) | 0.161 |
| HDL-C | 56 (±14) | 55 (±14) | 0.860 |
| LDL-C | 118 (±31) | 134 (±40) | 0.020 |
| Triglycerides | 117 (91–156) | 111 (83–133) | 0.207 |
| TC/HDL ratio | 3.8 (±1.0) | 4.1 (±1.3) | 0.143 |
| Glucose | 91 (83−98) | 97 (89−107) | - |
| Insulin | 10.1 (7−13.8) | 9.1 (7.4−16.2) | 0.887 |
| HOMA-IR | 2.3 (1.5−3.3) | 2.5 (1.6−4.1) | 0.482 |
| Hypertension | 34 (47%) | 12 (23%) | 0.007 |
| Diabetes mellitus | 11 (15%) | 3 (6%) | 0.104 |
| Smoking | 15 (21%) | 12 (23%) | 0.735 |
| Family history of coronary-arterial disease | 5 (7%) | 12 (23%) | 0.009 |
| ROS | NO | |||||
|---|---|---|---|---|---|---|
| Normal (n = 15) | High (n = 57) | P | Normal (n = 22) | Low (n = 50) | P | |
| Disease duration (years) | 15.2 (±11.2) | 14.0 (±9.3) | 0.674 | 15.3 (±7.9) | 14.2 (±10.4) | 0.644 |
| Prednisolone dose (mg/day) | 8.7 (±2.3) | 8.2 (±2.9) | 0.553 | 9.0 (±2.8) | 8.0 (±2.7) | 0.176 |
| Tender joints | 2.3 (±3.8) | 3.2 (±4.0) | 0.436 | 3.2 (±3.5) | 3.0 (±4.1) | 0.748 |
| Swollen joints | 2.6 (±3.0) | 2.7 (±3.6) | 0.938 | 3.0 (±3.1) | 2.5 (±3.6) | 0.598 |
| Global assessment (mm) | 36.7 (±24.1) | 49.1 (±23.7) | 0.076 | 43.2 (±24.2) | 47.6 (±24.0) | 0.482 |
| VAS (mm) | 42.7 (±24.0) | 54.8 (±24.6) | 0.091 | 56.0 (±28.1) | 50.9 (±23.3) | 0.435 |
| DAS28 | 3.4 (±1.1) | 3.8 (±1.2) | 0.248 | 4.0. (±1.0) | 3.6 (±1.2) | 0.123 |
| HAQ | 1.0 (±0.9) | 1.2 (±0.9) | 0.209 | 1.4 (±1.0) | 1.3 (±0.9) | 0.775 |
| ESR (mm/h) | 20.6 (±10.2) | 22.7 (±16.7) | 0.548 | 24.0 (±12.7) | 20.8 (±16.7) | 0.420 |
| Anti-CCP (UA/mL) | 37.0 (±83.9) | 52.6 (±93.1) | 0.570 | 72.6 (±95.4) | 38.8 (±87.1) | 0.152 |
| RF (mg/dL) | 90.1 (±132.6) | 282.8 (±493.2) | 0.012 * | 325.0 (±690.7) | 203.8 (±288.6) | 0.436 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herlitz-Cifuentes, H.; Vejar, C.; Flores, A.; Jara, P.; Bustos, P.; Castro, I.; Poblete, E.; Saez, K.; Opazo, M.; Gajardo, J.; et al. Plasma from Patients with Rheumatoid Arthritis Reduces Nitric Oxide Synthesis and Induces Reactive Oxygen Species in A Cell-Based Biosensor. Biosensors 2019, 9, 32. https://doi.org/10.3390/bios9010032
Herlitz-Cifuentes H, Vejar C, Flores A, Jara P, Bustos P, Castro I, Poblete E, Saez K, Opazo M, Gajardo J, et al. Plasma from Patients with Rheumatoid Arthritis Reduces Nitric Oxide Synthesis and Induces Reactive Oxygen Species in A Cell-Based Biosensor. Biosensors. 2019; 9(1):32. https://doi.org/10.3390/bios9010032
Chicago/Turabian StyleHerlitz-Cifuentes, Herbert, Camila Vejar, Alejandra Flores, Paola Jara, Paulina Bustos, Irene Castro, Evelyn Poblete, Katia Saez, Marina Opazo, Jorge Gajardo, and et al. 2019. "Plasma from Patients with Rheumatoid Arthritis Reduces Nitric Oxide Synthesis and Induces Reactive Oxygen Species in A Cell-Based Biosensor" Biosensors 9, no. 1: 32. https://doi.org/10.3390/bios9010032
APA StyleHerlitz-Cifuentes, H., Vejar, C., Flores, A., Jara, P., Bustos, P., Castro, I., Poblete, E., Saez, K., Opazo, M., Gajardo, J., Aguayo, C., Nova-Lamperti, E., & Lamperti, L. (2019). Plasma from Patients with Rheumatoid Arthritis Reduces Nitric Oxide Synthesis and Induces Reactive Oxygen Species in A Cell-Based Biosensor. Biosensors, 9(1), 32. https://doi.org/10.3390/bios9010032






