Functionalized Thick Film Impedance Sensors for Use in In Vitro Cell Culture
Abstract
1. Introduction
2. Materials and Methods
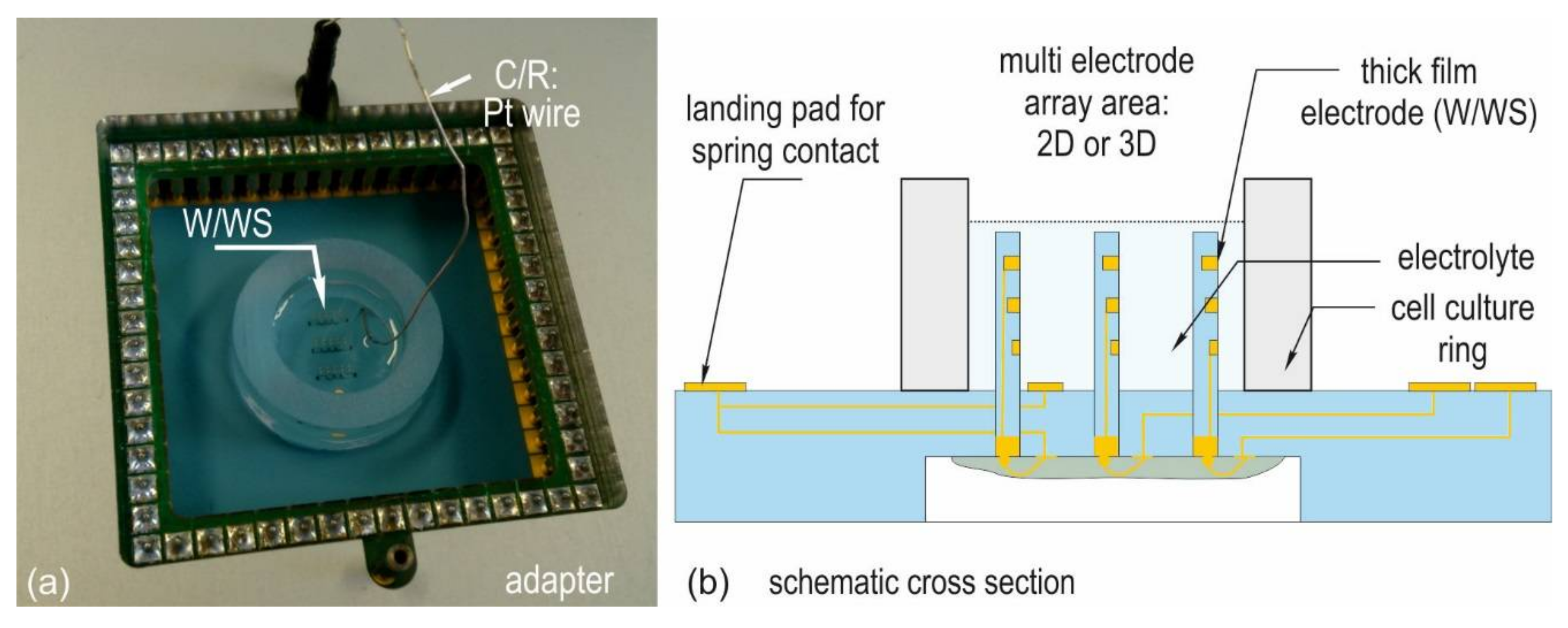
2.1. Test Chip Types
2.2. Test Procedure
2.2.1. Cleaning
2.2.2. Laminin Coating
2.2.3. Functionalization with Printed PEDOT:PSS
2.2.4. Functionalization with Sputtered Titanium Oxynitride
2.3. Impedance Measurement
3. Results and Discussion
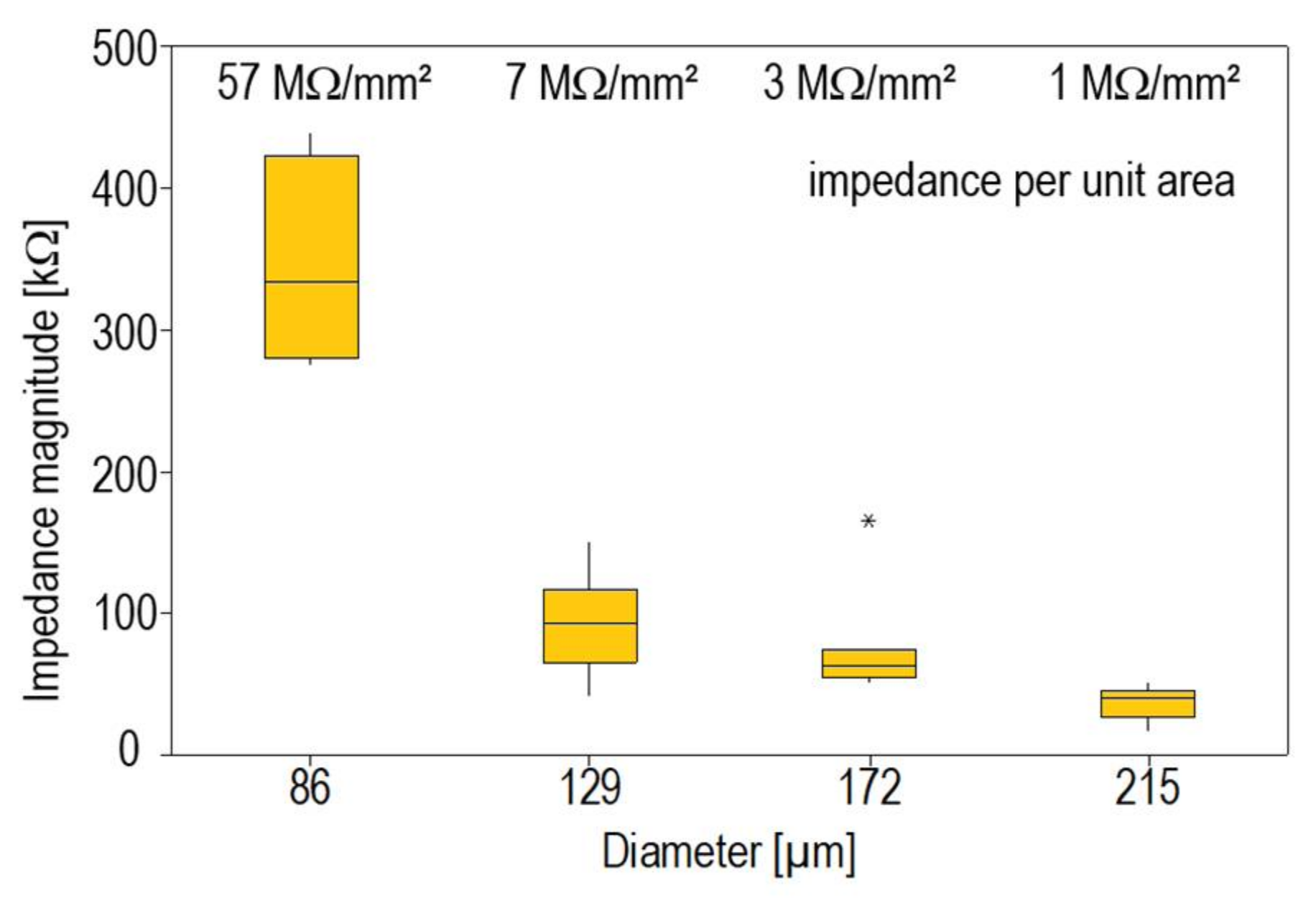
3.1. Influence of Electrode Size
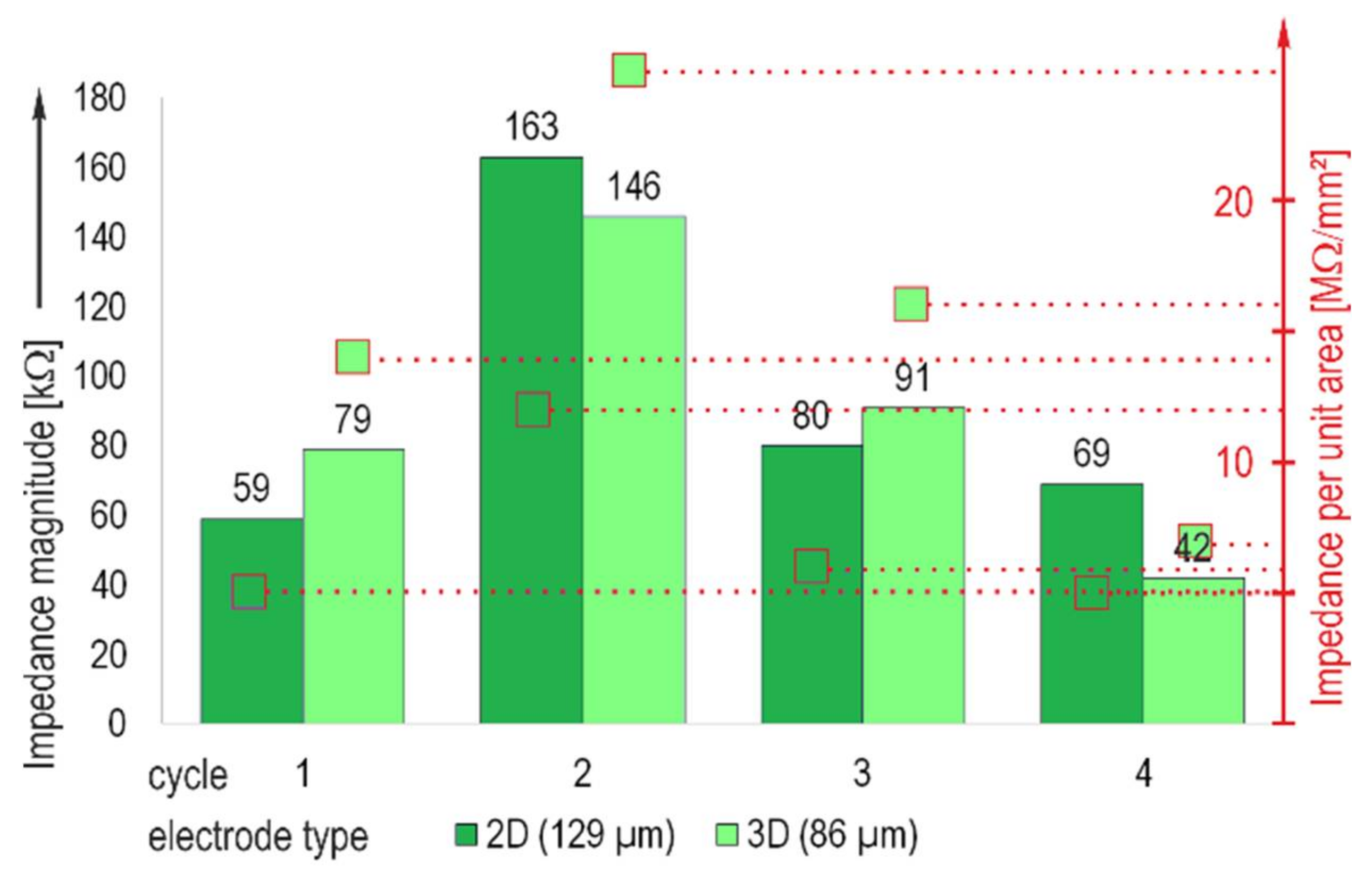
3.2. Influence of the Laminin Coating and Repeated Applications
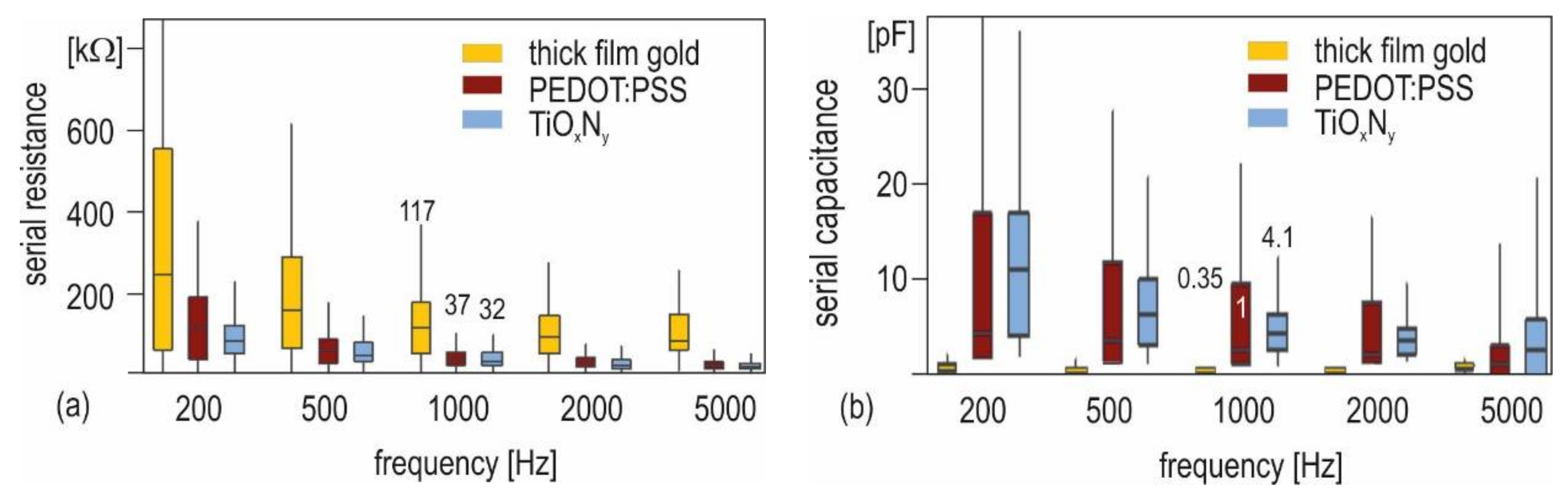
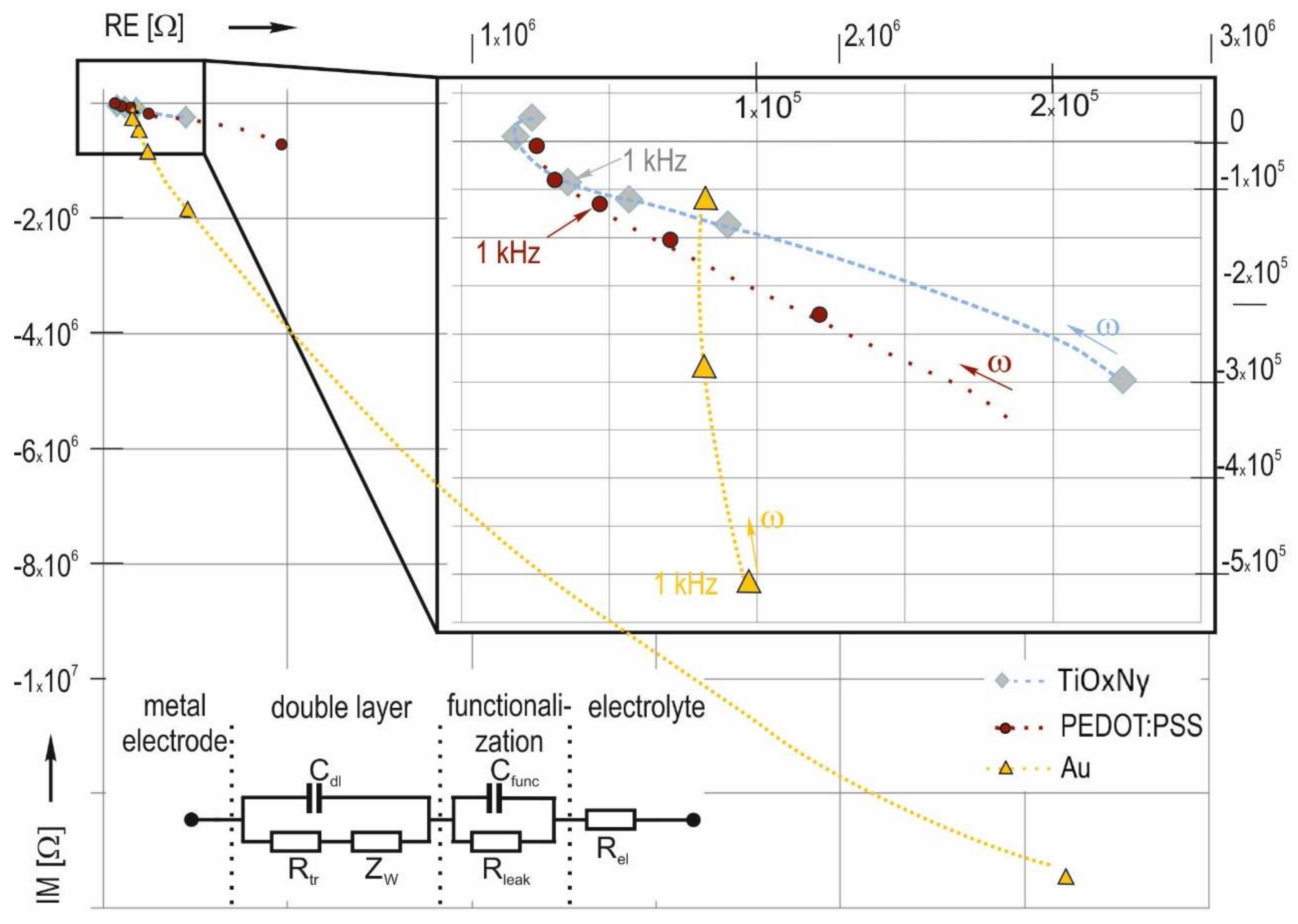
3.3. Influence of Functionalization
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wheeler, B.C.; Brewer, G.J. Designing Neural Networks in Culture: Experiments are described for controlled growth, of nerve cells taken from rats, in predesigned geometrical patterns on laboratory culture dishes. Proc. IEEE Inst. Electr. Electron. Eng. 2010, 98, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Cho, H.-Y.; Choi, J.-W. Microdevice platform for in vitro nervous system and its disease model. Bioengineering 2017, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Khoong, L.E.; Tan, Y.M.; Lam, Y.C. Overview on fabrication of three-dimensional structures in multi-layer ceramic substrate. J. Eur. Ceram. Soc. 2010, 30, 1973–1987. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Arasa, E.; Puyol, M.; Martinez-Cisneros, C.S.; Alonso-Chamarro, J.; Montenegro, M.C.B.S.M.; Sales, M.G.F. Novel LTCC-potentiometric microfluidic device for biparametric analysis of organic compounds carrying plastic antibodies as ionophores: Application to sulfamethoxazole and trimethoprim. Biosens. Bioelectron. 2011, 30, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, A.; Kaushik, A.; Tomizawa, Y.; Norena, N.; Bhansali, S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators B Chem. 2013, 182, 139–146. [Google Scholar] [CrossRef]
- Malecha, K.; Remiszewska, E.; Pijanowska, D.G. Surface modification of low and high temperature co-fired ceramics for enzymatic microreactor fabrication. Sens. Actuators B Chem. 2014, 190, 873–880. [Google Scholar] [CrossRef]
- Jędrychowska, A.; Malecha, K.; Cabaj, J.; Sołoducho, J. Laccase biosensor based on low temperature co-fired ceramics for the permanent monitoring of water solutions. Electrochim. Acta 2015, 165, 372–382. [Google Scholar] [CrossRef]
- Bartsch, H.; Welker, T.; Welker, K.; Witte, H.; Müller, J. LTCC based bioreactors for cell cultivation. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 12001. [Google Scholar] [CrossRef]
- Chabowski, K.; Junka, A.F.; Piasecki, T.; Nowak, D.; Nitsch, K.; Smutnicka, D.; Bartoszewicz, M.; Moczała, M.; Szymczyk, P. Impedance Sensors Made in PCB and LTCC Technologies for Monitoring Growth and Degradation of Pseudomonal Biofilm. Metrol. Meas. Syst. 2017, 24, 369–380. [Google Scholar] [CrossRef]
- Ciosek, P.; Zawadzki, K.; Łopacińska, J.; Skolimowski, M.; Bembnowicz, P.; Golonka, L.J.; Brzózka, Z.; Wróblewski, W. Monitoring of cell cultures with LTCC microelectrode array. Anal. Bioanal. Chem. 2009, 393, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Groß, G.A.; Thelemann, T.; Schneider, S.; Boskovic, D.; Köhler, J.M. Fabrication and fluidic characterization of static micromixers made of low temperature cofired ceramic (LTCC). Chem. Eng. Sci. 2008, 63, 2773–2784. [Google Scholar] [CrossRef]
- Bartsch de Torres, H.; Rensch, C.; Fischer, M.; Schober, A.; Hoffmann, M.; Müller, J. Thick film flow sensor for biological microsystems. Sens. Actuators A Phys. 2010, 160, 109–115. [Google Scholar] [CrossRef]
- Bartsch, H.; Himmerlich, M.; Fischer, M.; Demkó, L.; Hyttinen, J.; Schober, A. LTCC-Based Multi-Electrode Arrays for 3D in Vitro Cell Cultures. J. Ceram. Sci. Technol. 2015, 06, 315–324. [Google Scholar] [CrossRef]
- Bartsch, H.; Stöpel, D.; Himmerlich, M.; Baca, M.; Stadie, P.; Hyttinen, J.; Müller, J.; Schober, A. LTCC Based Multi-Electrode Arrays for In-Vitro Cell Culture. In Proceedings of the 11th International Conference and Exhibition on Ceramic Interconnect and Ceramic Microsystems Technologies (CICMT), Dresden, Germany, 20–23 April 2015; pp. 269–274. [Google Scholar]
- Fakunle, E.S.; Aguilar, Z.P.; Shultz, J.L.; Toland, A.D.; Fritsch, I. Evaluation of screen-printed gold on low-temperature co-fired ceramic as a substrate for the immobilization of electrochemical immunoassays. Langmuir 2006, 22, 10844–10853. [Google Scholar] [CrossRef] [PubMed]
- Blau, A. Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 2013, 18, 481–492. [Google Scholar] [CrossRef]
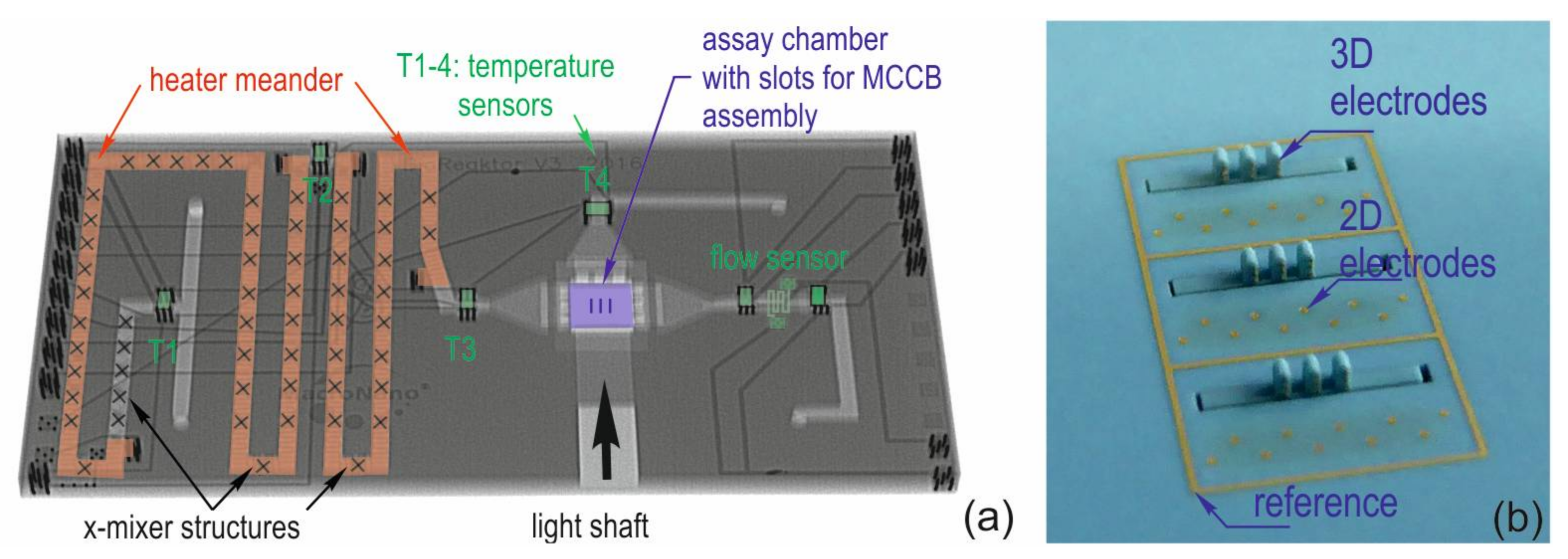
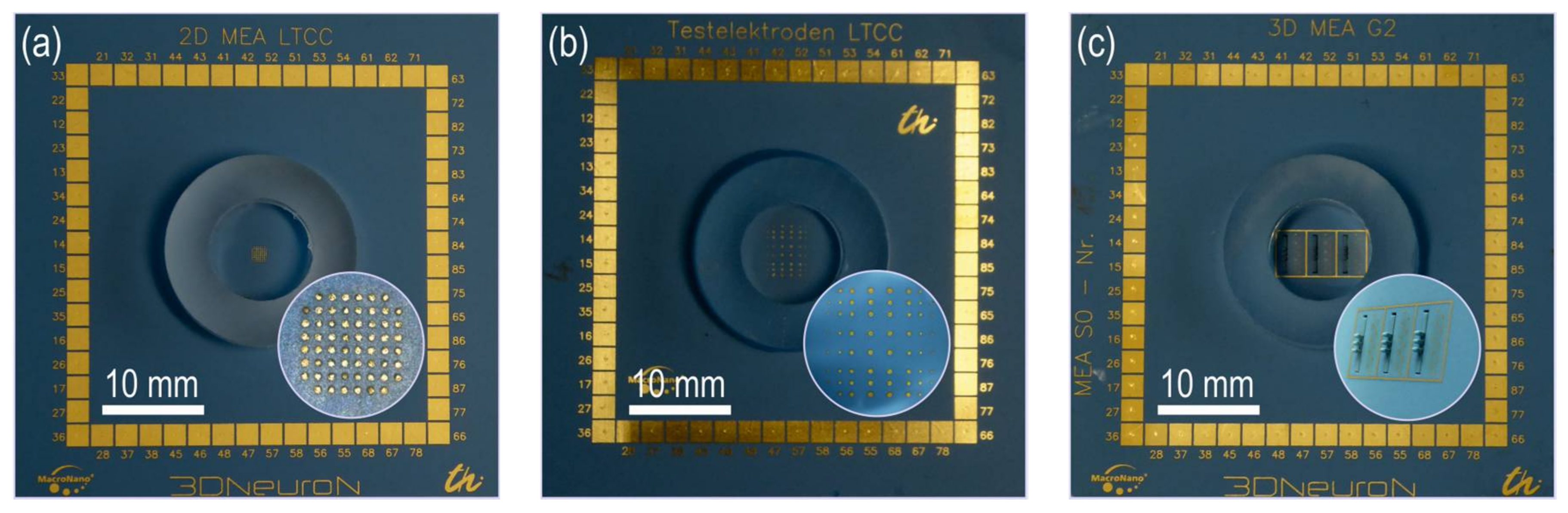
| Type A | Type B | Type C |
|---|---|---|
| Array of 60 electrodes Diameter 86 µm Pitch 200 µm | Array with different electrode sizes: Column 1 and 8: 6 × 86 µm Column 2 and 7: 8 × 129 µm Column 3 and 6: 8 × 172 µm Column 4 and 5: 8 × 215 µm Pitch Column 1–2, 2–3, 6–7, 7–8: 546 µm Pitch Column 3–4, 4–5, 5–6: 893 µm Rows pitch is equivalent | 3D MEA: 27 tower electrodes on MCCB: Diameter 86 µm Pitch in z-direction 150 µm Finger distance 500 µm 30 base plate electrodes Diameter 129 µm Pitch (x-y): 425 µm, Diagonal distance: 600 µm |
| Functionalization | ||
| TiOxNy and PEDOT:PSS | Without | Laminin coating |
| Related to results in section | ||
| 3.3 | 3.1 | 3.1/3.2/3.3 |
| Chip N° | Blade Gap [µm] | Wetting Agent [%] | Drying Temperature [°C] | Mg Imp @ 1 kHz [kΩ] | Phase Angle @ 1 kHz [°] | Rs @ 1 kHz MW [kΩ] | Cs [pF] |
|---|---|---|---|---|---|---|---|
| 22 | 150 | no | 65° | 81 | 54.5 | 37 | 2.5 |
| 23 | 300 | 0.01 | 65° | 281 | 67 | 55 | 1.0 |
| 24 | 300 | 0.01 | 75° | 333 | 70 | 53 | 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartsch, H.; Baca, M.; Fernekorn, U.; Müller, J.; Schober, A.; Witte, H. Functionalized Thick Film Impedance Sensors for Use in In Vitro Cell Culture. Biosensors 2018, 8, 37. https://doi.org/10.3390/bios8020037
Bartsch H, Baca M, Fernekorn U, Müller J, Schober A, Witte H. Functionalized Thick Film Impedance Sensors for Use in In Vitro Cell Culture. Biosensors. 2018; 8(2):37. https://doi.org/10.3390/bios8020037
Chicago/Turabian StyleBartsch, Heike, Martin Baca, Uta Fernekorn, Jens Müller, Andreas Schober, and Hartmut Witte. 2018. "Functionalized Thick Film Impedance Sensors for Use in In Vitro Cell Culture" Biosensors 8, no. 2: 37. https://doi.org/10.3390/bios8020037
APA StyleBartsch, H., Baca, M., Fernekorn, U., Müller, J., Schober, A., & Witte, H. (2018). Functionalized Thick Film Impedance Sensors for Use in In Vitro Cell Culture. Biosensors, 8(2), 37. https://doi.org/10.3390/bios8020037








