“The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space
Abstract
1. Introduction
1.1. Need for Miniaturized Sensors for Future Space Missions
1.2. Smartphone Based Devices for Facile Crew Health Monitoring during Deep Space Missions
1.3. Obstacles to Overcome to Enable SBD Use in Space
2. Existing SBDs Useful for Space Missions
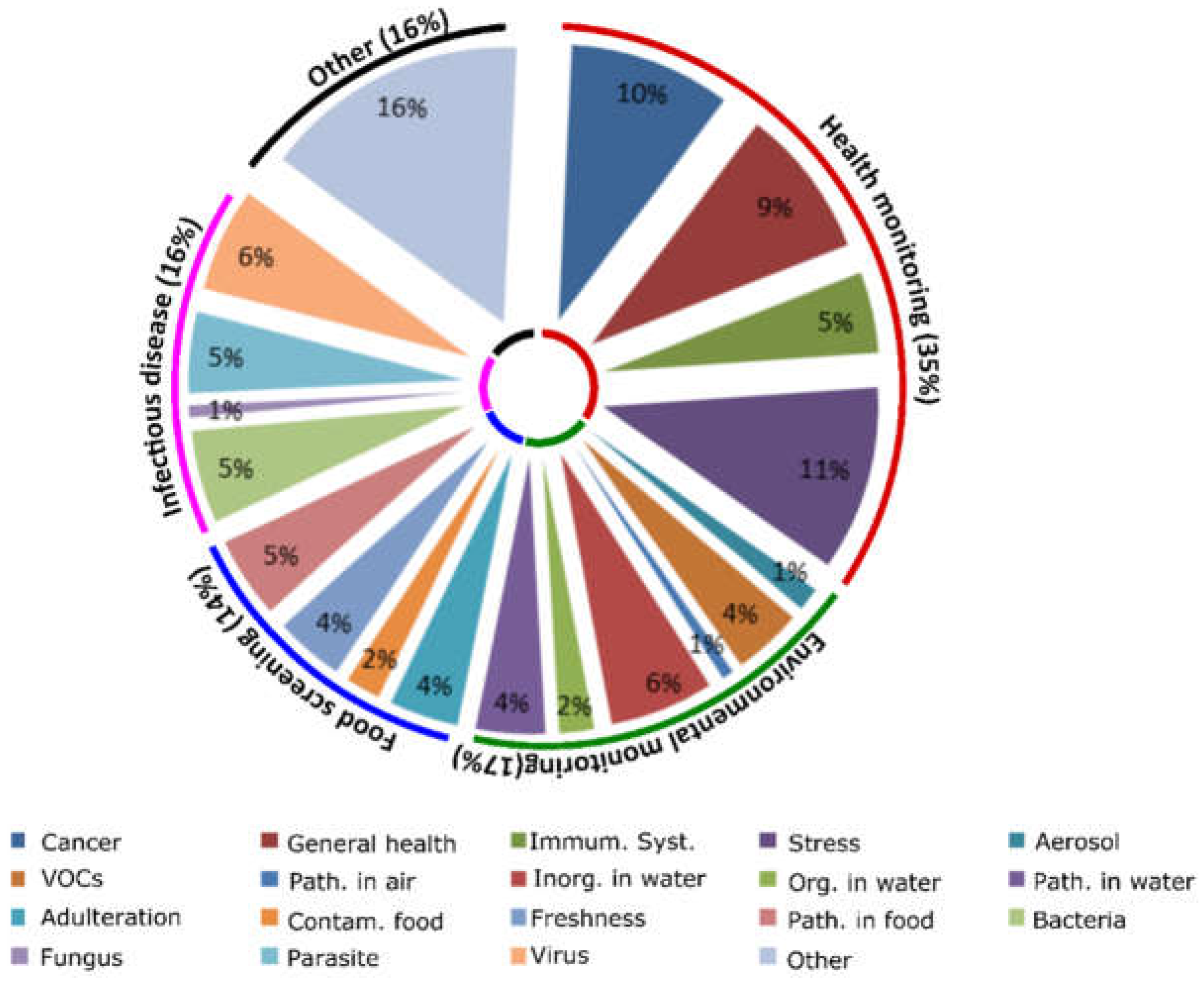
2.1. General Overview of Available SBDs to Monitor the Crew’s Health
2.2. SBDs for Health Monitoring in Space
2.2.1. Detecting Stress
2.2.2. Detecting Reduced Immune Response and General Health Monitoring
2.2.3. Detecting Cancer
2.3. Environmental Monitoring
2.3.1. Inorganic and Organic Compounds in Water
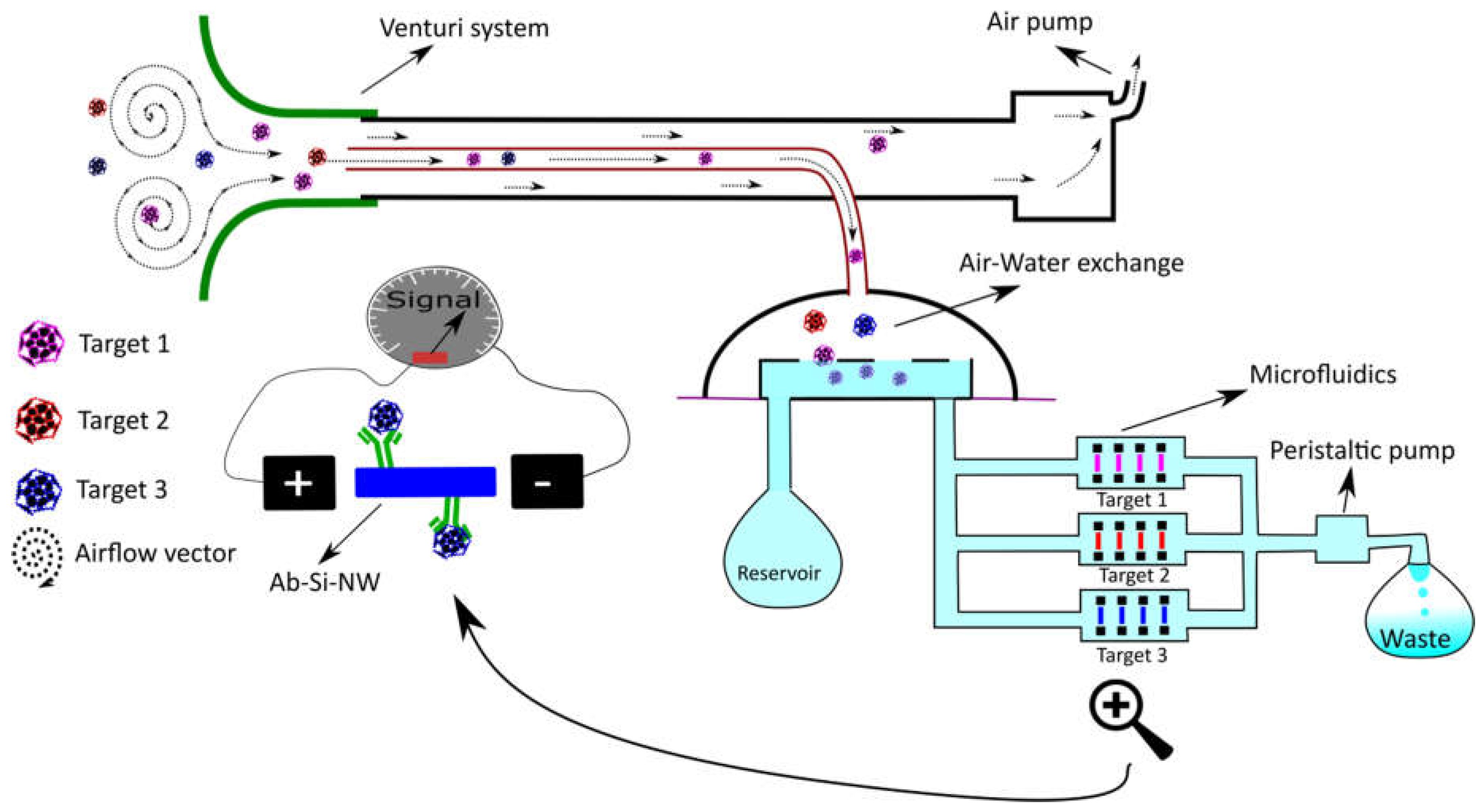
2.3.2. Aerosols, Pathogens and Volatile Organic Compounds (VOCs) in Air
2.4. Food Screening
2.5. Infectious Disease Detection
2.6. Other, Unclassified SBDs
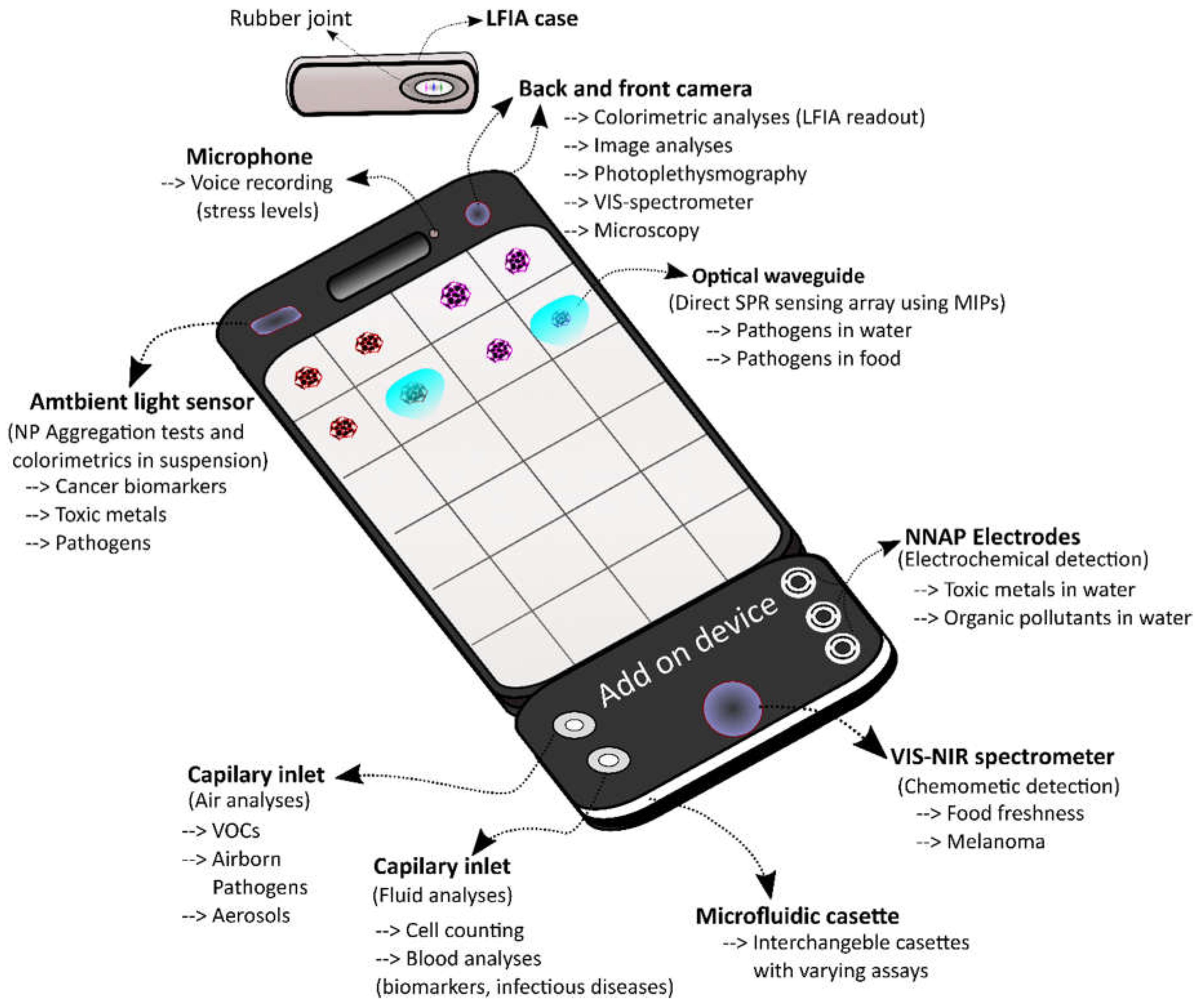
3. Limitations of the Smartphone for In Situ Analysis in Space
3.1. Novel Recognition Elements
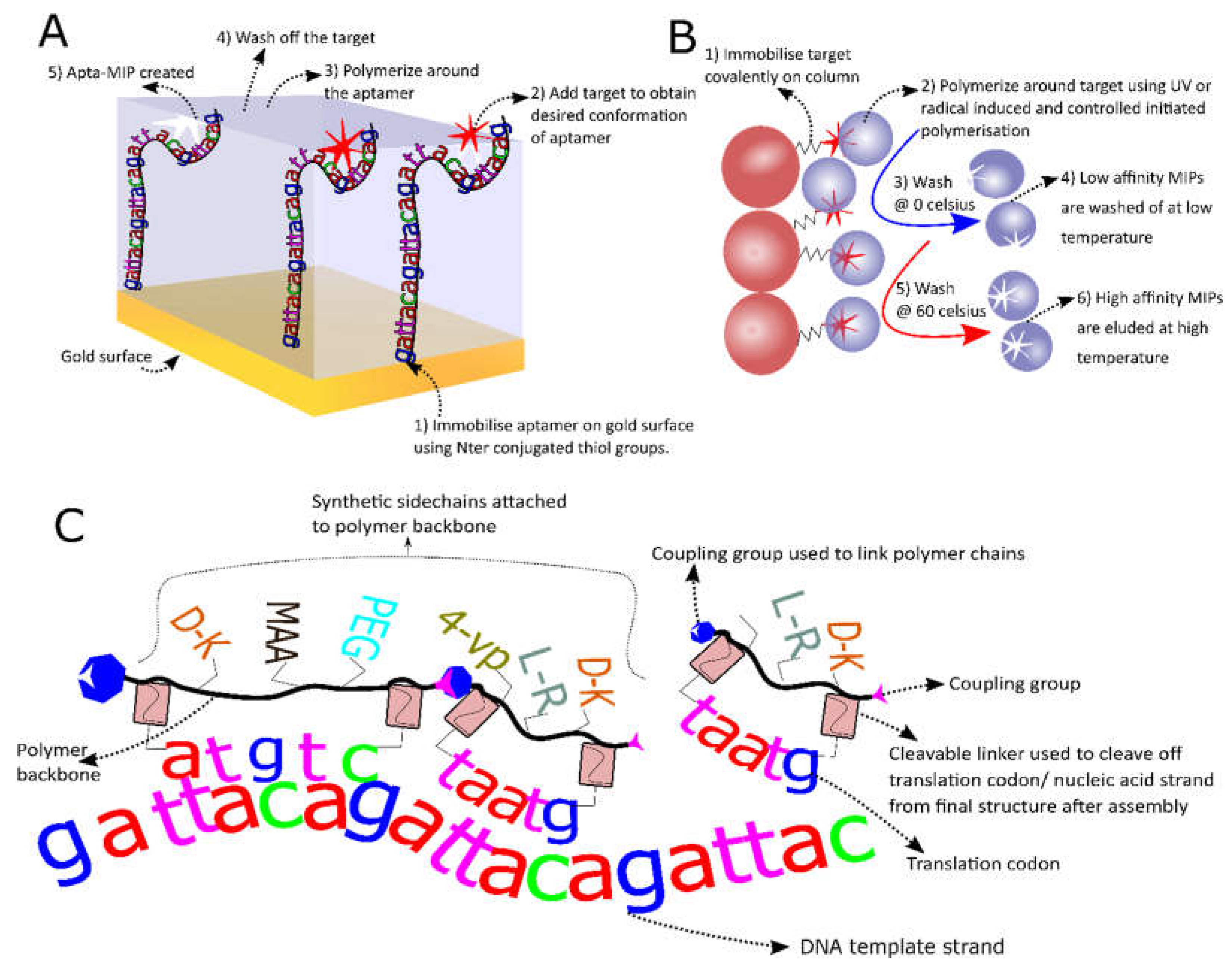
3.2. MIP-Aptamer Hybrids
3.3. Solid Phase MIPs
3.4. In Vitro Selection of More Diverse Polymers
3.5. Cell Free Synthetic Biology, the Answer to Long-Term Storage?
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Hawkey, A. Physiological and biomechanical considerations for a human Mars mission. J. Br. Interplanet. Soc. 2005, 58, 117–130. [Google Scholar]
- Ade, C.J.; Broxterman, R.M.; Moore, A.D.; Barstow, T.J. Decreases in maximal oxygen uptake following long-duration spaceflight: Role of convective and diffusive O2 transport mechanisms. J. Appl. Physiol. 2017, 122, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Drinnan, N.R.; de Juniac, A.B. The effects of microgravity on the urological system: A review. J. Clin. Urol. 2013, 6, 391–394. [Google Scholar] [CrossRef]
- Human Research Program Integrated Research Plan HRP 47065. Available online: https://www.nasa.gov/pdf/651214main_hrp47065_revc_IRP.pdf (accessed on 28 June 2011).
- Pierson, D.L. Microbial contamination of spacecraft. Graviatational Space Biol. Bull. 2001, 14, 1–6. [Google Scholar]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect. Drug Resist. 2015, 8, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial Responses to Microgravity and Other Low-Shear Environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. Npj Microgr. 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Crucian, B.E.; Stowe, R.P.; Simpson, R.J.; Ott, C.M.; Sams, C.F.; Pierson, D.L. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine 2013, 61, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Roberts, M.; Castro, S.; Oubre, C.; Makimura, K.; Leys, N.; Grohmann, E.; Sugita, T.; Ichijo, T.; Nasu, M. Microbial Monitoring of Crewed Habitats in Space—Current Status and Future Perspectives. Microbes Environ. 2014, 29, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Boguraev, A.-S.; Christensen, H.C.; Bonneau, A.R.; Pezza, J.A.; Nichols, N.M.; Giraldez, A.J.; Gray, M.M.; Wagner, B.M.; Aken, J.T.; Foley, K.D.; et al. Successful amplification of DNA aboard the International Space Station. Npj Microgr. 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Castro-Wallace, S.L.; Chiu, C.Y.; John, K.K.; Stahl, S.E.; Rubins, K.H.; McIntyre, A.B.R.; Dworkin, J.P.; Lupisella, M.L.; Smith, D.J.; Botkin, D.J.; et al. Nanopore DNA Sequencing and Genome Assembly on the International Space Station. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.C.; Damon, M.; Maule, J.; Monaco, L.A.; Wainwright, N. Rapid Culture-Independent Microbial Analysis Aboard the International Space Station (ISS) Stage Two: Quantifying Three Microbial Biomarkers. Astrobiology 2012, 12, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.F.; Greer, F.; Stockton, A.M.; Bryant, S.; Willis, P.A. Toward Total Automation of Microfluidics for Extraterrestrial in Situ Analysis. Anal. Chem. 2011, 83, 8636. [Google Scholar] [CrossRef] [PubMed]
- Parro, V.; de Diego-Castilla, G.; Rodríguez-Manfredi, J.A.; Rivas, L.A.; Blanco-López, Y.; Sebastián, E.; Romeral, J.; Compostizo, C.; Herrero, P.L.; García-Marín, A.; et al. SOLID3: A Multiplex Antibody Microarray-Based Optical Sensor Instrument for In Situ Life Detection in Planetary Exploration. Astrobiology 2011, 11, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.E.; Mojarro, A.; Hachey, J.; Saboda, K.; Tani, J.; Bhattaru, S.A.; Smith, A.; Pontefract, A.; Zuber, M.T.; Doebler, R.; et al. Towards in situ sequencing for life detection. In Proceedings of the 2017 IEEE Aerospace Conference, Big Sky, MT, USA, 4–11 March 2017. [Google Scholar] [CrossRef]
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic lab-on-a-chip platforms for environmental monitoring. TrAC Trends Anal. Chem. 2017, 95, 62–68. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors (Switzerland) 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Kim, B. Lab-on-a-chip pathogen sensors for food safety. Sensors (Switzerland) 2012, 12, 10713–10741. [Google Scholar] [CrossRef] [PubMed]
- Maule, J.; Wainwright, N.; Steele, A.; Monaco, L.; Morris, H.; Gunter, D.; Flroes, G.; Effinger, M.; Damon, M.; Wells, M.; et al. LOCAD-PTS: Operation of a new system for microbial monitoring aboard the International Space Station (ISS). In Proceedings of the AIAA SPACE 2008 Conference & Exposition, San Diego, CA, USA, 9–11 September 2008; pp. 1–9. [Google Scholar] [CrossRef]
- Van Houdt, R.; Mijnendonckx, K.; Leys, N. Microbial contamination monitoring and control during human space missions. Planet. Space Sci. 2012, 60, 115–120. [Google Scholar] [CrossRef]
- Long, K.D.; Woodburn, E.V.; Le, H.M.; Shah, U.K.; Lumetta, S.S.; Cunningham, B.T. Multimode smartphone biosensing: The transmission, reflection, and intensity spectral (TRI)-analyzer. Lab Chip 2017, 17, 3246–3257. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.K.J.; Tokarski, C.; Lang, S.N.; Van Ginkel, L.A.; Zhu, H.; Ozcan, A.; Nielen, M.W.F. Calling biomarkers in milk using a protein microarray on your smartphone. PLoS ONE 2015, 10, e134360. [Google Scholar] [CrossRef] [PubMed]
- Laksanasopin, T.; Guo, T.W.; Nayak, S.; Sridhara, A.A.; Xie, S.; Olowookere, O.O.; Cadinu, P.; Meng, F.; Chee, N.H.; Kim, J.; et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7, 273re1. [Google Scholar] [CrossRef] [PubMed]
- Priye, A.; Bird, S.W.; Light, Y.K.; Ball, C.S.; Negrete, O.A.; Meagher, R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maule, J.; Fogel, M.; Steele, A.; Wainwright, N.; Pierson, D.L.; McKay, D.S. Antibody binding in altered gravity: Implications for immunosorbent assay during space flight. J. Gravit. Physiol. 2003, 10, 47–55. [Google Scholar] [PubMed]
- Korth, D.W. Exercise Countermeasure Hardware Evolution on ISS: The First Decade. Aerosp. Med. Hum. Perform. 2015, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Fortunato, A.; Wolff, M.; Oliveira, D.M. mobiPV: A new, wearable real-time collaboration software for Astronauts using mobile computing solutions. In Proceedings of the SpaceOps 2016 Conference, Daejeon, Korea, 16–20 May 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Nambiar, S.; Zheng, W.; Yeow, J.T.W. PDMS/single-walled carbon nanotube composite for proton radiation shielding in space applications. Mater. Lett. 2013, 108, 79–83. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Nambiar, S.; Sun, Y.; Zhang, M.; Zheng, W.; Yeow, J.T.W. PMMA/MWCNT nanocomposite for proton radiation shielding applications. Nanotechnology 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Atxaga, G.; Marcos, J.; Jurado, M.; Carapelle, A.; Orava, R. Radiation Shielding of Composite Space Enclosures. Available online: https://orbi.uliege.be/bitstream/2268/132394/1/IAC-12%2CC2%2C6%2C6%2Cx13735.pdf (accessed on 1 June 2012).
- de Diego-Castilla, G.; Cruz-Gil, P.; Mateo-Martí, E.; Fernández-Calvo, P.; Rivas, L.A.; Parro, V. Assessing Antibody Microarrays for Space Missions: Effect of Long-Term Storage, Gamma Radiation, and Temperature Shifts on Printed and Fluorescently Labeled Antibodies. Astrobiology 2011, 11, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S.; Berlenbach, P.; Langenfelder, S.; Hörl, D.; Lehn, N.; Hiller, K.A.; Schmalz, G.; Durchschlag, H. Integrity of proteins in human saliva after sterilization by gamma irradiation. Appl. Environ. Microbiol. 2011, 77, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Coussot, G.; Moreau, T.; Faye, C.; Vigier, F.; Baqué, M.; Le Postollec, A.; Incerti, S.; Dobrijevic, M.; Vandenabeele-Trambouze, O. Biochip-based instruments development for space exploration: Influence of the antibody immobilization process on the biochip resistance to freeze-drying, temperature shifts and cosmic radiations. Int. J. Astrobiol. 2016, 16, 190–199. [Google Scholar] [CrossRef]
- Baqué, M.; Le Postollec, A.; Ravelet, C.; Peyrin, E.; Coussot, G.; Desvignes, I.; Incerti, S.; Moretto, P.; Dobrijevic, M.; Vandenabeele-Trambouze, O. Investigation of Low-Energy Proton Effects on Aptamer Performance for Astrobiological Applications. Astrobiology 2011, 11, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S. Measurements of Energetic Particle Radiatino in Transit to Mars on the Mars Science Laboratory. Am. Assoc. Adv. Sci. 2013. [Google Scholar] [CrossRef]
- Carr, C.E.; Rowedder, H.; Vafadari, C.; Lui, C.S.; Cascio, E.; Zuber, M.T.; Ruvkun, G. Radiation Resistance of Biological Reagents for In Situ Life Detection. Astrobiology 2013, 13, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.-Y.; Ling, H.-C.; Lo, Y.-C.; Yap, Z.-H.; Pua, J.-S.; Phan, R.C.-W.; Goh, V.-T. Android based self-diagnostic electrocardiogram system for mobile healthcare. Technol. Heal. Care 2015, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, P.; Garde, A.; Karlen, W.; Petersen, C.L.; Wensley, D.; Dumont, G.A.; Mark Ansermino, J. Evaluation of cardiac modulation in children in response to apnea/hypopnea using the Phone OximeterTM. Physiol. Meas. 2016, 37, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kwon, S.; Yoo, C.; Seo, S.; Park, K.; Song, J.; Lee, Y. Sinabro: Opportunistic and unobtrusive mobile electrocardiogram monitoring system. Assoc. Comput. Mach. 2014, 15, 1–6. [Google Scholar] [CrossRef]
- Kennedy, A.P.; Epstein, D.H.; Jobes, M.L.; Agage, D.; Tyburski, M.; Phillips, K.A.; Ali, A.A.; Bari, R.; Hossain, S.M.; Hovsepian, K.; et al. Continuous in-the-field measurement of heart rate: Correlates of drug use, craving, stress, and mood in polydrug users. Drug Alcohol Depend. 2015, 151, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; Le, V.; Albert, D.; Moreno, F.L.; Anderson, J.L.; Yanowitz, F.; Vranian, R.B.; Barsness, G.W.; Bethea, C.F.; Severance, H.W.; et al. Smartphone ECG for evaluation of STEMI: Results of the ST LEUIS Pilot Study. J. Electrocardiol. 2015, 48, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pierleoni, P.; Pernini, L.; Belli, A.; Palma, L. An android-based heart monitoring system for the elderly and for patients with heart disease. Int. J. Telemed. Appl. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rachim, V.P.; Chung, W.-Y. Wearable Noncontact Armband for Mobile ECG Monitoring System. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Sinddhuja, A.K.; Mounika, M.; Dass, P. A heartbeat and temperature measuring system for remote health monitoring using gsm technology. Int. J. Pharm. Technol. 2016, 8, 20847–20855. [Google Scholar] [CrossRef]
- Agarwal, T.; Bandivadekar, P.; Satpathy, G.; Sharma, N.; Titiyal, J.S. Detection of fungal hyphae using smartphone and pocket magnifier: Going cellular. Cornea 2015, 34, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial Spoilage of Foods: Fundamentals. Microbiol. Qual. Food Foodborne Spoilers 2016, 1–21. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.X.H.; Liu, F.S.F.; Yu, H.-Z. Mobile app-based quantitative scanometric analysis. Anal. Chem. 2014, 86, 11966–11971. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aztiria, A.; Basarab, A. Towards an automatic early stress recognition system for office environments based on multimodal measurements: A review. J. Biomed. Inform. 2016, 59, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, A.; Pioggia, G.; Tartarisco, G.; Baldus, G.; Ferro, M.; Cipresso, P.; Serino, S.; Popleteev, A.; Gabrielli, S.; Maimone, R.; et al. A system for automatic detection of momentary stress in naturalistic settings. Annu. Rev. CyberTherapy Telemed. 2012, 10, 182–186. [Google Scholar] [CrossRef]
- Muaremi, A.; Arnrich, B.; Tröster, G. Towards Measuring Stress with Smartphones and Wearable Devices During Workday and Sleep. Bionanoscience 2013, 3, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Kizakevich, P.N.; Hubal, R.; Brown, J.; Lyden, J.; Spira, J.; Eckhoff, R.; Zhang, Y.; Bryant, S.; Munoz, G. PHIT for duty, a mobile approach for psychological health intervention. Annu. Rev. CyberTherapy Telemed. 2012, 10, 268–272. [Google Scholar] [CrossRef]
- Gregoski, M.J.; Vertegel, A.; Shaporev, A.; Treiber, F.A. Tension tamer: Delivering meditation with objective heart rate acquisition for adherence monitoring using a smart phone platform. J. Altern. Complement. Med. 2013, 19, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Doule, O.; Poulet, L. Ergonomy of Head Mounted Displays Inside Analog. In Proceedings of the AIAA SPACE 2014 Conference and Exposition, San Diego, CA, USA, 4–7 August 2014; pp. 1–26. [Google Scholar] [CrossRef]
- Chintamani, K.; Lierde, B.V.; Maloney, S.; Kiernan, P. Wearable crew support technology on the International Space Station: The mobile Procedure Viewer (mobiPV). HFES Eur. 2014, 4959, 1–11. [Google Scholar]
- Benjamin, C.L.; Stowe, R.P.; St. John, L.; Sams, C.F.; Mehta, S.K.; Crucian, B.E.; Pierson, D.L.; Komanduri, K.V. Decreases in thymopoiesis of astronauts returning from space flight. JCI Insight 2016, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.M.; Pitts, K.P.; Feldkamp, J.; Kirschbaum, C.; Wolf, J.M. Cortisol-dependent stress effects on cell distribution in healthy individuals and individuals suffering from chronic adrenal insufficiency. Brain. Behav. Immun. 2015, 50, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mavandadi, S.; Coskun, A.F.; Yaglidere, O.; Ozcan, A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal. Chem. 2011, 83, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tasoglu, S.; Chen, P.Z.; Chen, M.; Akbas, R.; Wach, S.; Ozdemir, C.I.; Gurkan, U.A.; Giguel, F.F.; Kuritzkes, D.R.; et al. Micro-a-fluidics ELISA for rapid CD4 cell count at the point-of-care. Sci. Rep. 2014, 4, 3796. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [PubMed]
- Manuscript, A.; Blood, W.; Count, C. Vitamin D and the Immune System. J. Investig. Med. 2009, 49, 1841–1850. [Google Scholar] [CrossRef]
- Lee, S.; Oncescu, V.; Mancuso, M.; Mehta, S.; Erickson, D. A smartphone platform for the quantification of vitamin D levels. Lab Chip Miniaturisation Chem. Biol. 2014, 14, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C. 3D printable retinal imaging adapter for smartphones could go global. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.E.; Koh, J.; Lin, J.; Di Carlo, D. Research highlights: Translating chips. Lab Chip 2015, 15, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef]
- Fink, C.A.; Bates, M.N.; Fink, C.A.; Bates, M.N. Melanoma and Ionizing Radiation: Is There a Causal Relationship? Radiat. Res. 2005, 164, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.Y.; George, K.A.; Cucinotta, F.A. Evaluation of skin cancer risk for lunar and Mars missions. Adv. Space Res. 2006, 37, 1798–1803. [Google Scholar] [CrossRef]
- Ramlakhan, K.; Shang, Y. A mobile automated skin lesion classification system. In Proceedings of the International Conference on Tools with Artificial Intelligence (ICTAI), Boca Raton, FL, USA, 7–9 November 2011; Volume 23, pp. 138–141. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zouridakis, G.; Wadhawan, T.; Situ, N.; Hu, R.; Yuan, X.; Lancaster, K.; Queen, C.M. Melanoma and other skin lesion detection using smart handheld devices. Methods Mol. Biol. 2015, 1256, 459–496. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, T.; Situ, N.; Lancaster, K.; Yuan, X.; Zouridakis, G. SkinScan©: A portable library for melanoma detection on handheld devices. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 Match–2 April 2011; Volume 11, pp. 133–136. [Google Scholar] [CrossRef]
- Oskouei, S.S.L.; Golestani, H.; Hashemi, M.; Ghiasi, S. CNNdroid: GPU-Accelerated Execution of Trained Deep Convolutional Neural Networks on Android. AC. ISBM 2015, 1201–1205. [Google Scholar] [CrossRef]
- Gregg, D.; Ionica, M.H. The Movidius Myriad Archetecture’s Potential for Scientific Computing. IEEE Micro 2009, 6–14. [Google Scholar] [CrossRef]
- Rallapalli, S.; Qiu, H.; Bency, A.J.; Karthikeyan, S.; Govindan, R.; Urgaonkar, R. Are Very Deep Neural Networks Feasible on Mobile Devices? In Usc Conference Proceedings; USC University of Southern California: Oakland, CA, USA, 2015. [Google Scholar]
- Jahan-Tigh, R.R.; Chinn, G.M.; Rapini, R.P. A comparative study between smartphone-based microscopy and conventional light microscopy in 1021 dermatopathology specimens. Arch. Pathol. Lab. Med. 2016, 140, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Swedish, T.; Wahi, A.; Moufarrej, M.; Noland, M.; Gurry, T.; Aranda-Michel, E.; Aksel, D.; Wagh, S.; Sadashivaiah, V.; et al. Mobile phone based mini-spectrometer for rapid screening of skin cancer. Proc. SPIE 2015, 9482, 1–5. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Khimji, I.; Akbas, R.; Qiu, W.; Edwards, D.; Cramer, D.W.; Ye, B.; Demirci, U. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab Chip 2011, 11, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Akbas, R.; Demirci, U. Microchip ELISA coupled with cell phone to detect ovarian cancer HE4 biomarker in urine. Methods Mol. Biol. 2015, 1256, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Adel Ahmed, H.; Azzazy, H.M.E. Power-free chip enzyme immunoassay for detection of prostate specific antigen (PSA) in serum. Biosens. Bioelectron. 2013, 49, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Long, K.D.; Yu, H.; Cunningham, B.T. Smartphone instrument for portable enzymelinked immunosorbent assays. Biomed. Opt. Express 2014, 5, 3792–3806. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.K.; Kulah, H. Android based portable cell counting system for label free quantification of dep manipulated cancer cells. In Proceedings of the 19th International Conference Solid-State Sensors, Kaohsiung, Taiwan, 19–22 June 2017; Volume 17, pp. 556–559. [Google Scholar] [CrossRef]
- Archibong, E.; Konnaiyan, K.R.; Kaplan, H.; Pyayt, A. A mobile phone-based approach to detection of hemolysis. Biosens. Bioelectron. 2017, 88, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Felton, E.J.; Velasquez, A.; Lu, S.; Murphy, R.O.; Elkhal, A.; Mazor, O.; Gorelik, P.; Sharda, A.; Ghiran, I.C. Detection and quantification of subtle changes in red blood cell density using a cell phone. Lab Chip 2016, 16, 3286–3295. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Gu, C.; Li, C.; Lin, J. Doppler radar noncontact vital sign monitoring. Neural Comput. Neural Devices Neural Prosthes. 2014, 41–62. [Google Scholar] [CrossRef]
- Reyes, B.A.; Reljin, N.; Kong, Y.; Nam, Y.; Ha, S.; Chon, K.H. Employing an incentive spirometer to calibrate tidal volumes estimated from a smartphone camera. Sensors (Switzerland) 2016, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, J.; Peretz-Soroka, H.; Zhu, L.; Li, Z.; Sang, Y.; Hipolito, J.; Zhang, M.; Santos, S.; Hillier, C.; et al. Mkit: A cell migration assay based on microfluidic device and smartphone. Biosens. Bioelectron. 2018, 99, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.; Nemade, H.B.; Bandyopadhyay, D. Nano-enabled paper humidity sensor for mobile based point-of-care lung function monitoring. Biosens. Bioelectron. 2017, 94, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.L.; Ouyang, Y.; Li, J.; Krauss, S.T.; Shukla, N.; Kessel, B.G.; Haverstick, D.M.; Garner, G.T.; Landers, J.P. Protein quantitation from whole blood on polyester-toner laser-printed microfluidic discs with cell phone image analysis. In Proceedings of the 18th International Conference Miniaturized Systems for Chemistry & Life Science MicroTAS, San Antonio, TX, USA, 26–30 October 2014; pp. 1434–1436. [Google Scholar]
- Harder, R.; Diedrich, A.; Whitfield, J.S.; Buchowski, M.S.; Pietsch, J.B.; Baudenbacher, F.J. Smart Multi-Frequency Bioelectrical Impedance Spectrometer for BIA and BIVA Applications. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Patnaik, R.; Kuhlmann, K.; Rai, A.J.; Sia, S.K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015, 15, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, H.; Dong, T. Smartphone-Based Rapid Screening of Urinary Biomarkers. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dweik, B.; Argun, A.; Tempelman, L.; Mackenzie, N.; Forchione, J.; Hamdan, M. Portable Sensor for Rapid Measurement of Trace Toxic Metals in Water. Techport NASA 2015, 1–4. [Google Scholar]
- Sun, H.; Li, W.; Dong, Z.-Z.; Hu, C.; Leung, C.-H.; Ma, D.-L.; Ren, K. A suspending-droplet mode paper-based microfluidic platform for low-cost, rapid, and convenient detection of lead(II) ions in liquid solution. Biosens. Bioelectron. 2018, 99, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Tseng, W.-L. Ultrasensitive detection of target analyte-induced aggregation of gold nanoparticles using laser-induced nanoparticle Rayleigh scattering. Talanta 2015, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xiao, M.; Fu, Q.; Yu, S.; Shen, H.; Bian, H.; Tang, Y. A portable smart-phone readout device for the detection of mercury contamination based on an aptamer-assay nanosensor. Sensors (Switzerland) 2016, 16, 1871. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Xu, F.; Shi, X.; Feng, D.; Wei, D.; Li, Y.; Feng, Y.; Wang, Y.; Jia, D.; et al. High-yield synthesis of strong photoluminescent N-doped carbon nanodots derived from hydrosoluble chitosan for mercury ion sensing via smartphone APP. Biosens. Bioelectron. 2016, 79, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.; Krishnan, S.; Rajkumar, S.; Halery, N.; Balkunde, P. Monitoring of fluoride in water samples using a smartphone. Sci. Total Environ. 2016, 551–552, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.-J.; Wei, J.-F.; Xu, J.-R.; Wang, Y.-H.; Zheng, G.-X. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gartia, M.R.; Jiang, J.; Chang, T.-W.; Qian, J.; Liu, Y.; Liu, X.; Liu, G.L. Audio jack based miniaturized mobile phone electrochemical sensing platform. Sens. Actuators B Chem. 2015, 209, 677–685. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bao, X.; Han, J.; Xia, J.; Tian, X.; Ni, L. A smartphone-based colorimetric reader coupled with a remote server for rapid on-site catechols analysis. Talanta 2016, 160, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, N.; Lukac, M.; Ahmed, T.; Kar, A.; Praveen, P.S.; Honles, T.; Leong, I.; Rehman, I.H.; Schauer, J.J.; Ramanathan, V. A cellphone based system for large-scale monitoring of black carbon. Atmos. Environ. 2011, 45, 4481–4487. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Shiledar, A.; Li, Y.-C.; Wong, J.; Feng, S.; Chen, X.; Chen, C.; Jin, K.; Janamian, S.; Yang, Z.; et al. Air quality monitoring using mobile microscopy and machine learning. Light Sci. Appl. 2017, 6, e17046. [Google Scholar] [CrossRef] [PubMed]
- Mirowsky, J.; Hickey, C.; Horton, L.; Blaustein, M.; Galdanes, K.; Peltier, R.E.; Chillrud, S.; Chen, L.C.; Ross, J.; Nadas, A.; et al. The effect of particle size, location and season on the toxicity of urban and rural particulate matter. Inhal. Toxicol. 2013, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tsow, F.; Campbell, K.D.; Iglesias, R.; Forzani, E.; Tao, N. A wireless hybrid chemical sensor for detection of environmental volatile organic compounds. IEEE Sens. J. 2013, 13, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Tan, M.; Wang, Z.; Yao, M.; Xu, Z.; Wu, Y.; Wang, J.; Guo, X.; Zhu, T. Integrating silicon nanowire field effect transistor, microfluidics and air sampling techniques for real-time monitoring biological aerosols. Environ. Sci. Technol. 2011, 45, 7473–7480. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. Infection prevention and control during prolonged human space travel. Clin. Infect. Dis. 2013, 56, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.P.; Staymates, M. Enhanced aerodynamic reach of vapor and aerosol sampling for real-time mass spectrometric detection using Venturi-assisted entrainment and ionization. Anal. Chim. Acta 2017, 957, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Das, A.J.; Wahi, A.; Kothari, I.; Raskar, R. Ultra-portable, wireless smartphone spectrometer for rapid, non-destructive testing of fruit ripeness. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Intaravanne, Y.; Sumriddetchkajorn, S.; Nukeaw, J. Cell phone-based two-dimensional spectral analysis for banana ripeness estimation. Sens. Actuators B Chem. 2012, 168, 390–394. [Google Scholar] [CrossRef]
- Yu, X.; Lu, Q.; Gao, H.; Ding, H. Development of a handheld spectrometer based on a linear variable filter and a complementary metal-oxide-semiconductor detector for measuring the internal quality of fruit. J. Near Infrared Spectrosc. 2016, 24, 69–76. [Google Scholar] [CrossRef]
- Bueno, L.; Meloni, G.N.; Reddy, S.M.; Paixão, T.R.L.C. Use of plastic-based analytical device, smartphone and chemometric tools to discriminate amines. RSC Adv. 2015, 5, 20148–20154. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.; Song, Y.; Zhu, M.-J.; Lin, Y.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Borysiak, M.D.; Kimura, K.W.; Posner, J.D. NAIL: Nucleic Acid detection using Isotachophoresis and Loop-mediated isothermal amplification. Lab Chip 2015, 15, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sikora, U.; Ozcan, A. Quantum dot enabled detection of Escherichia coli using a cell-phone. Analyst 2012, 137, 2541. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-S.; Park, T.S.; Yoon, J.-Y. Rapid and reagentless detection of microbial contamination within meat utilizing a smartphone-based biosensor. Sci. Rep. 2014, 4, 5953. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Li, W.; McCracken, K.E.; Yoon, J.-Y. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip 2013, 13, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.K.; Bakthavathsalam, P.; Jaffar Ali, B.M. Smartphone based bacterial detection using biofunctionalized fluorescent nanoparticles. Microchim. Acta 2014, 181, 1815–1821. [Google Scholar] [CrossRef]
- Dallet, C.; Kareem, S.; Kale, I. Real time blood image processing application for malaria diagnosis using mobile phones. In Proceedings of the IEEE International Symposium on Circuits & Systems (ISCAS), Melbourne, Australia, 1–5 June 2014; pp. 2405–2408. [Google Scholar] [CrossRef]
- Lillehoj, P.B.; Huang, M.-C.; Ho, C.-M. A handheld, cell phone-based electrochemical biodetector. In Proceedings of the 26th IEEE International Conference on Micro Electro Mechanical Systems, Taipei, Taiwan, 20–24 January 2013; pp. 53–56. [Google Scholar] [CrossRef]
- Stemple, C.C.; Angus, S.V.; Park, T.S.; Yoon, J.-Y. Smartphone-Based Optofluidic Lab-on-a-Chip for Detecting Pathogens from Blood. J. Lab. Autom. 2014, 19, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Liu, C.; Sadik, M.; Bau, H.H. Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection. Methods Mol. Biol. 2015, 1256, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, P.A.; Coskun, A.F.; Chung, A.J.; Weaver, W.M.; Adeyiga, O.; Khodadadi, D.; Ozcan, A.; Di Carlo, D. Digital readout platform for water-in-oil droplet immunoassays running on a cell-phone for point of care viral load sensing. In Proceedings of the The 16th International Conference on Microsystems for Chemistry and Life Sciences MicroTAS, Okinawa, Japan, 28 October–1 November 2012; pp. 338–340. [Google Scholar]
- Coulibaly, J.T.; Ouattara, M.; D’Ambrosio, M.V.; Fletcher, D.A.; Keiser, J.; Utzinger, J.; N’Goran, E.K.; Andrews, J.R.; Bogoch, I.I. Accuracy of Mobile Phone and Handheld Light Microscopy for the Diagnosis of Schistosomiasis and Intestinal Protozoa Infections in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0005550. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, R.K.D.; Cybulski, J.S.; Duah, E.; Prakash, M.; D’Ambrosio, M.V.; Fletcher, D.A.; Keiser, J.; Andrews, J.R.; Bogoch, I.I. Diagnosis of Schistosoma haematobium infection with a mobile phone-mounted Foldscope and a reversed-lens CellScope in Ghana. Am. J. Trop. Med. Hyg. 2015, 92, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Holmen, S.D.; Kjetland, E.F.; Taylor, M.; Kleppa, E.; Lillebø, K.; Gundersen, S.G.; Onsrud, M.; Albregtsen, F. Colourimetric image analysis as a diagnostic tool in female genital schistosomiasis. Med. Eng. Phys. 2015, 37, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Jacob, J.M.; Costa, M.N.; Santos, D.S.; Viveiros, M.; Inácio, J.; Martins, R.; Barquinha, P.; Fortunato, E.; Baptista, P.V. Gold on paper-paper platform for Au-nanoprobe TB detection. Lab Chip 2012, 12, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Fortunato, E.; Baptista, P.V. Mobile based gold nanoprobe TB diagnostics for point-of-need. Methods Mol. Biol. 2015, 1256, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Balagon, M.F.; Maghanoy, A.; Orcullo, F.M.; Cang, M.; Dias, R.F.; Collovati, M.; Reed, S.G. Rapid quantitative serological test for detection of infection with Mycobacterium leprae, the causative agent of leprosy. J. Clin. Microbiol. 2014, 52, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.-L.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Cesarman, E.; Erickson, D. Detection of Kaposi’s sarcoma associated herpesvirus nucleic acids using a smartphone accessory. Lab Chip Miniaturisation Chem. Biol. 2014, 14, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Qi, H.; Luo, W.; Tseng, D.; Ki, S.J.; Wan, Z.; Göröcs, Z.; Bentolila, L.A.; Wu, T.-T.; Sun, R.; et al. Fluorescent imaging of single nanoparticles and viruses on a smart phone. ACS Nano 2013, 7, 9147–9155. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, D.; Long, K.D.; Yu, H.; Clark, P.P.; Lin, Y.; George, S.; Nath, P.; Cunningham, B.T. Label-free biodetection using a smartphone. Lab Chip 2013, 13, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Yang, C. A smartphone-based chip-scale microscope using ambient illumination. Lab Chip Miniaturisation Chem. Biol. 2014, 14, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R. Multiplexed homogeneous assays of proteolytic activity using a smartphone and quantum dots. Anal. Chem. 2014, 86, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.; Parent, F.; De Lima Filho, E.S.; Loranger, S.; Kashyap, R. Toward the integration of optical sensors in smartphone screens using femtosecond laser writing. Opt. Lett. 2015, 40, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- DuVall, J.A.; Borba, J.C.; Shafagati, N.; Luzader, D.; Shukla, N.; Li, J.; Kehn-Hall, K.; Kendall, M.M.; Feldman, S.H.; Landers, J.P. Optical imaging of paramagnetic bead-DNA aggregation inhibition allows for low copy number detection of infectious pathogens. PLoS ONE 2015, 10, e0129830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ozcan, A. Wide-field fluorescent microscopy and fluorescent imaging flow cytometry on a cell-phone. J. Vis. Exp. 2013, e50451. [Google Scholar] [CrossRef] [PubMed]
- Gantelius, J.; Bass, T.; Sjöberg, R.; Nilsson, P.; Andersson-Svahn, H. A lateral flow protein microarray for rapid and sensitive antibody assays. Int. J. Mol. Sci. 2011, 12, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; van Oordt, T.; Schneider, E.M.; Zengerle, R.; von Stetten, F.; Luong, J.H.T. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wu, Z.; Xu, F.; Li, X.; Yao, C.; Xu, M.; Sheng, L.; Yu, S.; Tang, Y. A portable smart phone-based plasmonic nanosensor readout platform that measures transmitted light intensities of nanosubstrates using an ambient light sensor. Lab Chip 2016, 16, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J.; Chu, K.; Wachsmann-Hogiu, S. Nanometer-Scale Sizing Accuracy of Particle Suspensions on an Unmodified Cell Phone Using Elastic Light Scattering. PLoS ONE 2012, 7, e46030. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R. Antibody-based sensors: Principles, problems and potential for detection of pathogens and associated toxins. Sensors (Switzerland) 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Mayer, G. Selection and Biosensor Application of Aptamers for Small Molecules. Front. Chem. 2016, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Griffete, N.; Lamouri, A.; Felidj, N.; Chehimi, M.M.; Mangeney, C. Nanocomposites of Gold Nanoparticles@Molecularly Imprinted Polymers: Chemistry, Processing, and Applications in Sensors. Chem. Mater. 2015, 27, 5464–5478. [Google Scholar] [CrossRef]
- Sharma, S.K.; Leblanc, R.M. Biosensors based on β-galactosidase enzyme: Recent advances and perspectives. Anal. Biochem. 2017, 535, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors (Switzerland) 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, Z.F.; Su, Y.; Kitto, R.Z.; Hammond, M.C. Engineering and In Vivo Applications of Riboswitches. Annu. Rev. Biochem. 2017, 86, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kintzios, S.; Prabhakarpandian, B. Biotoxin detection using cell-based sensors. Toxins (Basel) 2013, 5, 2366–2383. [Google Scholar] [CrossRef] [PubMed]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer-MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016, 75, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, J.; Su, L.; Gao, X.; Tang, Y.; Ma, T.; Zhu, L.; Li, J. Novel hybrid probe based on double recognition of aptamer-molecularly imprinted polymer grafted on upconversion nanoparticles for enrofloxacin sensing. Biosens. Bioelectron. 2017, 87, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J. Molecularly Imprinted Polymers with DNA Aptamer Fragments as Macromonomers. ACS Appl. Mater. Interfaces 2016, 8, 6371–6378. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template—“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [PubMed]
- Moczko, E.; Poma, A.; Guerreiro, A.; Perez, I.; Sansalvador, D.V.; Caygill, S.; Canfarotta, F.; Whitcombe, M.J.; Piletsky, S. Surface-modified multifunctional MIP nanoparticles. Nanoscale 2016, 5, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Basozabal, I.; Guerreiro, A.; Gomez-Caballero, A.; Aranzazu Goicolea, M.; Barrio, R.J. Direct potentiometric quantification of histamine using solid-phase imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2014, 58, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Korposh, S.; Chianella, I.; Guerreiro, A.; Caygill, S.; Piletsky, S.; James, S.W.; Tatam, R.P. Selective vancomycin detection using optical fibre long period gratings functionalised with molecularly imprinted polymer nanoparticles. Analyst 2014, 139, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; De Vargas Sansalvador, I.M.P.; Whitcombe, M.J.; Piletsky, S.A. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA—Development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Gittens, M.; Guerreiro, A.; Thompson, K.-A.; Walker, J.; Piletsky, S.; Tothill, I.E. Detection of Waterborne Viruses Using High Affinity Molecularly Imprinted Polymers. Anal. Chem. 2015, 87, 6801–6807. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Caygill, S.; Moczko, E.; Piletsky, S. Automatic reactor for solid-phase synthesis of molecularly imprinted polymeric nanoparticles (MIP NPs) in water. RSC Adv. 2014, 4, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Cowen, T.; Guerreiro, A.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. A Protocol for the Computational Design of High Affi nity Molecularly Imprinted Polymer Synthetic Receptors. Glob. J. Biotechnol. Biomater. Sci. 2017, 1, 001–007. [Google Scholar] [CrossRef]
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Nucleic Acid Ligands With Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol. Ther. Nucleic Acids 2014, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Brudno, Y.; Birnbaum, M.E.; Kleiner, R.E.; Liu, D.R. An in vitro translation, selection and amplification system for peptide nucleic acids. Nat. Chem. Biol. 2010, 6, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, S.; Chaput, J.C. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 2012, 4, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Hili, R.; Liu, D.R. Enzyme-free translation of DNA into sequence-defined synthetic polymers structurally unrelated to nucleic acids. Nat. Chem. 2013, 5, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.A.; Cumbers, J.; Hogan, J.A.; Arkin, A.P. Towards synthetic biological approaches to resource utilization on space missions. J. R. Soc. Interface 2015, 12, 20140715. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J. Synthetic biology meets bioprinting: Enabling technologies for humans on Mars (and Earth). Biochem. Soc. Trans. 2016, 44, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Lasseur, C.; Brunet, J.; Weever, H.D.; Dixon, M.; Dussap, G.; Godia, F.; Leys, N.; Mergeay, M.; Straeten, D. Van Der Melissa: The European project of closed life support system. Gravitational Sp. Biol. 2010, 23, 3–12. [Google Scholar]
- Verseux, C.N.; Paulino-Lima, I.G.; Baqué, M.; Billi, D.; Rothschild, L.J. Synthetic Biology for Space Exploration: Promises and Societal Implications. Ambivalences Creat. Life Soc. Philos. Dimens. Synth. Biol. 2016, 73–100. [Google Scholar] [CrossRef]
- Lu, Y. Cell-free synthetic biology: Engineering in an open world. Synth. Syst. Biotechnol. 2017, 2, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Bundy, B.C.; Swartz, J.R. Efficient disulfide bond formation in virus-like particles. J. Biotechnol. 2011, 154, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Slomovic, S.; Nguyen, P.Q.; Lee, J.W.; Donghia, N.; Burrill, D.; Ferrante, T.; McSorley, F.R.; Furuta, Y.; Vernet, A.; et al. Portable, On-Demand Biomolecular Manufacturing. Cell 2016, 167, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Armstrong, S.; Rivers, P.J.; Zhang, J.; Yang, J.; Green, E.; Rozzelle, J.; Liang, S.; Kittle, J.D.; Steiner, A.R.; et al. Engineering toward a bacterial “endoplasmic reticulum” for the rapid expression of immunoglobulin proteins. MAbs 2014, 6, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Z.; Hayes, C.A.; Shin, J.; Caschera, F.; Murray, R.M.; Noireaux, V. Protocols for Implementing an Escherichia coli Based TX-TL Cell-Free Expression System for Synthetic Biology. J. Vis. Exp. 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Ferrante, T.; Cameron, D.E.; DaleyKeyser, A.; Yin, P.; Collins, J.J. Resource Paper-Based Synthetic Gene Networks. Cell 2014, 159, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Berkheimer, S.D.; Werner, C.J.; Bundy, B.C. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques 2014, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Mirasoli, M.; Guardigli, M.; Zangheri, M.; Caliceti, C.; Calabria, D.; Simoni, P. Advanced biosensors for monitoring astronauts’ health during long-duration space missions. Biosens. Bioelectron. 2018, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, S.M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
| Target and Device Working Conditions | Detection Method | Pros and Cons |
|---|---|---|
| Hemolysis in blood [85]. LOD: 1.39 mg/dL hemoglobin. Matrix: Plasma. System showed higher accuracy as conventional methods (Roche Cobas c501 and Siemens Dimension Vista 1500) and fast analyses time (10 min versus 4 h for conventional lab-based methods) | Colorimetric detection of free hemoglobin levels in plasma. Plasma is imaged and image-analyses is used to determine the amount of free hemoglobin levels present. | Pro: fast (10 min), cheap (few dollar), and relevant (astronaut anemia can be measured) |
| Con: Blood separation based on gravitation in capillary | ||
| Cell density detection [86]. System able to distinguish between normal red blood cells (RBCs) and RBCs from anemia patient. It was suggested as method to detect low-density neutrophils as well but this was not tested. | Magnetic levitation. Cells in a capillary filled with a paramagnetic medium are placed between 2 rare earth magnets and their levitation position is determined solely by their density. | Pro: Fast and facile identification of astronaut anemia and other diseases that evoke cell density changes |
| Con: Proof of principle only | ||
| Non-contact vital sign detection such as sleep apnea, pulse wave velocity measurements and respiration monitoring [87]. | Doppler radar sensor. Integration of demodulation techniques and miniaturization (System on chip) to enable SBD detection. | Pro: basic vital signals can be remotely measured and analyzed |
| Con: Experimental and not robust, sensitive for noise from movement | ||
| Tidal volume, V(T), estimator [88]. V(T) was estimated using a commercial spirometer for simple calibration. Method enables V(T) estimation with about 18% error compared to spirometer data. | Video analyses of chest movement | Pro: non-invasive monitoring of lung volume |
| Con: Other simple and more direct methods exist as well | ||
| Mobile cell migration assay for neutrophil and cancer cell chemotaxis [89]. System achieves 3 µm resolution and was validated for detection of chronic obstructive pulmonary disease in clinical samples. | Test kit consists of a smartphone-imaging platform using microfluidic channels, LED illumination, emission filters and image analyses. | Pro: Neutrophil chemotaxis can be tested directly from a drop of blood. |
| Con: System still at proof of principle stage | ||
| Detection of chronic obstructive pulmonary diseases [90]. System showed high correlation with breathing frequency and peak flow rate. | Resistance relative humidity sensor. Nanoparticle doped paper (NDP) resistance was measured during NDP exposure to breathe channeled through mouthpiece. | Pro: Quick way to detect chronic obstructive pulmonary diseases |
| Con: System still at proof of principle stage | ||
| Quantitative clinical method for total protein, albumin, and hematocrit analysis [91]. Calibration curves showed good dynamic range and RSD values under 5%. | Colorimetric detection on polyester-toner, laser printed, microfluidic disks. Test enables both whole blood separation and component detection using SBD image analyses. | Pro: System is quick and fully integrated. |
| Con: The system is complex (production costs) | ||
| Determine water-fat ratio in the body [92]. Method was compared to dual-energy X-ray absorptiometry (DXA) in healthy volunteers and showed a maximum absolute error of 6.5%. | Bioelectrical impedance analysis using a miniature multi-frequency impedance spectrometer for whole body impedance measurements. | Pro: Non-invasive, rapid and accurate |
| Con: System still at proof of principle stage | ||
| Determine hemoglobin concentration and detect HIV virus [93]. System was validated in clinical trial (n = 38) showing 95% limit of agreement for hemaglobin and 95% sensitivity and specificity for HIV immune assay. | Microfluidic device with colorimetric detection to determine hemoglobin concentration and absorbance (silver enhanced precipitation of colloid gold) for HIV related antibody detection. | Pro: System is simple does not require expertise for use |
| Con: System still at proof of principle stage | ||
| Urinary tract infection detection [94]. Application functions independently of room illumination and smartphone type (6 phones both Android and iPhone tested). | Colorimetric detection using image analyses. Device needs reference values for training set. Device is equipped with auto-localization to classify and detect ± 100 spots of 12 biomarkers simultaneously | Pro: multiplex detection of 12 biomarkers within one picture |
| Con: semi-quantitative only, varying illumination can effect results |
| Target | Method | Advantages | Limitation | Reference |
|---|---|---|---|---|
| Escherichia coli, Salmonella enterica, Rift valley fever virus with sensitivity close to single target copy. Method was validated using RT-qPCR. | Inhibition of DNA-paramagnetic silica bead aggregation, otherwise induced in longer strand DNA mixtures, by centrifugation after LAMP (a). | Single copy detection of DNA using simple device replacing fluorescent detection with simple aggregation assay measurable directly with SBD camera. | No guaranty regarding specificity in assay. Any short DNA amplicons will shield the beads from aggregation | [141] |
| Water born parasites, CD4+ T-Cells are detected in an 81 mm2 wide view with 10 µm resolution. An experimental protocol is included. | Fluorescent imaging flow cytometry using microfluidics, LED excitation and time-lapse video recording using the digital frames for cell counting. Also wide view microscopy using the smartphone camera is demonstrated. | Wide field of view for good diagnostics at low copy number and mobile cell counting. | Target must be fluorescently labeled prior to analyses | [142] |
| Multiplex (384) lateral flow protein micro array for clinically relevant biomarkers. Accuracy was 98% compared to established glass microarray for 26 antigen specific antibodies. | Paper based lateral flow protein microarray using biotin conjugated secondary Abs and anti-biotin coated GNPs | High multiplexing possibility and sensitive detection (30 ng/mL) in 10 min. | Multiple amplification steps can impede accurate quantification. High multiplexing can reduce signal to noise ratio. | [143] |
| DNA or RNA detection of multiple analytes in diverse matrixes (blood and water) using various microfluidic devices is described. | Microchip combining filtration, cell lysis, isothermal amplification and fluorescent detection for virus and bacteria. | Sensitivity and specificity comparable to conventional bench top methods | Complex matrix can impede enzyme assisted isothermal amplification | [126] |
| Human C-reactive protein (CRP) detection by sandwich ELISA, HRP detection for direct ELISA and BCA total protein estimation assays were performed for the SBD and compared to conventional microtiter plate readers (MTPR). | Standard ELISA tests read out by smartphone camera. SBD showed equal performance to conventional MTPR for LOD, LOQ, dynamic range, sensitivity and precision for all 3 assays. | Simple application using already existing established methods with low cost and miniaturized material. | Analyses requires same time frame and expertise as conventional ELISA | [144] |
| Carcinoembryonic antigen (CEA) (1) and (2), adenosine triphosphate (ATP). LOD for CEA was 6.1 pg/mL. LOD for ATP was 11 µM. Normal range of CEA is < 2.5 ng/mL and ATP roughly 1 mM. Thus mentioned LODs show usefulness’ of the device. | Inhibition of peroxide induced etching of nanoprisms and color change by presence of more Ab-NPs at high target concentrations (1). GNP aggregation inhibition by ssDNA stabilization after target binding with aptamer and dsDNA dissociation (2) (b). | Simple system using the ambient light sensor to detect the color changes in the suspension. | Complicated setup. Especially using dsDNA which dissociates to ssDNA (for GNP stabilization) and aptamer-target complex. The functioning of the system might be very dependent on the salt concentration in the matrix | [145] |
| Relative particle number densities determined in food (fat droplets in milk, yeast in water) and medical (RBCs in whole blood) matrixes. | ELS (c) with diode laser is used to create angular resolved scattering patterns which are imaged by the SBD camera. Mie theory is then used to calculate particle size. | Cheap determination of size distribution of particles in blood, yeast and milk. | Poor accuracy (±20 nm) and at proof of principle stage. | [146] |
| RE | Description | Advantage | Limitations | Reference |
|---|---|---|---|---|
| Antibody | Specialized immune protein capable to recognize its antigen via a key-lock principle. Antibody antigen binding is based on Van der waals, hydrophobic and hydrogen bonds making it quite a stable complex. | Highly developed protocols exist, LOD often in pM range. Antibodies can often operate in quite varying conditions (pH, Salinity, complex matrix) and many protocols exist, making antibody based detection often the method of choice. | Production cost of monoclonal Ab is high. Protein can degrade limiting long-term storage. Setting up a reliable hybridoma line for monoclonal antibody is costly and can take years. Antibodies are primarily produced in animals. | [147,148] |
| Aptamer | Oligonucleotide designed to specifically bind its target (often upon conformation change) via subsequent systematic selection of the best binders available in a randomized pool of oligonucleotides. This selection process is called SELEX (systematic evolution by exponential enrichment). Many varieties of the process exist. | Developed protocols exist, LOD in the nm and even pM range is reported. Production is synthetic and cheaper as antibodies. Aptamer-target complexation often results in a significant conformational change of the aptamer which can be used as a label-free sensing principle. | Often binding specificity is sensitive to salt concentration. Degradation sensitive due to nucleases, hard to use in complex matrix. | [149,150] |
| MIP | Polymers with functional groups capable to interact with target functional groups are polymerized around the target. Next the target is eluded leaving a functionalized pocket behind to act with the target via a key-lock interaction principle | MIPs are cheap to produce if the target is not expensive. MIPs are very stable, leading to long shelf life. Detection limits in the pM range are reported but less common. | Washing out the template molecule can prove difficult. Target affinity can change between batches. Higher amounts of template is needed which can increase production costs. | [151,152] |
| Enzyme activity inhibition | The ability of an enzyme to catalyze its reaction is inhibited by the presence of a pollutant. The method is often used to detect organophosphorus pesticides. In such assays the enzymatic catalyzed conversion of a substrate to a colored product is often measured. Absence or reduction of the intensity of reaction indicates enzyme inhibition. | OPA (a), OPAA (b) and ACHE (c) enzyme inhibition assays are cheap and fast tests ideal for on-site screening. Especially OPH and OPAA enzymes are good candidates since they allow sensitive 1 step only detection. Moreover, genetically engineered recombinant enzymes of these groups exist and result in higher sensitivity. | Enzymatic activity can be reduced by many different compounds. Thus the specificity of this system can be compromised if real samples are used. Work remains to be done to further engineer OP and OPAA enzymes for optimal results. | [155] |
| Enzymatic substrate conversion | Enzymatic catalyzes of a compound leading to direct or indirect electron transport to an electrode used in electrochemical detection or conversion to a fluorochrome or colored compound for optical detection. | A wide variety of sensors based on this principle exist some of which like glucose sensors have proven to be fast, sensitive, low cost and reliable. | The inhibition of catalytic activity can lead to false negatives. Especially in matrices from patients containing ROS (d) and or inflamed tissues containing proteases capable to degrade the enzymes. | [153,154] |
| Riboswitches | RNA based system comprising 2 domains, a recognition domain (aptamer) and signaling domain. Upon recognition the conformational change frees an area of the signaling domain that can inhibit or promote translation of a protein or transcription of a reporter gene, triggering a fluorescent response. In some cases fluorescent response even occurs directly upon binding the analyte. These riboswitches are called fluorogenic riboswitches. | This system is very effective to enable small molecule induced gene regulation and can be used with synthetic aptamers to create fluorescent RNA based biosensors as internal validation for CBBs. Moreover, synthesis is synthetic and cheap compared to antibodies. | The best functioning riboswitches are prokaryotic. They will need to be adapted to use in eukaryotic cells to prevent rapid degradation of the RNA. For this non-natural nucleic acids, equally used for aptamer construction, might proof useful. | [156] |
| Affibodies | Synthetically constructed peptide scaffolds combined with a specific peptide sequence used as the RE. The Scaffold sequence (around 6.5 kDa) contains no cysteine and often stays the same. The variable region classically contains 13 amino acids and can be specifically engineered for a given target. | Smaller then antibodies thus closer to surface of transduction element leading to low LODs. Scaffold can be engineered to allow orientated immobilization. Absence of cysteine avoids artificial sulfur-bridge formation. | The method is relatively undeveloped. Some initial successes are booked but more research is needed. | [157,159] |
| CBBs | Living cells are integrated in the sensor. Their shape change, cell membrane damage or dead caused by interaction with the target are reported through optical or electrochemical detection. | CBBs have the unique ability to offer a measurable response to a pollutant related to actual physiologic responses of the subject to the substance. | The cells must be kept alive to function making long-term storage difficult. Many structurally different compounds can cause a similar response making downstream identification complex. Moreover, CBB sensors often require lengthy incubation and measuring steps in an incubator seriously limiting portability. | [158] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelis, J.; Elliott, C.; Campbell, K. “The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space. Biosensors 2018, 8, 96. https://doi.org/10.3390/bios8040096
Nelis J, Elliott C, Campbell K. “The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space. Biosensors. 2018; 8(4):96. https://doi.org/10.3390/bios8040096
Chicago/Turabian StyleNelis, Joost, Christopher Elliott, and Katrina Campbell. 2018. "“The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space" Biosensors 8, no. 4: 96. https://doi.org/10.3390/bios8040096
APA StyleNelis, J., Elliott, C., & Campbell, K. (2018). “The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space. Biosensors, 8(4), 96. https://doi.org/10.3390/bios8040096






