Abstract
Analytical chemistry is now developing mainly in two areas: automation and the creation of complexes that allow, on the one hand, for simultaneously analyzing a large number of samples without the participation of an operator, and on the other, the development of portable miniature devices for personalized medicine and the monitoring of a human habitat. The sensor devices, the great majority of which are biosensors and chemical sensors, perform the role of the latter. That last line is considered in the proposed review. Attention is paid to transducers, receptors, techniques of immobilization of the receptor layer on the transducer surface, processes of signal generation and detection, and methods for increasing sensitivity and accuracy. The features of sensors based on synthetic receptors and additional components (aptamers, molecular imprinted polymers, biomimetics) are discussed. Examples of bio- and chemical sensors’ application are given. Miniaturization paths, new power supply means, and wearable and printed sensors are described. Progress in this area opens a revolutionary era in the development of methods of on-site and in-situ monitoring, that is, paving the way from the “test-tube to the smartphone”.
1. Sensors Characteristics
In analytical chemistry, two main types of sensors are usually considered: biological and chemical ones. Generally, biosensors/sensors are classified according to the type of the receptor:
- enzymes,
- affinity receptors (antigens/antibodies, DNA probes),
- cells and cell organelles,
- artificial receptors (Molecularly Imprinted Polymers, biomimetics, aptamers).
The sensors also differ by the method for immobilization of the receptor layer (physical sorption, immobilization in a polymer matrix, covalent binding, affinity immobilization) and method for an analytical signal’s detection (optical, electrochemical, fluorescent, etc.).
Any sensor contains a transducer that changes one form of energy into another. In an electrochemical sensor, an electrode plays the role of a transducer. Another important part of the sensor is the receptor-sensitive recognition element. The main difference between biological and chemical sensors is the nature of a receptor. In the first case it is a biomaterial, and in the second case it is a chemical compound or a group of compounds selectively interacting with an analyte.
A variety of biological and synthetic materials and ways of their immobilization on the transducer and the use of nanotechnologies lead to the appearance of a steadily increasing number of biosensors/sensors.
1.1. Thin- and Thick-Film Transducers
In electrochemical sensors, electrodes perform the role of transducers. Requirements for working electrodes are high electroconductivity, inertness over a wide potential range, non-toxicity, and, finally, low cost. The rejection of an extremely toxic mercury electrode opened the era of solid-phase sensors [1,2,3]. In the development of sensors today, as a rule, planar electrodes and electrode systems made by screen printing (thick-film screen-printed electrodes) or thin-layer deposition are used [4,5]. As an alternative to mercury, low-toxicity solid-phase compounds [6,7], noble metals [8,9], carbon [10], or composite (nano)materials [11,12] are used.
Planar electrodes, based on noble metals, are made by thin-layer sputtering. Photolithography, vacuum deposition, etching, and stepwise firing are used to obtain thin metal films of gold, silver, platinum, etc. on the surface of the substrate [13,14].
Electrodes based on carbon paste are prepared by mixing carbon powder with paraffin, petroleum oils, dioctylphthalate, α-bromnaphthalene, or tricresyl phosphate (“carbon paste electrodes”) and by a screen-printing process. Pastes for electrode printing consist of fine carbon material and epoxy resins, etc. [15,16]. The non-working area of the transducer is insulated using a dielectric. This technology makes it possible to obtain thick films (about 20 μm).
Thus, the main advantages of thick-film electrodes are their low cost, simplicity of manufacturing, compactness, and providing a wide choice of materials and designs [17]. In addition, it is not necessary to perform the procedure for cleaning and preparing the electrode surface before each measurement, since the electrode is intended for one-time use. The disadvantages include the inability to analyze organic media capable of dissolving paste components in the conductive layer. However, examples of the use of organic-solvent-compatible screen-printed electrodes have been published [18].
Planar electrodes have found a wide application in easy-to-use portable point-of-care sensors, which can be performed at the bedside and in a “home clinic”.
1.2. Receptors
The main focus in the development of receptors is currently on enzyme and affinity biosensors.
Enzyme biosensors. In this case, the receptor properties are determined by the ability of natural enzymes to participate in ultraspecific catalytic reactions with certain substrates–analytes [19,20]. Enzymes are protein macromolecules that have a substrate-binding site and an active site providing a catalytic reaction. Some enzymes have in their structure a cofactor, which is a non-protein component responsible for catalysis (metal ions, iron-sulfur clusters, flavins, gems, etc.). The enzyme activity strongly depends on environmental conditions, such as temperature, ionic strength and pH of the solution, and the presence/absence of inhibitors. [21,22]. The disadvantages of enzyme sensors are determined by their low stability, short shelf life, and high cost.
Tissue and cells in some cases serve as receptors in biosensors, while the receptor functions perform the reactions with enzymes contained in them [23,24,25,26]. The simplest microorganisms, such as bacteria, yeast, and fungi, and their separate organelles, for example, cell membranes, are the most frequently used as recognition elements in cell biosensors. Tissue biosensors contain homogenates or thin sections of plant and animal tissues immobilized on the surface of the transducer. Such receptors do not require the use of expensive procedures for the isolation and purification of enzymes. Furthermore, in them enzymes are more stable than those in pure form due to the “habitual” environment [25,27,28,29,30].
In affinity biosensors, reversible biochemical processes for the formation of complementary complexes are used. DNA sensors and immunosensors relate to this group. In the first case, the receptor is a DNA fragment. Examples are DNA probes or DNA primers [31,32]. In an immunoassay, an antibody or an antigen serves as a receptor. The formation of an immunocomplex is provided by a Fab fragment of the antibody. Variable and hypervariable regions in their structure are energetically and conformationally complementary to the antigen [33,34]. The library of antibodies and DNA probes is enormous today, and the processes for obtaining, isolating, and purifying such receptors are automated. In addition, DNA primers and antibodies are more stable and universal than enzymes.
In chemical sensors, receptors are synthetic molecules having in their structure functional groups able to selectively interact with an analyte. They are used in sensors for the determination of both individual chemical compounds and groups of substances, which are implemented in chemometric systems, such as an “electronic nose” or an “electronic tongue” [35,36].
This kind of sensor for bioanalytes determination contains the following types of compounds as receptors:
- (1)
- non-biological molecules modeling the active sites of an enzyme–redox-active metal centers and suitable ligand environment that provide electrocatalytic processes simulating the action of enzymes [37],
- (2)
- molecularly imprinted polymers [38,39],
- (3)
- calixarenes [40,41], and
- (4)
- nanomaterials and others [42].
Synthetic receptors are more stable than biological ones, have a relatively small molecular weight, and bind more firmly to the analyte. Their main disadvantage is their relatively low biocompatibility.
1.3. Methods of Immobilization
To improve the analytical characteristics of sensors and expand the range of analytes, the surface of the transducer is modified with organic compounds, nanomaterials, enzymes, affinity agents [43,44,45], etc. A modifier is either “grafted” onto the surface (surface modification) or mixed with components of electrode pastes (bulk modification).
The basic requirements for immobilization methods are maximum preservation of specificity and exclusion of receptor degradation (the activity of a recognition component in its immobilized state should be comparable with its activity in a solution). The processes for immobilization of a receptor layer in chemical sensors and biosensors are based on general principles. However, in the case of biosensors it is especially important to perform the procedure in the most “soft”, close to physiological, conditions [46,47]. The main methods for immobilization of the receptor are physical sorption, incorporation into a polymer matrix, covalent fixation, and immobilization using affinity reagents.
Physical sorption is the simplest way to immobilize a receptor based on its electrostatic and Van der Waals interaction with the transducer material [48,49]. This method is widely used due to the simplicity of implementation and low cost. However, the low stability of these sensors during storage and the inability to re-use them and regenerate their surface are essential disadvantages of this type of immobilization [50].
Immobilization of a bioreceptor into a polymer matrix is most often performed by photopolymerization [51], electrochemical polymerization, or incorporation into a sol-gel matrix [52]. Polymers are increasingly frequently being further modified with various functional groups (-COOH, -NH2, etc.) to hold the bioreceptor/receptor more firmly and orient it. Thus, the receptor located in the polymer pores, on the one hand, is reliably retained on the transducer’s surface, and on the other hand, its reactivity is preserved. This method, as well as physical sorption, is most often used for the immobilization of enzymes [53,54] and living cells [55]. In addition, the method is simple and it significantly prolongs the receptor’s activity. However, polymerization requires strict precision in the experimental conditions, specific pore size, the polymer’s composition, and the sorption-desorption equilibrium of the system.
The method of covalent immobilization consists in modifying the transducer’s surface by compounds capable of forming chemical (in particular, covalent) bonds with components of the receptor layer. Cross-linked reagents–thiols [56], amines [57], carboxyl compounds [58], alkynes, azides [59], and so on are most frequently used as modifiers. This method provides a firm and oriented fixing of the receptor on the transducer’s surface. It promotes a significant increase in the sensitivity and accuracy of detection, and allows for surface regeneration, for example, by changing the stability of the “analyte-receptor” complex [60,61]. This increases the shelf life of the sensor. However, the use of toxic reagents and extreme environmental conditions (high/low pH values, a non-physiological temperature, and organic solvents) often does not allow for using this method for the immobilization of biomaterial.
Affinity immobilization. In this case, the layer applied on the transducer’s surface includes reagents capable of interacting with the bioreceptor, thereby fixing the bioreceptor on the surface. Complementary sites of DNA [62], genetically modified polypeptides [63], and individual amino acids and aptamers [64] act as such agents. The method is universal and highly specific; it does not lead to degradation of the bioreceptor, but it is labor-consuming. In addition, massive protein molecules on the transducer’s surface negatively affect the sensitivity of the analysis, for example, in the case of electrochemical sensors.
Despite the wide choice of existing methods for immobilization, the listed problems cannot be considered solved. The search for and synthesis of new linkers that are non-toxic and biocompatible with the living cell and/or its individual organelles as well as improving immobilization techniques are highly relevant.
The use of synthetic receptors opens new possibilities for their immobilization, mainly by expanding the range of potential linkers and by the high tolerance of such molecules to aggressive environmental conditions.
1.4. Signal-Forming Processes
The signal-forming process in enzyme sensors, as a rule, includes one or several catalytic transformations of a substrate (analyte) [65]. Several generations of enzyme sensors exist (Figure 1). In the first generation of enzyme sensors (I), the product of a catalytic interaction enters into an electrochemical signal-forming reaction. In the second generation, the signal-forming process includes the interaction of the product of the enzymatic reaction with a mediator (II), and an analytical signal forms because of the exchange of electrons between the mediator and a transducer. In the third generation (III) of enzyme sensors, direct electron transfer between the transducer and the active center of the enzyme determines the analytical signal [20].
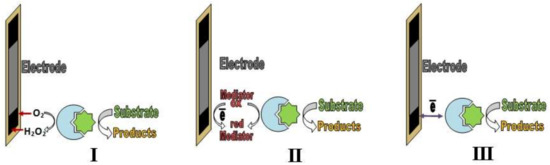
Figure 1.
First (I), second (II) and third (III) generations of enzyme sensors
DNA hybridization and DNA intercalation sensors are described as follows. DNA hybridization sensors are based on the formation of double-stranded DNA between a DNA probe and an analyte of a sample. The basic principle of intercalation devices is the ability of the analyte to embed into the structure of a DNA probe.
Immunoassay methods are based on the detection of either an antigen–antibody complex (Ag–Ab) or excessive amounts of immunoreagents (antigens or antibodies) [62]. Unlike enzyme sensors, the components of affinity complexes are not able to generate a distinct and reproducible analytical signal.
In this case, an enzyme is used as a label, which takes part in the formation of the response. As a rule, antigens, antibodies, and DNA primers are labeled. Homogenous (formation of an immunocomplex antigen–antibody in solution) and heterogeneous (formation of an immunocomplex using a solid template) methods are known for enzyme immunoassays.
The main disadvantage of an enzyme immunoassay is the labor-consuming and multi-stage analysis. For example, classical ELISA includes, in addition to the formation of the “antigen–antibody” immunocomplex, the subsequent localization of an enzyme-labeled conjugate of anti-antibodies on an immunocomplex. It is possible to reduce the number of stages by implementing a competitive and non-competitive format for heterogeneous analysis immunoassays using signal-forming label (able to generate an analytical signal by itself) and label-free procedures [66].
1.5. Methods of Analytical Signal Detection
Methods of analytical signal detection depend on the physicochemical properties of the receptor, and its ability to generate one or the other type of signal correlates with analyte concentration. The type of the signal and available detector determines the measurement method. There are many phenomena, effects, and types of energy converters that can be used in sensor designs. Detectors based on changes in thermal conductivity [67], the appearance/suppression of thermal radiation [68,69,70], the appearance of an electromotive force with rising temperature [71], Faraday [72] and Doppler [73] effects, the piezoelectric effect [74,75], etc. are known. The most common are optical and electrochemical detectors.
In optical sensors, the signal-forming process involves a change in phase, amplitude, polarization, and frequency of the light because of the “analyte–receptor” complex’s formation. Signal-forming labels (colorimetric and luminescent) and label-free ones (based on surface plasmon resonance (SPR), combinatorial light scattering, etc.) are used in optical sensors.
Colorimetric sensors are available and easy to use, but are suitable for qualitative/semi-quantitative analysis and much less sensitive than, for example, luminescent sensors. Photo-/fluo-/bio-/chemiuminescences can be distinguished and have become popular [76]. Moreover, in many sensors, a strategy of amplified detection, for example electrochemiluminescence, is used. Оrganic dyes [77,78,79] and quantum dots [80,81,82,83,84] are applied as signal-forming labels in optical affinity biosensors. Due to the high sensitivity and wide range of detectable analytes, the sensors of this kind have become popular. However, the main problem is the nonspecific luminescence, which limits the use of luminescent sensors in the analysis of real samples.
Optical sensors have several advantages: (i) each type of analyte can be determined using the appropriate spectrometric method; (ii) they create the possibility for remote monitoring; and (iii) they can be used in the implementation of non-invasive formats of biosensors [85,86]. However, some disadvantages complicate their development. These are: interference with ambient light; possible bleaching of dyes and other auxiliary components; a high signal of a background; a rather long measurement time; and a limited accessibility of accessories.
SPR sensors are a relatively new class of devices based on the reflection of incident light followed by the activation of “surface plasmons” on the metal–dielectric interface. An analytical signal is a shift of the SPR’s minimum due to affinity complex formation. This shift is greater for the more specific interactions that occur on the sensor [87,88,89,90,91]. The enormous increase in the efficiency of light scattering by molecules adsorbed on the surface of silver, gold, or copper—the phenomenon of combinatorial light scattering (CLS)—has also found a use in the development of sensors based on the interaction of a receptor and an analyte [92,93,94,95]. SPR and CLS methods are ultrasensitive, but require strict observance of the sensor’s preparation and analysis conditions, especially when treating of the transducer’s surface. Therefore, the prospects for the use of SPR and CLS sensors are not very wide.
Great attention has been paid to biosensors/sensors based on electrochemical methods of signal recording (about 80% of publications according to Scopus). Several types of electrochemical sensors are described in monographs [96,97], reviews [98], and original papers [99,100,101].
Voltammetric and amperometric sensors have been developed [102,103,104,105,106,107]. Amperometric sensors have found the widest application as they are the most sensitive, selective, and simple measuring devices. Voltammetric measurements include linear (LSV), pulse (DPPV), and square wave (SWV) [108,109,110,111,112,113] modes. Better selectivity and sensitivity of sensors are observed in DPPV and SWV in comparison with LSV. To increase the sensitivity, an additional step of “accumulation” of an analyte with subsequent detection of products of its electrochemical transformation—inverse voltammetry—can be introduced into the analysis procedure [3,114,115]. The potential range where the signal is observed is a qualitative characteristic and the limiting or peak current is quantitatively related to the concentration of an electroactive compound.
In potentiometric sensors, ion-selective electrodes are used as the indicator electrodes. Their potential serves as an analytical signal [116,117,118,119].
Conductometric and coulometric sensors are described as well [120,121,122,123,124,125].
The impedimetric method is based on measuring the working electrode’s resistance before and after affinity complex formation [126,127,128,129]. The electrochemical impedance spectra are recorded in the presence of electron-transfer mediators (potassium/ruthenium hexacyanoferrates, ferrocene) by applying a potential at which the redox system is electroactive and varies with a small amplitude. Impedimetric methods are similar to the methods for conductometric sensors, but signal processing is estimated using the different contribution of the capacitance and the resistance of a double electrical layer.
Field-effect transistors are actively used as miniature devices today. The work of such devices is based on a change of the electrical resistance of the conductive channel that changes due to the formation of specific “analyte–receptor” complexes. The electrons move from source to drain, and the control of the conductivity is regulated by the gate [130,131,132,133,134]. The analytical signal does not directly depend on the mass/dimensions of the complex but is determined by the charge redistribution at the gate of the transistor after interaction between the analyte and receptor. Because of this, field-effect transistors are more sensitive and accurate as sensors compared to impedimetric ones.
However, the chemical purity of the sensitive element and the immobilization of the receptor layer in this case represent a more serious problem due to an extremely small size of the transducer working area and the need for targeted immobilization of receptors only within these limits. In addition, the sample matrix effect is much more pronounced so surface protection is necessary.
The electrochemical methods are extremely attractive. The reasons are a wide choice of electrochemical reactions that can be used to form a signal, simple and relatively low-cost detectors, reliability, the low detection limits and small operating volumes of a sample, and the possibility to analyze colored samples. The possibility to analyze colored samples is especially important in the analysis of biological samples [135,136,137]. In addition, electrochemical methods do not assume any strict requirements for the shape or size of transducers and can be used in flowing, portable, and automated analytical systems. Therefore, the most commercially successful sensors are electrochemical devices, such as amperometric enzyme glucose meters (OneTouch, AccuCheck, Contour, etc.).
2. Non-Enzymatic Sensors
The development of biosensors is limited by their low stability and the rapid degradation of biological components during storage and use. This makes calibration difficult and reduces reliability. In contrast to biological components, their synthetic equivalents are stable chemically and thermally. Therefore, at present, the attention of researchers is being increasingly drawn to the creation of artificial analogues of enzymes: biomimetics. Recent research has been devoted to new strategies for creating biomimetics based on a deeper understanding of the fundamental principles of “guest-host” supramolecular systems design [138,139]. Natural enzymes have two important centers: (i) the cofactor; and (ii) the protein environment that provide both selectivity as a result of interaction between the analyte and receptor and a catalytic chemical reaction followed by the formation of an analytical signal.
In non-enzyme methods and sensors, the task of analytical signal formation is usually solved using non-protein catalysts. Providing selectivity in non-enzyme methods and sensors requires the creation of materials capable of molecular recognition. Sensors based on non-enzyme signal-forming labels or mediator systems (label-free sensors) in immuno- and DNA sensors are discussed in Section 3. About one third of all enzymes are “metal enzymes” and contain metal ions, which play an important role in catalysis [140]. One of the approaches to the synthesis of non-biological molecules is modeling an active site of an enzyme: a redox-active metal center and a suitable ligand environment. In these sites, catalytic processes simulating the action of hydrogenases and metal enzymes are possible [141]. For example, the authors of [142] developed a biomimetic for the heterogeneous catalysis of imines hydrogenation. A metal-organic framework modified with Brønsted acid was used as the basis. Encapsulated Cr3+ ions and sulfonic acid provide for high reactivity of the catalyst and the possibility of its re-use. Application of the developed biomimetic makes it possible to efficiently convert both aldimines and ketimines to their hydrogenated counterparts in a similar way to enzymatic catalysis.
The novel paddle-wheel Ni complex was synthesized due to the electro-oxidation of a dinickel precursor [143]. It allowed for the production of nickel (II) hydroxide nanoparticles on an electrode’s surface for the electrocatalytic detection of sugars. The small size (1–3 nm) and effective electrodeposition of the nanoparticles played key roles in the improvement of the sensitivity for sugars detection (Clim = 0.4 µM for glucose). Macrocyclic complexes of Ni (II) were synthesized [144] and their catalytic activity in the oxidation of amines was studied. A chronopotentiometric method for urea and creatinine determination was developed [145]. Many papers devoted to the electrocatalytic oxidation of alcohols, vitamins, amino acids, insulin, and urea in the presence of complexes of transition metal ions have been published [146,147,148,149]. Particularly, Ni(OH)2 films formed on the nickel anode in a strongly alkaline medium were used for the electrocatalytic detection of alcohols and amines [150]. Complexes of zinc with 1,4,7,10 tetraazacyclododecane (cyclen) incorporated into poly (ethylene glycol) dimethylacrylate were applied to the potentiometric detection of creatinine [151]. The main advantage of metal complexes is the ability not only to catalyze the transformation of the substrate, but also to coordinate it due to the ligands near the metal center of the biomimetic.
Many works on the investigation of the catalytic activity of organic polymers without metal in their structure have been published [152,153], but their catalysis efficiency is relatively low. Therefore, organic polymers are not widely used as biomimetics.
Nanomaterials are actively used in the creation of synthetic enzymes, both as a carrier for catalyst molecules and using their own catalytic activity. Investigations in this area are described in a new review [96]. Two aspects are considered: the impact of surface Gibbs free energy on decreasing the chemical reaction barrier and catalytic effects. An interesting experimental work [154] in which the synthesis of several types of nanoparticles (gold, silver of various structure and composition, including individual metals, core–shell particles, and nanoalloys) and their application in a reaction of cholesterol oxidation has been published. A biomimetic based on Fe3O4 nanoparticles for non-enzymatic H2O2 detection was created [155]. The selectivity of the sensor is provided with a molecularly imprinted polymer (MIP), which includes cetyltrimethylammonium bromide (CTAB) and poly (sodium 4-styrenesulfonate). The detection limit of the sensor is 103 µM/L. Silver nanoparticles were used in moxifloxacin hydrochloride determination [156] using a modified carbon-paste electrode. The detection limit of the sensor in human urine is 29 nM. Papers concerning the non-enzymatic detection of hydrogen peroxide and glucose on a base of carbon nanotubes and graphene catalytic activity have been published [157,158,159,160]. The large surface area of the nanoparticles makes it possible to successfully use them as a platform for the immobilization of agents for molecular recognition. High electrical conductivity contributes to an increase in the sensitivity of the sensor [135,155]. A serious difficulty in the commercialization of such sensors is their low reproducibility, especially when using nanomaterials. The main reason is the variation in the size, shape, or composition of nanoparticles and the general instability of their suspensions. Therefore, it is necessary to strictly control the structure of nanomaterials at the stage of their synthesis and when including them into the structure of sensors.
An alternative to enzymes in the field of selective extraction of an analyte from a sample matrix are molecularly imprinted polymers (MIPs). Technologies of molecular imprinting are aimed at creating recognition sites in synthetic polymers. Inside the polymer matrix, a “template” (atoms, ions, molecules, complexes, or even microorganisms) creates cavities that correspond to the target molecule in shape, size, and energy [161,162]. In this way, the molecular imprints of the template in the polymer matrix provide exceptional selectivity for separation of the target molecules [163,164]. Organic monomers (acrylic and methacrylic acids, vinylpyridine, vinylimidazole, pyrrole, etc.) and cross-linking agents (ethylene glycol dimethacrylate, divylbenzene, etc.) can be used for the synthesis of MIPs [165,166]. The matrix can also include micro- or nanoparticles of an inorganic material (metal, SiO2, etc.) coated with a polymer shell [167,168,169,170,171]. The main types of molecular imprinting for the synthesis of MIPs are shown in Figure 2.
Figure 2.
Main types of molecular imprinting [163].
Increasing interest is currently being given to the strategy of synthesizing MIPs on the surface of nano/microparticles [172,173,174]. The use of this method allows us to obtain monodisperse particles with a high specific surface area. In addition, the application of this synthesis methodology makes it possible to use an MIP not only as a non-enzyme receptor but also as a signal-forming layer. Nanoparticles of silica [175,176], magnetite [177,178], and carbon nanotubes [179] previously modified with vinyl groups using sol-gel methods [180] and obtained by grafting [181,182] are used to implement this approach. Yet another application of MIPs is the development of new non-enzyme systems capable of catalyzing chemical reactions for which enzymes do not exist (for example, Diels–Alder reactions [183] and others) or improving the reliability, selectivity, and efficiency of already existing enzyme sensors [184].
Prospects for the development and use of MIPs are obvious, especially for the creation of simple portable devices. They will significantly improve the quality and reliability of an analysis. However, it is necessary (i) to increase the affinity to the target analyte, (ii) to effectively remove the template molecules after the synthesis of the MIP, and (iii) to exclude the non-specific adsorption of both the molecules of the analyte and those of interfering components (iv). This will allow for the wide implementation of such devices in analytical practice [185].
Selectivity of non-enzymatic sensors can be provided by aptamers. Aptamers are synthetic single-stranded DNA or RNA fragments of several tens of nucleic acids in length. The idea of using short sequences of nucleic acids as receptors for the selective capture of biomolecules first appeared in 1980 and continues to be developed intensively. This is due firstly to the high affinity of aptamers comparable to monoclonal antibodies (the dissociation constant in the nanomolar and picomolar range) [186]. Aptamers have in their structure clefts and grooves of target molecules, a higher receptor density, and a smaller spatial blockage that allows for increasing the binding efficiency. Secondly, aptamers are more stable in a wide range of temperatures and other extreme conditions for their storage and use in comparison with proteins [187]. Thirdly, aptamers can be synthesized chemically, which favorably distinguishes them, for example, from monoclonal antibodies, in the production of which complex and expensive technologies are used. Aptamers are universal: they can bind not only large organic ligands but also low-molecular compounds (amino acids, organic dyes, metal ions) [188,189]. Examples of practical applications of aptamers in sensory technologies are given in Section 5.
Thus, the use of supramolecular compounds, nanomaterials, electrically conductive polymers, MIPs, and aptamers as biomimetics makes non-enzymatic sensors not only a good alternative to traditional enzyme biosensors, but allows in some cases for the ability to exceed them in terms of universality, tolerance to environment, and shelf life. However, biomimetics are inferior to natural enzymes in the efficiency of their catalysis and their selectivity in extracting the substrate. Thus, the catalytic activity of metal complexes does not always correspond to enzymes, the suspension of nanomaterials is often unstable, and MIPs are not always able to provide the required affinity. There is a continuous search for new biomimetics and rational ways of obtaining them.
3. Methods of Sensors Characteristics Improvement
Nanomaterials are actively used as components of the modifying layer of transducers to improve the analytical characteristics of biosensors/sensors [96]. The creation of a nanomaterial layer on the transducer’s surface opens wide possibilities for the immobilization of various compounds, including biological ones. This leads to an increase in sensitivity. Recent advances in materials science and technology have made it possible to obtain nanostructured materials of various geometries that are used for conjugation with many biomolecules. The most widely used ones are quantum dots, carbon nanomaterials (nanotubes, graphene), metal nanoparticles, and hybrid core–shell nanomaterials [190,191].
Quantum dots are luminescent semiconductor nanocrystals that have attracted the attention of researchers due to their high quantum yield and molar extinction coefficients, wide excitation spectra, and narrow and symmetrical emission bands (30–50 nm) [192,193]. The main advantage of quantum dots is the possibility of high-precision control over their size as well as availability. The main disadvantages are their high toxicity and complexities in their synthesis [194]. In biosensors and sensors, such nanomaterials, as a rule, are used as signal-forming labels [195,196,197]. For example, a simple, fast, ultra-sensitive electrochemiluminescent competitive assay has been developed [198], where the conjugate of antibodies with CdSe acted as a signal-forming label. Gold nanoparticles were used as a carrier. Gold nanoparticles effectively accelerate the electron transfer to the transducer. The detection limit is 0.0084 ng/cm3 for clenbuterol detection.
Carbon nanomaterials (graphene and its oxides, fullerenes, single- and multi-walled carbon nanotubes, nanowires, fibers, sheets, etc.) are widely represented in sensors as the main material and/or a transducer modifier [199,200,201]. Currently, many reviews [39,202,203] and original papers [204,205,206] on various aspects of carbon nanomaterials application in sensors and biosensors have been published. These materials have a developed surface and high conductivity and mechanical strength. The morphology of carbon materials promotes an increase in the sensitivity, selectivity, and stability of the response compared with an unmodified transducer [207]. In the work [208], multi-walled carbon nanotubes have been successfully used as an electrically conductive layer of a developed DNA sensor for determination of hepatitis B.
The main disadvantages observed in using such materials are hydrophobicity and the tendency to aggregate.
Nanoparticles of metals or their oxides are characterized by electrochemical activity as well as a large surface. In biosensors and sensors, nanoparticles of noble (Au, Ag, Pt, Pd) and transition metals (Cu, Ni, Co., Fe, etc.) are actively used [209,210]. They act as signal-forming labels [211,212], carriers for the receptor layer, and transducer components [213,214,215]. The use of metal nanoparticles makes it possible to improve the analytical characteristics of the sensors due to increasing the conductivity of the transducer, the possibility of amplifying the analytical signal, and the covalently oriented immobilization of the receptor layer (usually according to the thiol-disulfide reaction). Therefore, gold and silver nanoparticles have found wide application as signal-forming labels in immunosensors for the determination of bacterial and viral agents [216]. The authors of [217] developed a device containing two separate graphite working electrodes, which was used to detect human IgG and goat IgG using gold nanoparticles as signal-forming labels. A similar device was used for the quantitative determination of a carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) using antibodies labeled with gold nanoparticles [218]. In the works [219,220,221], previously thiolated gold and silver nanoparticles were used to covalently immobilize antibodies on the transducer surface. Ag-SiO2 nanoparticles were applied as a carrier for a fluorescent signal-forming label in sensor construction for the detection of Escherichia coli [222]. An original approach to the determination of Staphylococcal enterotoxin B has been proposed [223]. “ZnS-quantum dots” were used as a signal-forming label. The developed method is characterized by Clim 0.2 ng/mL for fluorescent detection and 0.24 ng/mL for electrochemical detection.
The use of magnetic nanomaterials (MNM) allows for including stages of magnetic separation and magnetic concentrating in the analysis procedure. Concentrating in a magnetic field is an express and simple method, which does not require the use of expensive and large-sized equipment.
Paramagnetics, such as iron oxides [224,225], ferrites of manganese, cobalt, and nickel, and their composites: metal–metal, metal oxide–metal, metal–(bio) polymer, etc. (for example, FePt, Fe3O4-Ag, Fe3O4-Au, and CdS-FePt), have been widely used in sensory devices [226,227,228,229,230]. Nanomaterials are functionalized to increase the efficiency of their binding to biomolecules [231,232,233,234] and to use the coating to form an analytical signal [235,236].
The authors in [237] developed an immunosensor for CEA detection on the base of magnetic nanoparticles (MNPs). Primary antibody conjugated with magnetic iron-oxide nanoparticles that were coated with silica capture the target antigen. After that, a secondary antibody modified with SiO2 nanoparticles was added. SiO2 provided more binding sites for the signal-forming label’s (cyanine fluorophore) immobilization. It resulted in a 6-fold increase in fluorescence and thus heightened sensitivity. Electrochemical immunosensors for the quantitative determination of Escherichia coli ATCC 25992 and Staphylococcus aureus B-1266 were proposed [238,239]. Fe3O4 MNPs, coated with chitosan or 3-aminopropyltriethoxysilane, have been used as a direct signal-forming label. The linear range for both immunosensors is estimated to be 10–105 CFU/cm3. The detection limit for Escherichia coli ATCC 25992 determination in water samples is 9.3 CFU/cm3. For Staphylococcus aureus determination, Clim = 8.7 CFU/cm3. An electrochemical immunosensor based on an Fe3O4-SiO2 nanocomposite as a direct signal-forming label for measles virus antigen detection is described in [240]. The detection limit is equal to 1.87 × 10−5 mg/cm3. Metal–polymer nanocomposites based on Fe3O4 were used as signal-forming labels for the quantitative determination of Escherichia coli ATCC 25922 in the environment [235]. Amorphous ferrocene-modified silica was used as an electrochemically active polymer coating. The detection limit for the developed immunosensors is 12 CFU/cm3 for E. coli detection [241].
Nevertheless, the problem of producing nanostructures containing sensors arises due to the instability of suspensions of nanoparticles. Such disadvantage is eliminated mainly by a preliminary modification of the surface of the nanoparticles or substrate with the aim to increase their binding to the surface or to cover them by polymer.
4. Sensors Design
4.1. Chips, Microfluidic Systems, and Lab-On-The-Chips
A chip is a microelectronic measuring device used both in laboratory practice and in the home clinic [242,243]. The sensitive element of the chip is a sensor. The design features of chips are determined by the nature of the response and the required analytical characteristics. Chips, as a rule, are built into microfluidic systems. Microfluidic systems are adopted to work with extremely small flows of liquids (less than 10−6 L). The systems differ in the way they organize the flow. Continuous Flow Based Microfluidics provide a continuous flow of reagents through microchannels [244,245]. They are suitable for solving analytical problems that do not require complex liquid manipulations (flow chemical monocomponent analysis). In Droplet Microfluidics Based Chips or Micro-Electrode-Dot-Array systems, the reagents pass as droplets suspended in the carrier phase [246,247]. Such systems are more labile, universal, and suitable for multiplex analysis of complex samples.
Of great interest today are analytical lab-on-the-chip systems, including a sensor integrated into a chip, microfluidic transport of the reagents, and a portable detector. Having regard to the many advantages of lab-on-a-chip technologies, including multiplex analysis, the small size of the device, the low reagent consumption, and easy integration, such systems are widely used in drug screening, cell engineering, and medical diagnostics [248,249]. Lab-on-the-chips are easy to integrate into smartphones. For example, a lab-on-the-chip for the detection of three important mycotoxins (ochratoxin A (OTA), aflatoxin B1 (AFB1), and deoxynivalenol (DON)) in spiked buffer was developed [250]. A colorimetric signal for performing a multiplexed semi-quantitative direct competitive ELISA has been detected using a smartphone camera. Horseradish peroxidase was used as an enzymatic label. The reagents have been flown though the reaction chambers using pumps. Detection limits of <40, 0.1–0.2, and <10 ng/mL were obtained for OTA, AFB1, and DON, respectively. A disposable paper–plastic hybrid microfluidic lab-on-a-chip has been developed for the colorimetric measurement of some components in urine [251]. The developed device is incorporated into a paper-based conventional reagent test strip inside a plastic-based microchannel. For the colorimetric reaction of glucose, protein, pH, and red blood cells, the device uses a small volume (40 μL) of urine and a finger-actuating micropump. The lab-on-the-chip is controlled by a smartphone-based optical platform using the “UrineAnalysis” Android app.
The use of synthetic receptors in designs of lab-on-the-chips for bioanalytes detection allows for the creation of sensitive and stable devices for in vitro and in vivo medical diagnostics.
4.2. Wearable and Written Sensors
Wearable sensors are presented by soft, flexible, and stretchable electronic devices intended for the monitoring of clinically important indices of human health [252,253,254]. Chemical wearable sensors, preferably with an electrochemical detection of the response, are used for the determination of individual chemical substances and groups of the components (biomarkers). Designs of electrochemical wearable devices have been distinguished, namely, tattoo, patch, and band [255]. Tattoo sensors are manufactured using specially engineered stress-enduring inks and biocompatible polymers. These are the most ergonomic and convenient-to-use sensors. Patch sensors made on a textile basis are more durable, have a more developed surface, and, therefore, have more options for immobilizing the receptor layer and integrating the accompanying electronics. Tattoo and patch sensors are low-cost and not intended for re-use. Band sensors based on silicone are the most durable and reliable of all the above devices, and there is the possibility of replacement/regeneration of the sensor layer as well as multiplex detection of analytes. Figure 3 shows real examples of such sensors.
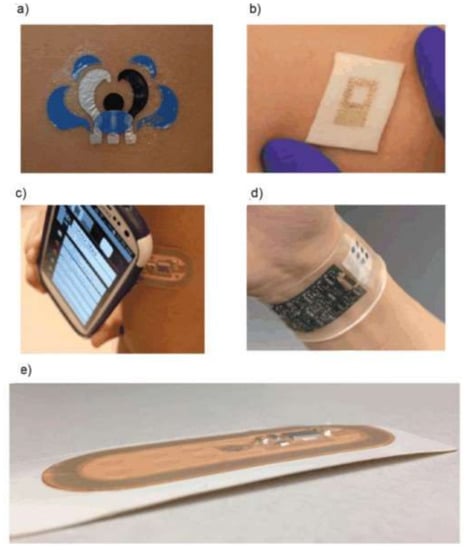
Figure 3.
Three types of wearable sensors: tattoo (a,b), patch (c), and band (d,e) [255].
The authors of [256] have developed an enzyme biosensor for the determination of lactate in sweat using a flexible printed temporary-transfer tattoo. The receptor layer based on lactate oxidase is coated with chitosan. Potentiometric sensor patches intended for the detection of calcium, ammonium, and sodium ions are presented in the paper [257,258,259]. It was shown that such skin sensors are resistant to mechanical deformations and demonstrate practically the Nernstian response in a wide range of concentrations.
An integrated multi-analyte potentiometric-amperometric sensor wristband platform has been developed [260]. This new platform has combined a plastic-based sensor array with silicon-integrated circuits consolidated on a flexible circuit board to improve signal conditioning, processing, and transmission. In addition to monitoring the general profile of human sweat, it is possible to independently determine glucose, lactate, potassium, and sodium in exercise-induced sweat.
Recent advances in screen-printed film and nanotechnologies allow for the integration of thin polymeric, metal, carbon, and composite materials into electronic soft and stretchable devices [261,262].
An interesting strategy is the use of “island-bridge” layers, where current-conducting tracers are intertwined with functionalized “islands” or “serpentine-shaped” structures consisting of periodic arcs and straight segments, which have been adopted to connect rigid areas on top of soft elastomers [263,264,265]. Sometimes, wearable sensors have a core–shell structure [266,267,268]. The core is an ultra-thin sensor module packaged in a soft shell based on textiles or silicone. Such a design allows for the provision of the most durable contact with the skin and resistance to wetting.
Amperometry, potentiometry, and impedimetry are used as measuring methods. Principles of field-effect transistors are used as well. An organic field-effect-transistor-type (OFET) sensor based on nanoparticle-decorated reduced graphene oxide (Pt_rGO) for the determination of dopamine has been proposed by the authors of [269]. A conducting-polymer source–drain electrode modified with graphene acts as a transducer. The OFET sensor is flexible and shows a high sensitivity to remarkably low dopamine concentrations (10−16 M) and selectivity to relatively interfering molecules. An ultrasensitive impedimetric wearable immunosensor for the determination of cortisol has been proposed [270]. Antibodies are immobilized on thin films of zinc oxide, and the analytical signal is formed as a result of a change in the thickness of the electrical double layer due to antibody–hormone interactions at diagnostically relevant concentrations. The detection limit is 1 ng/mL and the linear range is 10–200 ng/mL.
A written electrochemical sensor for the determination of methyl parathion and nitrite in food in situ has been proposed [271]. Written electrodes were made of a mixture of chitosan, graphite, and silver powders. The porous structure of the sensors makes it possible to perform an analysis without preliminary extraction of an analyte. The written sensor (Figure 4) was tested on model systems and real objects (Fuji apples, Chinese onions, and cabbage). The detection limit is 15 ng and 18.4 μg for methyl parathion and nitrite, respectively.
Figure 4.
An electrochemical written sensor for the determination of methyl parathion and nitrite in food [271].
A biotransferrable graphene wireless nanosensor for bacteria detection in saliva was developed [272]. Figure 5 shows the design of the sensor.
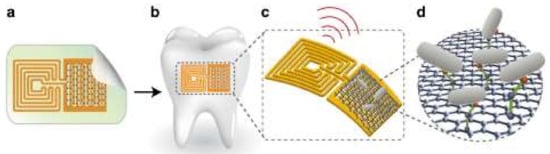
Figure 5.
Design of the graphene-based hybrid nanosensor: (a) printing of graphene onto a silk film; (b) biotransfer of the nanosensor onto a tooth; (c) schematic of the sensing element; (d) binding of the bacteria by a self-assembling antimicrobial peptide [272].
Firstly (Figure 5a), a graphene-based sensing element with a wireless readout coil was generated on silk fibroin. Secondly, the graphene-based transducer was biotransferred onto a tooth surface followed by dissolution of the supporting silk film (Figure 5b). Next, the specificity of detection was provided by the use of self-assembling antimicrobial peptides (odorranin-HP) onto the graphene monolayer. When recognition and binding of the target bacteria (Helicobacter pylori) are realized, electroconductivity of the graphene film is modulated and wirelessly monitored using an inductively coupled radio frequency reader device (Figure 5c,d). The developed hybrid nanosensor was demonstrated to have a low detection limit (100 CFU/mL), battery-free operation, and a remote wireless sensing capability.
The authors of [273] developed a smart bandage for uric acid (UA) detection. UA level in wound exudate is highly correlated with wound severity and indicates the bacterial infection of it (a significant decrease because of catabolysis by microbial uricase). The device is based on a urease/prussian blue amperometric screen-printed biosensor and a custom-designed wearable potentiostat, which allowed for on-demand wireless data transfer to a computer/smartphone using radio frequency identification or near-field communication (Figure 6). The authors demonstrated high sensitivity, selectivity, operational stability, and mechanical robustness for the developed sensor. The use of the smart bandage allows for optimizing the process of wound healing and reducing a patient’s stress and pain.
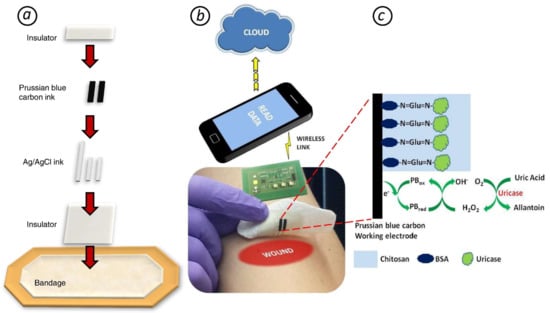
Figure 6.
(a) Screen printing the smart bandage. (b) Wearable potentiostat determines uric acid (UA) concentration and wirelessly communicates with a computer or smartphone. (c) The principle of UA amperometric detection (BSA - Bovine serum albumin) [273].
Prospects for the development of wearable and written sensors are extensive, and the scope of their potential application is enormous. Miniature and wireless sensors allow for constant monitoring of a patient’s health both at the bedside and far beyond the hospital. At the same time, due to modern built-in data exchange systems, a doctor’s recommendations can be received at a distance. Thus, the development of wearable and written sensors is an important step towards personalized medicine.
However, some problems exist that should be solved before the sensors of this kind find application in practice. They are the low reliability of such modern devices and the dependence of measurement results on the efficiency of biological fluids transport (for example, sweat) and analytes to the surface of the sensor. The authors also point out problems with the selectivity of the analysis and the possibility of the sensors to be re-used [274,275]. Data interpretation as a rule is ambiguous. It is necessary to establish if a correlation between measurement parameters that are characteristic for the skin and blood is observed.
4.3. Powering of Sensors
Processes occurring in the immediate environment, directly on the body of the carrier, can serve as the source of energy for a sensor. A strategy for supplying power to portable electronics from the energy of human body movement using a triboelectric nanogenerator (TENG) has been proposed [276,277,278]. Devices operating from solar panel, vibratory, radio frequency, electromagnetic, thermal, chemical-to-electric, and biofuel cells are described. However, the complexity of the power suppliers’ designs and the need to take into account a large number of factors (the location of the device, the degree of physical activity of the user, the duration of daylight, etc.) to ensure the reliable operation of the sensor, especially in the monitoring mode, does not yet allow them to be widely implemented. Today, flexible and stretchable batteries are also used as a source of energy [279,280,281]. Such batteries should be thin, but at the same time, they should have a sufficient charge capacity. Technologies of manufacturing thin-layer printed batteries make it possible to successfully solve these problems [282,283]. For example, the authors in [284] developed a stretchable lithium ion battery on a base of thin, low-modulus silicone elastomers segmented with metal electrodes and electrolyte layers (Figure 7). Self-similar serpentine interconnecting geometries allowed the authors to achieve extremely high stretchability while maintaining capacity densities of 1.1 mAh/cm2.
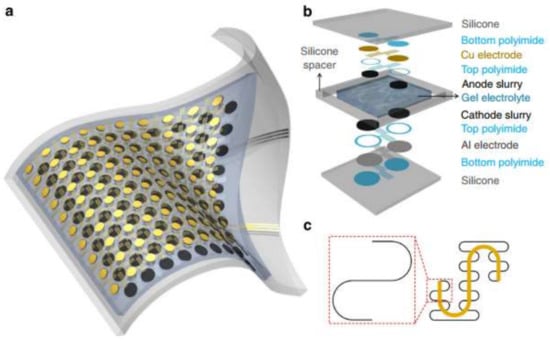
Figure 7.
Battery layout and design. (a) Scheme of a battery’s construction; (b) Illustration of the various layers in the battery’s structure; (c) ‘Self-similar’ serpentine geometries scheme (black: 1st level serpentine; yellow: 2nd level serpentine) [284].
In the last few years, researchers have been interested in self-powered sensors based on the biofuel cells principle [285]. The transformation of biocatalytic reaction energy into electrical energy makes it possible to create miniature, portable, and energetically autonomous sensors. Enzyme, organelle, and microbial biofuel cells and mediated electron transfer can be realized (see Figure 8).
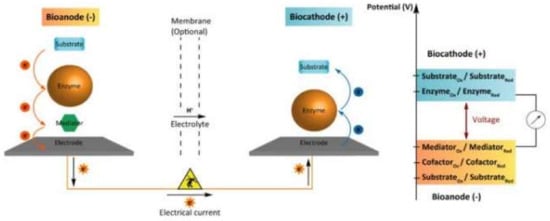
Figure 8.
Schematic of an enzymatic biofuel cell involving a mediated bioanode and a direct electron transfer-based biocatode [285].
The mediator transfers electrons from the enzyme to the anode. At the cathode, a direct electron transfer from the electrode to the enzyme occurs. Small molecules or redox polymers are used as mediators of electron transfer. For example, a self-powered biosensor system for the determination of cholesterol is described in [286]. At the anode, enzymatic oxidation of cholesterol occurs. The hydrogen peroxide formed during the reaction is catalytically reduced on the cathode modified with Prussian blue.
One sensitive element of organelle-based biofuel sensors are cellular organelles: membranes, mitochondria, etc. The principles of forming the response of organelle-based biofuel sensors are similar to those for enzymatic biofuel cells. However, due to the habitual microcellular environment, organelle-based sensors are more stable but significantly inferior to enzymatic sensors for selectivity. In addition, organelles are extremely sensitive to toxins. In [287], a sensor for cyanide detection based on the toxic effect of organelles on the mitochondria of protozoa microorganisms is described. A similar effect was used in [288] for the development of sensors sensitive to herbicides. The main component of the receptor layer in this case was thylakoid membranes of plants.
Microbial biofuel sensors use microbial fuel cells (MFCs) as a receptor layer. MFCs perform the function of the biocatalyst of substrate oxidation and carry out the transfer of electrons to the anode by intracellular mechanisms. Then, electrons move to the cathode along the external circuit, which generates power [289]. There are turn-on and turn-off microbial biofuel sensors. Thus, in the pioneering work [290] Clostridium butyricum are used in the design of a turn-on self-powered biosensor for the monitoring of biological oxygen demand. The inhibitory effect of organic toxicants on microorganisms underlies turn-off microbial biofuel sensors [291]. Such self-powered biosensors have been published in [292,293,294,295,296]. For example, the authors of [297] have developed a single-chamber MFC that could be operated in flow-mode for bacteriological wastewater analysis.
The main problem of self-powered biosensors is the low stability of the enzymes. The use of synthetic receptors will improve the analytical characteristics and extend the shelf life of these sensors.
5. Applications of Sensors
Biosensors are developed for a range of applications. Nevertheless, only some of them have found real practical application today [298]. Examples are “Contour” for glucose determination, “OneTouch” for glucose and cholesterol determination [299,300], test strips sensitive to hCG [301], a lactose biosensor [302,303], and portable systems for enzyme immunoassay and polymerase chain reaction analysis [304,305,306,307]. Despite a large number of publications, the potential field of application for biosensors is huge, and many existing developments are far from where we should expect them to be, usually due to their low reliability and the complexity of their design. In our opinion, sensors with synthetic receptors (MIPs, aptamers, biomimetics) have great prospects for commercial implementation due to their stability and sensitivity.
5.1. Medical Diagnostics
Medical diagnostics, of course, is the main field of practical application for sensors. Simplicity of use, short response time, and high sensitivity and selectivity make them indispensable for onsite and in situ application, for example, in the home clinic. Special attention is paid to the diagnostics of such socially significant diseases as diabetes, cancer, and infectious and cardiovascular diseases. A continuous search for new biomarkers and the development of methods for their detection is in progress.
Oncological diseases occur in a healthy organism suddenly and develop very intensively, so early diagnosis in this case plays a key role. The level of oncomarkers in a patient’s blood at the initial stages of the disease does not exceed a few pM, so not many methods and sensors allow for their detection. Aptasensors have been successfully applied in this field. For example, a voltammetric sensor [308] based on a methylene blue-labeled aptamer for determination of the cancer marker vascular endothelial growth factor (VEGF) has been described. The receptor layer is immobilized on the surface of a gold-plated screen-printed electrode due to thiol-disulfide interaction. The detection limit is 190 ng/L. An aptasensor [309] sensitive to platelet-derived growth factor BB (PDGF-BB) has been developed. The authors have used the strategy of amplifying the response of hydroxyapatite nanoparticles (HAP-NPs), which were a support for the deposition of the aptamer. Because of the aptamer’s immobilization on the electrode surface, a redox-active molybdophosphate precipitate was formed. The developed amperometric aptasensor is characterized by high sensitivity (Clim = 50 fg/mL) and a wide linear range (0.1 pg/mL–10 ng/mL).
No less important is the detection of cardiomarkers. An aptasensor for the determination of myoglobin has been proposed. A myoglobin-specific aptamer was immobilized on the surface of a printed electrode and modified with graphene oxide and carbon nanotubes, which were used in this device [310]. The sensor provides a low detection limit (34 ng/L) and a linearity range of (1–4000 ng/mL) was observed.
The risk of a cardiovascular disease is directly related to the cholesterol concentration in the blood. An enzyme-free sensor sensitive to cholesterol has been proposed [311]. Nanohybrid silica-chitosan oligosaccharide lactate particles were coated with a curcumin layer. They fluoresce in the presence of cholesterol interacting with it electrostatically. A non-enzymatic method for cholesterol determination based on cobalt chloride has been proposed [312]. The analytical signal is an increase in the oxidation peak of cobalt chloride in an aprotic medium in the presence of cholesterol. The detection limit of the method is 2 μM and its linear range is 25–200 μM. The results obtained are lower than the cholesterol content in venous blood. The difference can be due to the fact that capillary blood was analyzed. This phenomenon should be thoroughly investigated before using the sensor.
Despite the commercial success of classical glucose meters, non-enzymatic sensors for the rapid determination of blood glucose are being actively developed today. A sensor based on three-dimensional porous Cu/Cu2O films decorated with Pd nanoparticles acting as an electrocatalyst was proposed [313]. Its high detection sensitivity (Clim = 1.157 mA cm−2 mM−1) is caused by the porous structure of the modifier and the high catalytic activity of Pd nanoparticles. A catalyst for the non-enzymatic determination of glucose based on copper nanoparticles electrodeposited on the surface of a Ni-based metal-organic framework derivate is described in [314]. The sensor has a low detection limit of 66.67 nM and a wide linear range of 0.20 to 2.72 mM. It is highly stable and suitable for re-use. A poly(m-phenylenediamine)/sliver (PmPD/Ag) composite electrode was used [315] for non-enzymatic glucose detection. An amperometric sensor was formed by the in situ synthesis of Ag particles on the surface of the polymer matrix. The sensor is sensitive (Clim = 7.2 μM), fast (the response time is 3 s), and stable for 30 days.
In the case of bacterial and viral agents diagnostics, more stable and sensitive sensors based on synthetic receptors (aptamers and MIPs) have replaced the traditional biosensors (immunosensors, DNA sensors). An impedimetric sensor for Staphylococcus epidermidis detection has been developed [316]. The selectivity of the sensor is caused by a cell-imprinted polymer based on 3-aminophenylboronic acid. Its linear range is 103–107 CFU/mL. Even better analytical characteristics were achieved in [317], where a very cost-effective, fast, sensitive, and specific electrochemical sensor for the detection of Escherichia coli modified with an imprinted polymer is described. The sensor is based on Ag-ZnO bimetallic nanoparticles and a graphene oxide nanocomposite, which serve as a platform for the imprinting of bacteria. The signal of the K3[Fe(CN)6]/K4[Fe(CN)6] mediator system was detected voltammetrically using a glassy carbon electrode. The detection limit of the sensor is less than 10 CFU/mL and the linear range is 10–105 CFU/mL. A fluorescent aptasensor for the determination of H5N1 influenza virus has been proposed [318]. Ag-SiO2 nanoparticles were used as a metal-enhanced fluorescence sensing platform, and thiazole orange served as a signal-forming label. The duration of analysis did not exceed 30 min, and the detection limit of H5N1 in serum is 3.5 ng/mL.
5.2. Environmental and Food Analysis
Сontaminants detection (pesticides, toxins, antibiotics, heavy metals, etc.) in food and the environment is a very important task. A wide application in environmental and food analysis is found for enzymatic sensors, in which an analyte is an inhibitor of the biocatalytic activity and immunosensors. The use of sensors allows for in situ and onsite determination, eliminating the sample preparation stage. This greatly simplifies and speeds up the analysis. In addition, there is the possibility of a multiplex analysis. Many papers on sensors for environmental and food analysis are published today [319,320,321]. Below are some examples of contaminants determination in environmental objects and food.
An electrochemiluminescent aptasensor for the detection of malachite green and chloramphenicol has been proposed [322]. Malachite green is potential carcinogenic and mutagenic agent, while chloramphenicol causes gray baby syndrome, leukemia, and aplastic anemia. CdS quantum dots and luminol-gold nanoparticles decorated on a homemade screen-printed carbon electrode were used as signal-forming labels. The detection limits for malachite green and chloramphenicol were 0.03 nM and 0.07 nM, respectively. Chen et al. [323] have developed a method for the detection of chloramphenicol and polychlorinated biphenyl-72 based on the displacement of a nanotracer (an aptamer-labeled magnetic dendritic probe) by the analytes. Thiolated aptamers were assembled on Fe3O4@Au nanoparticles which were used for concentrating the analyte. At the second stage, nanotracers having in their structure hybridized complementary strands (cDNA1 or cDNA2) and quantum dots (CdS or PbS) were formed. Next, a hybridization reaction between aptamers and cDNAs produces composite magnetic probes. Once the analytes are recognized, the nanotracers are released and detected simultaneously by voltammetry of the metal ions Cd(II) and Pb(II).
At present, many sensors for the determination of mycotoxins in food and environmental objects have been developed. Thus, nanostructured polymer membranes synthesized in situ have become the basis of a fluorescent sensor for the selective determination of aflatoxin B1 [324]. The detection limit of the sensor is 14 ng/mL and its linear range is 20–160 ng/mL. A label-free impedimetric aptasensor for ochratoxin A detection based on immobilized thiol-modified aptamers on Ag nanoparticles, which were decorated on the gold surface, is proposed in [325]. The analytical signal is detected in the presence of a ferricyanide redox mediator. The sensor is tested on model solutions and real beer samples. The detection limit of the sensor is 0.02 nM.
6. Trends and Prospects
Bio- and chemical sensors play an essential role in the implementation of innovative and integrated techniques for the monitoring of health, applications in food analysis and quality control, and environmental applications, for example, the assessment and monitoring of the quality of water resources, as an alternative to the classical chemical analytical methods. Vikas, Anjum, and C S Pundir in the review published in 2007 [326] wrote: “Biosensors offer considerable promises for attaining analytic information in a faster, simpler, and cheaper manner compared to conventional assays. The biosensing approach is rapidly advancing, and applications ranging from metabolite, biological/chemical warfare agent, food pathogens, and adulterant detection to genetic screening and programmed drug delivery have been demonstrated. Innovative efforts, coupling micromachining and nanofabrication may lead to even more powerful devices that would accelerate the realization of large-scale and routine screening. With gradual increase in commercialization, a wide range of new biosensors are thus expected to reach the market in the coming years”. Their prediction turned out to be correct.
A great majority of the biosensors described earlier and used today are based on an application of enzymes as the recognition component. Their advantage is the high selectivity. Their disadvantages are instability, cost, and the need in some cases to use them in combination with a mediator system. These disadvantages are why the tendency to get rid of enzymes can be observed. Researchers are focused on the search for and synthesis of compounds that exhibit the properties of receptors (sensing elements of sensors), catalysts, and compounds that provide selectivity of detection and signal-forming labels. Nanostructures, organic and inorganic compounds containing transition metals, electrochemically active compounds, synthetic analogues of enzymes, and MIPs are used as such materials as an alternative to enzymes. Moreover, magnetic materials are used as carriers accelerating the delivery of a signal-forming complex to the electrode surface. Replacement of enzymes with alternative materials makes it possible to create a fundamentally new generation of non-enzymatic biological and chemical sensors that are not inferior to the classical ones in terms of sensitivity, selectivity, and accuracy of determination of target analytes, but that are much more stable during storage, are commercially available, and are versatile in use.
A significant role in the development of sensorics is played by new approaches to the design of sensors, such as a lab-on-a-chip, printed (written), and wearable sensors. Using the principles of fuel cells can reduce the energy consumption of measuring systems and provide the possibility to create miniature versions of them. The electrochemical method of detection remains the preferred one. The results of these studies will serve as a basis for creating devices that are needed not only in laboratory clinical trials, but also in point-of-care diagnostics, the home clinic, and telemedicine.
Acknowledgments
The study was supported by a grant from the RSF (Russian Science Foundation, Grant #17-13-01096).
Authors Contribution
A.N.K. wrote Section 1 and Section 3 and edited the text of the paper. T.S.S. wrote part of Section 4 and edited the text. N.N.M. wrote part of Section 2 and edited the text. A.V.O. wrote part of Section 4 and and edited the text. M.B.V. wrote part of Section 2. Idea of the paper and its general plan were proposed by K.Z.B. She wrote Sections 1, 5, “Trends and prospects” part and edited the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ariño, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z. Film stripping voltammetry. Talanta 1971, 18, 513–539. [Google Scholar] [CrossRef]
- Brainina, K.Z. Inverse voltammetry (Stripping analysis) in the investigation of biologically important compounds. J. Electroanal. Chem. Interfacial Electrochem. 1981, 128, 479–485. [Google Scholar] [CrossRef]
- Nascimento, V.B.; Angnes, L. Screen-printed electrodes. Química Nova 1998, 21, 614–629. [Google Scholar] [CrossRef]
- Mir, M.; Dondapati, S.K.; Duarte, M.V.; Chatzichristidi, M.; Misiakos, K.; Petrou, P.; Kakabakos, S.E.; Argitis, P.; Katakis, I. Electrochemical biosensor microarray functionalized by means of biomolecule friendly photolithography. Biosens. Bioelectron. 2010, 25, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z. Sensors and sample preparation in stripping voltammetry. Anal. Chim. Acta 1995, 305, 146–153. [Google Scholar] [CrossRef]
- Stojko, N.Y.; Brainina, K.Z.; Faller, C.; Henze, G. Stripping voltammetric determination of mercury at modified solid electrodes: I. Development of the modified electrodes. Anal. Chim. Acta 1998, 371, 145–153. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Wang, X.; Han, X.; Liu, S.; Zhao, C. Electrochemical synthesis of gold nanostructure modified electrode and its development in electrochemical DNA biosensor. Biosens. Bioelectron. 2011, 30, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Skotadis, E.; Voutyras, K.; Chatzipetrou, M.; Tsekenis, G.; Patsiouras, L.; Madianos, L.; Chatzandroulis, S.; Zergioti, I.; Tsoukalas, D. Label-free DNA biosensor based on resistance change of platinum nanoparticles assemblies. Biosens. Bioelectron. 2016, 81, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Alshehri, N.; Rahman, A.M.A.; Dasouki, M.; Abu-Salah, K.M.; Zourob, M. Electrochemical immunosensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens. Bioelectron. 2018, 101, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Sanayeei, M.; Hemmati, S. Label-free electrochemical DNA biosensor for guanine and adenine by ds-DNA/poly(L-cysteine)/Fe3O4 nanoparticles-graphene oxide nanocomposite modified electrode. Biosens. Bioelectron. 2018, 102, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Tsigara, A.; Benkhial, A.; Warren, S.; Akkari, F.; Wright, J.; Frehill, F.; Dempsey, E. Metal microelectrode nanostructuring using nanosphere lithography and photolithography with optimization of the fabrication process. Thin Solid Films 2013, 537, 269–274. [Google Scholar] [CrossRef]
- Rauf, S.; Shiddiky, M.J.A.; Trau, M. “Drill and fill” lithography for controlled fabrication of 3D platinum electrodes. Sens. Actuators B Chem. 2013, 185, 543–547. [Google Scholar] [CrossRef]
- Chu, Z.; Peng, J.; Jin, W. Advanced nanomaterial inks for screen-printed chemical sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
- Trojanowicz, M. Impact of nanotechnology on design of advanced screen-printed electrodes for different analytical applications. TrAC Trends Anal. Chem. 2016, 84, 22–47. [Google Scholar] [CrossRef]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
- Almeida, E.S.; Silva, L.A.J.; Sousa, R.M.F.; Richter, E.M.; Foster, C.W.; Banks, C.E.; Munoz, R.A.A. Organic-resistant screen-printed graphitic electrodes: Application to on-site monitoring of liquid fuels. Anal. Chim. Acta 2016, 934, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prodromidis, M.I.; Karayannis, M.I. Enzyme Based Amperometric Biosensors for Food Analysis. Electroanalysis 2002, 14, 241–261. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezi–Renault, N. Amperometric enzyme biosensors: Past, present and future. ITBM-RBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Chaubey, A.; Malhotra, B.D. Mediated biosensors. Biosens. Bioelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z. Biosensing environmental pollution. Curr. Opin. Biotechnol. 2007, 18, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Du, D.; Zhang, X.; Ju, H. Trends in cell-based electrochemical biosensors. Curr. Med. Chem. 2008, 15, 3160–3170. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, G.; Qin, L.; Xu, Y.; Li, Y.; Li, R. Cell-based biosensors and its application in biomedicine. Sens. Actuators B Chem. 2005, 108, 576–584. [Google Scholar] [CrossRef]
- Hu, L.; Zou, L.; Qin, Z.; Fang, J.; Huang, L.; Wang, P. A novel label-free bioengineered cell-based biosensor for salicin detection. Sens. Actuators B Chem. 2017, 238, 1151–1158. [Google Scholar] [CrossRef]
- May, K.M.L.; Wang, Y.; Bachas, L.G.; Anderson, K.W. Development of a whole-cell-based biosensor for detecting histamine as a model toxin. Anal. Chem. 2004, 76, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, R.; Neufeld, T.; Biran, D.; Ron, E.Z.; Rishpon, J.; Shacham-Diamand, Y. Novel integrated electrochemical nano-biochip for toxicity detection in water. Nano Lett. 2005, 5, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Teles, F.R.R.; Fonseca, L.P. Trends in DNA biosensors. Talanta 2008, 77, 606–623. [Google Scholar] [CrossRef]
- Harris, L.J.; Larson, S.B.; Hasel, K.W.; McPherson, A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry 1997, 36, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D. Using Antibodies: A Laboratory Manual; CSHL Press: New York, NY, USA, 1999; ISBN 978-0-87969-544-6. [Google Scholar]
- Hong, K.Y.; de Albuquerque, C.D.L.; Poppi, R.J.; Brolo, A.G. Determination of aqueous antibiotic solutions using SERS nanogratings. Anal. Chim. Acta 2017, 982, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Marx, Í.M.G.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Assessment of table olives’ organoleptic defect intensities based on the potentiometric fingerprint recorded by an eectronic tongue. Food Bioprocess. Technol. 2017, 10, 1310–1323. [Google Scholar] [CrossRef]
- Patil, A.; Saha, D.; Ganguly, S. A Quantum biomimetic electronic nose sensor. Sci. Rep. 2018, 8, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, H.; Gao, J.; Liu, X.; Gao, X.; Lu, X.; Fang, G.; Wang, J.; Li, J. Upconversion particle@Fe3O4@molecularly imprinted polymer with controllable shell thickness as high-performance fluorescent probe for sensing quinolones. Talanta 2018, 181, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Song, X.; Yu, H.; Hu, C.; Liu, M.; Cai, J.; Zhao, L.; Chen, Y.; Yang, P. Supramolecular recognition of A-tracts DNA by calix[4]carbazole. Sens. Actuators B Chem. 2018, 259, 177–182. [Google Scholar] [CrossRef]
- Athar, M.; Lone, M.Y.; Jha, P.C. Recognition of anions using urea and thiourea substituted calixarenes: A density functional theory study of non-covalent interactions. Chem. Phys. 2018, 501, 68–77. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Golmohammadi, H.; Abbasi-Moayed, S.; Nejad, M.A.F.; Fahimi-Kashani, N.; Jafarinejad, S.; Shahrajabian, M.; Hormozi-Nezhad, M.R. Nanoparticle-based optical sensor arrays. Nanoscale 2017, 9, 16546–16563. [Google Scholar] [CrossRef] [PubMed]
- Slepchenko, G.B.; Gindullina, T.M.; Deryabina, V.I.; Akeneev, Y.A.; Otmakhov, V.I. Voltammetric Determination of Organic Ecotoxicants on Modified Electrodes. Procedia Chem. 2015, 15, 350–354. [Google Scholar] [CrossRef][Green Version]
- Filippov, A.P.; Strizhak, P.E.; Il’in, V.G. Quartz crystal microbalance modified with Cu(II) stearate and octadecylamine co-ordination chemical compounds for detection of volatile organic compounds. Sens. Actuators B Chem. 2007, 126, 375–381. [Google Scholar] [CrossRef]
- Kumar, B.; Feller, J.-F.; Castro, M.; Lu, J. Conductive bio-Polymer nano-Composites (CPC): Chitosan-carbon nanotube transducers assembled via spray layer-by-layer for volatile organic compound sensing. Talanta 2010, 81, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Casuso, I.; Pla-Roca, M.; Gomila, G.; Samitier, J.; Minic, J.; Persuy, M.A.; Salesse, R.; Pajot-Augy, E. Immobilization of olfactory receptors onto gold electrodes for electrical biosensor. Mater. Sci. Eng. C 2008, 28, 686–691. [Google Scholar] [CrossRef]
- Du, L.; Zou, L.; Wang, Q.; Zhao, L.; Huang, L.; Wang, P.; Wu, C. A novel biomimetic olfactory cell-based biosensor with DNA-directed site-specific immobilization of cells on a microelectrode array. Sens. Actuators B Chem. 2015, 217, 186–192. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Z. Multiplexed electrochemical immunoassay of biomarkers using chitosan nanocomposites. Biosens. Bioelectron. 2014, 55, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.S.; Sales, M.G.F. Disposable immunosensor using a simple method for oriented antibody immobilization for label-free real-time detection of an oxidative stress biomarker implicated in cancer diseases. Biosens. Bioelectron. 2014, 53, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Bock, D.; Seeger, S. One-step immobilization of immunoglobulin G and potential of the method for application in immunosensors. Sens. Actuators B Chem. 1995, 28, 143–149. [Google Scholar] [CrossRef]
- Chen, H.; Liu, F.; Qi, F.; Koh, K.; Wang, K. Fabrication of Calix[4]arene Derivative Monolayers to Control Orientation of Antibody Immobilization. Int. J. Mol. Sci. 2014, 15, 5496–5507. [Google Scholar] [CrossRef] [PubMed]
- Esawy, M.A.; Awad, G.E.A.; Wahab, W.A.A.; Elnashar, M.M.M.; El-Diwany, A.; Easa, S.M.H.; El-beih, F.M. Immobilization of halophilic Aspergillus awamori EM66 exochitinase on grafted k-carrageenan-alginate beads. 3 Biotech 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; An, J.; Liu, X.; Wang, H.; Dai, R.; Deng, Y. New drug screening model using enzymes immobilized on mesoporous materials: A proof-of-concept study using immobilized α-glucosidase and acarbose. J. Nanosci. Nanotechnol. 2016, 16, 12460–12469. [Google Scholar] [CrossRef]
- López-Gallego, F.; Jackson, E.; Betancor, L. Heterogeneous systems biocatalysis: The path to the fabrication of self-sufficient artificial metabolic cells. Chem. Eur. J. 2017, 23, 17841–17849. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Sharma, A.L.; Kim, K.-H.; Deep, A. Antibody conjugated glycine doped polyaniline nanofilms as efficient biosensor for atrazine. Mater. Res. Express 2017, 4, 125022. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Tu, F.; Yao, C. Ultrasensitive enzyme-free electrochemical immunoassay for free thyroxine based on three dimensionally ordered macroporous chitosan–Au nanoparticles hybrid film. Biosens. Bioelectron. 2014, 59, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microbial Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Rusling, J.F.; Dixit, C.K. Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development. Methods 2017, 116, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G. Biosensors: Essentials, 1st ed.; Lecture Notes in Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-40241-8. [Google Scholar]
- Dutta, P.; Sawoo, S.; Ray, N.; Bouloussa, O.; Sarkar, A. Engineering bioactive surfaces with Fischer carbene complex: Protein a on self-assembled monolayer for antibody sensing. Bioconjugate Chem. 2011, 22, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Adumeau, P.; Sharma, S.K.; Brent, C.; Zeglis, B.M. Site-Specifically Labeled Immunoconjugates for Molecular Imaging—Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol. 2016, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wollenberger, U.; Schubert, F.; Pfeiffer, D.; Scheller, F.W. Enhancing biosensor performance using multienzyme systems. Trends Biotechnol. 1993, 11, 255–262. [Google Scholar] [CrossRef]
- Drijvers, J.M.; Awan, I.M.; Perugino, C.A.; Rosenberg, I.M.; Pillai, S. Chapter 7—The Enzyme-Linked Immunosorbent Assay: The application of ELISA in clinical research. In Basic Science Methods for Clinical Researchers; Jalali, M., Saldanha, F.Y.L., Jalali, M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 119–133. ISBN 978-0-12-803077-6. [Google Scholar]
- ElAfandy, R.T.; AbuElela, A.F.; Mishra, P.; Janjua, B.; Oubei, H.M.; Büttner, U.; Majid, M.A.; Ng, T.K.; Merzaban, J.S.; Ooi, B.S. Nanomembrane-based, thermal-transport biosensor for living cells. Small 2017, 13, 1603080. [Google Scholar] [CrossRef] [PubMed]
- Khaw, M.K.; Mohd-Yasin, F.; Nguyen, N.T. Microcalorimeter: Design considerations, materials and examples. Microelectron. Eng. 2016, 158, 107–117. [Google Scholar] [CrossRef]
- Davaji, B.; Lee, C.H. A paper-based calorimetric microfluidics platform for bio-chemical sensing. Biosens. Bioelectron. 2014, 59, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Ramanathan, K.; Danielsson, B. Mini/micro thermal biosensors and other related devices for biochemical/clinical analysis and monitoring. TrAC Trends Anal. Chim. 2000, 19, 340–349. [Google Scholar] [CrossRef]
- Nestorova, G.G.; Adapa, B.S.; Kopparthy, V.L.; Guilbeau, E.J. Lab-on-a-chip thermoelectric DNA biosensor for label-free detection of nucleic acid sequences. Sens. Actuators B Chem. 2016, 225, 174–180. [Google Scholar] [CrossRef]
- Lu, J.; Wu, L.; Hu, Y.; Wang, S.; Guo, Z. Faraday cage-type electrochemiluminescence biosensor based on multi-functionalized graphene oxide for ultrasensitive detection of microRNA-21. J. Electrochem. Soc. 2017, 164, B421–B426. [Google Scholar] [CrossRef]
- Gruhl, F.J.; Länge, K. Surface modification of an acoustic biosensor allowing the detection of low concentrations of cancer markers. Anal. Biochem. 2012, 420, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Skládal, P. Piezoelectric biosensors. TrAC Trends Anal. Chim. 2015, 79. [Google Scholar] [CrossRef]
- Karaseva, N.; Ermolaeva, T.; Mizaikoff, B. Piezoelectric sensors using molecularly imprinted nanospheres for the detection of antibiotics. Sens. Actuators B Chem. 2016, 225, 199–208. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chim. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, R.; Chen, P.R. Genetically encoded fluorescent sensors for measuring transition and heavy metals in biological systems. Curr. Opin. Chem. Biol. 2017, 43, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Wei, T.-B.; Li, W.-T.; Qu, W.-J.; Leng, Y.-L.; Zhang, J.-H.; Lin, Q.; Zhang, Y.-M.; Yao, H. Phenazine-based colorimetric and fluorescent sensor for the selective detection of cyanides based on supramolecular self-assembly in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Quenching-recovery fluorescent biosensor for DNA detection based on mercaptopropionic acid-capped cadmium telluride quantum dots aggregation. Sens. Actuators B Chem. 2017, 249, 149–155. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Sarswat, P.K.; Free, M.L. Quantum dots and carbon dots based fluorescent sensors for TB biomarkers detection. Vacuum 2017, 146, 606–613. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Li, B.; Li, D.; Zhang, S.; Wu, Y. Differentiation of proteins and cancer cells using metal oxide and metal nanoparticles-quantum dots sensor array. Sens. Actuators B Chem. 2017, 250, 69–75. [Google Scholar] [CrossRef]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene quantum dots prepared from glucose as optical sensor for glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Ozhukil Valappil, M.K.; Pillai, V.; Alwarappan, S. Spotlighting graphene quantum dots and beyond: Synthesis, properties and sensing applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Wang, E. Chapter 20a Optical sensors. In Comprehensive Analytical Chemistry; Modern Instrumental Analysis; Ahuja, S., Jespersen, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 47, pp. 755–775. [Google Scholar]
- Marose, S.; Lindemann, C.; Ulber, R.; Scheper, T. Optical sensor systems for bioprocess monitoring. Trends Biotechnol. 1999, 17, 30–34. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Based Sensors, 1st ed.; Springer Series on Chemical Sensors and Biosensors; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; Volume 4, ISBN 978-3-540-33918-2. [Google Scholar]
- Michel, D.; Xiao, F.; Alameh, K. A compact, flexible fiber-optic Surface Plasmon Resonance sensor with changeable sensor chips. Sens. Actuators B Chem. 2017, 246, 258–261. [Google Scholar] [CrossRef]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Rithesh Raj, D.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, Y.; Bai, J.; Zhang, X.; Cui, Y.; Ning, B.; Cui, J.; Gao, Z. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B Chem. 2018, 254, 629–635. [Google Scholar] [CrossRef]
- Song, C.; Yang, B.; Zhu, Y.; Yang, Y.; Wang, L. Ultrasensitive sliver nanorods array SERS sensor for mercury ions. Biosens. Bioelectron. 2017, 87, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 2013, 63, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Genova, E.; Pelin, M.; Decorti, G.; Stocco, G.; Sergo, V.; Ventura, A.; Bonifacio, A. SERS of cells: What can we learn from cell lysates? Anal. Chim. Acta 2018, 1005, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kurzątkowska, K.; Santiago, T.; Hepel, M. Plasmonic nanocarrier grid-enhanced Raman sensor for studies of anticancer drug delivery. Biosens. Bioelectron. 2017, 91, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N.; Shchyogolev, S.; Goryacheva, I.; Rusanova, T.; Smirnova, T.; Zhelobitskaya, E.; Brainina, K.; Stozhko, N.; Bukharinova, M.; et al. Nanoanalytics: Nanoobjects and Nanotechnologies in Analytical Chemistry; Shtykov, S., Ed.; De Gruyter: Berlin, Germany, 2018; ISBN 978-3-11-054006-2. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley-VCH: Hoboken, NJ, USA, 2006; ISBN 978-0-471-67879-3. [Google Scholar]
- Warsinke, A.; Benkert, A.; Scheller, F.W. Electrochemical immunoassays. Fresenius J. Anal. Chem. 2000, 366, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sens. Actuators B Chem. 2018, 257, 570–578. [Google Scholar] [CrossRef]
- Liu, X.; Lillehoj, P.B. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens. Bioelectron. 2017, 98, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.G.; Silva, M.S.V.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3). Sens. Actuators B Chem. 2018, 256, 905–912. [Google Scholar] [CrossRef]
- Sinha, A.; Lu, X.; Wu, L.; Tan, D.; Li, Y.; Chen, J.; Jain, R. Voltammetric sensing of biomolecules at carbon based electrode interfaces: A review. TrAC Trends Anal. Chem. 2018, 98, 174–189. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Voltammetric aptasensors for protein disease biomarkers detection: A review. Biotechnol. Adv. 2016, 34, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, Y.; Siangproh, W.; Khampieng, T.; Chailapakul, O.; Apilux, A. Paper-based amperometric sensor for determination of acetylcholinesterase using screen-printed graphene electrode. Talanta 2018, 178, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, M.-A.; Craciunescu, I.; Gligor, D. Amperometric sensor based on HEMA hydrogels modified with Toluidine Blue for nitrite detection in water samples. Mater. Chim. Phys. 2017, 200, 233–240. [Google Scholar] [CrossRef]
- Singh, S.; Kaushal, A.; Khare, S.; Kumar, A. DNA chip based sensor for amperometric detection of infectious pathogens. Int. J. Biol. Macromol. 2017, 103, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ribet, F.; Stemme, G.; Roxhed, N. Ultra-miniaturization of a planar amperometric sensor targeting continuous intradermal glucose monitoring. Biosens. Bioelectron. 2017, 90, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Mettakoonpitak, J.; Miller-Lionberg, D.; Reilly, T.; Volckens, J.; Henry, C.S. Low-cost reusable sensor for cobalt and nickel detection in aerosols using adsorptive cathodic square-wave stripping voltammetry. J. Electroanal. Chim. 2017, 805, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.B.; Brahim, M.B.; Abdelhédi, R.; Samet, Y. Boron doped diamond sensor for sensitive determination of metronidazole: Mechanistic and analytical study by cyclic voltammetry and square wave voltammetry. Mater. Sci. Eng. C 2016, 59, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Terbouche, A.; Ait-Ramdane-Terbouche, C.; Djebbar, S.; Benali-Baitich, O.; Hauchard, D. Effectiveness study of sensor based on modified cavity microelectrode by Algerian humic acid–polyaniline composites using square wave voltammetry. Sens. Actuators B Chem. 2012, 169, 297–304. [Google Scholar] [CrossRef]
- Guerreiro, G.V.; Zaitouna, A.J.; Lai, R.Y. Characterization of an electrochemical mercury sensor using alternating current, cyclic, square wave and differential pulse voltammetry. Anal. Chim. Acta 2014, 810, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Rahman, M.M.; Siddiquey, I.A.; Asiri, A.M.; Hasnat, M.A. Efficient hydroquinone sensor based on zinc, strontium and nickel based ternary metal oxide (TMO) composites by differential pulse voltammetry. Sens. Actuators B Chem. 2018, 256, 383–392. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z. Organic reagents in inverse voltammetry: A review. Z. Anal. Chem. 1982, 312, 428–437. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Topcu, C.; Caglar, B.; Onder, A.; Coldur, F.; Caglar, S.; Guner, E.K.; Cubuk, O.; Tabak, A. Structural characterization of chitosan-smectite nanocomposite and its application in the development of a novel potentiometric monohydrogen phosphate-selective sensor. Mater. Res. Bull. 2018, 98, 288–299. [Google Scholar] [CrossRef]
- Sun, L.; Sun, C.; Sun, X. Screening highly selective ionophores for heavy metal ion-selective electrodes and potentiometric sensors. Electrochimica Acta 2016, 220, 690–698. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, K.H.; Bae, N.H.; Sim, G.S.; Oh, Y.-J.; Lee, S.J.; Lee, T.J.; Lee, K.G.; Choi, B.G. Fabrication of newspaper-based potentiometric platforms for flexible and disposable ion sensors. J. Colloid Interface Sci. 2017, 508, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC Trends Anal. Chim. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Joseph, M.B.; Colburn, A.; Mollart, T.P.; Palmer, N.; Newton, M.E.; Macpherson, J.V. A synthetic diamond conductivity sensor: Design rules and applications. Sens. Actuators B Chem. 2017, 238, 1128–1135. [Google Scholar] [CrossRef]
- Grysiński, T.; Moroń, Z. Planar sensors for local conductivity measurements in biological objects—Design, modelling, sensitivity maps. Sens. Actuators B Chem. 2011, 158, 190–198. [Google Scholar] [CrossRef]
- Anshori, I.; Suzuki, H. Microfluidic device for high-sensitivity coulometric detection of proteins. Sens. Actuators B Chem. 2018, 256, 835–838. [Google Scholar] [CrossRef]
- Van der Schoot, B.; Bergveld, P. Coulometric sensors, the application of a sensor-actuator system for long-term stability in chemical sensing. Sens. Actuators 1988, 13, 251–262. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Gao, Q.; Qi, H.; Zhang, C. Ultrasensitive DNA detection based on coulometric measurement of enzymatic silver deposition on gold nanoparticle-modified screen-printed carbon electrode. Sens. Actuators B Chem. 2012, 162, 384–390. [Google Scholar] [CrossRef]
- Wang, J.; Suzuki, H.; Satake, T. Coulometric microdevice for organophosphate pesticide detection. Sens. Actuators B Chem. 2014, 204, 297–301. [Google Scholar] [CrossRef]
- Ali, M.A.; Jiang, H.; Mahal, N.K.; Weber, R.J.; Kumar, R.; Castellano, M.J.; Dong, L. Microfluidic impedimetric sensor for soil nitrate detection using graphene oxide and conductive nanofibers enabled sensing interface. Sens. Actuators B Chem. 2017, 239, 1289–1299. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of printed sensors for taste sensing applications. Sens. Actuators A Phys. 2018, 269, 53–61. [Google Scholar] [CrossRef]
- Aicher, M.; Grothe, H.; Wolf, B. A novel thin film impedance Ca ion sensor for drinking water. Sens. Actuators B Chem. 2017, 244, 1103–1112. [Google Scholar] [CrossRef]
- Guan, J.-G.; Miao, Y.-Q.; Zhang, Q.-J. Impedimetric biosensors. J. Biosci. Bioeng. 2004, 97, 219–226. [Google Scholar] [CrossRef]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.-S.; Hahn, Y.-B. Recent advances in nanowires-based field-effect transistors for biological sensor applications. Biosens. Bioelectron. 2018, 100, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-U.-J.; Ahmad, R.; Hahn, Y.-B. Nonenzymatic flexible field-effect transistor based glucose sensor fabricated using NiO quantum dots modified ZnO nanorods. J. Colloid Interface Sci. 2018, 512, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.L.; Nguyen, T.T.; Huyen Tran, T.T.; Chu, V.T.; Thinh Tran, Q.; Tuan Mai, A. Detection of influenza A virus using carbon nanotubes field effect transistor based DNA sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 93, 83–86. [Google Scholar] [CrossRef]
- Adzhri, R.; Md Arshad, M.K.; Gopinath, S.C.B.; Ruslinda, A.R.; Fathil, M.F.M.; Ayub, R.M.; Nor, M.N.M.; Voon, C.H. High-performance integrated field-effect transistor-based sensors. Anal. Chim. Acta 2016, 917, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nehra, A.; Pal Singh, K. Current trends in nanomaterial embedded field effect transistor-based biosensor. Biosens. Bioelectron. 2015, 74, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Higson, S. Biosensors for Medical Applications; WOODHEAD PUB: Sawston, Cambridge, UK, 2018; ISBN 978-0-08-101638-1. [Google Scholar]
- Ahmed, M.U.; Hossain, M.M.; Tamiya, E. Electrochemical Biosensors for Medical and Food Applications. Electroanalysis 2008, 20, 616–626. [Google Scholar] [CrossRef]
- Motherwell, W.B.; Bingham, M.J.; Six, Y. Recent progress in the design and synthesis of artificial enzymes. Tetrahedron 2001, 57, 4663–4686. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P.W.N.M. Supramolecular catalysis. Part 2: Artificial enzyme mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Berggren, G.; Adamska, A.; Lambertz, C.; Simmons, T.R.; Esselborn, J.; Atta, M.; Gambarelli, S.; Mouesca, J.M.; Reijerse, E.; Lubitz, W.; et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 2013, 499, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Bao, Z.; Su, Y.; Xing, H.; Yang, Q.; Ren, Q. Functionalized metal–organic framework as a biomimetic heterogeneous catalyst for transfer hydrogenation of imines. ACS Appl. Mater. Interfaces 2017, 9, 9772–9777. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Periñán, E.; Gennari, M.; Revenga-Parra, M.; Abad, J.M.; Mateo-Martí, E.; Pariente, F.; Castillo, O.; Mas-Ballesté, R.; Zamora, F.; Lorenzo, E. Highly dense nickel hydroxide nanoparticles catalyst electrodeposited from a novel Ni(II) paddle-wheel complex. J. Catal. 2015, 329, 22–31. [Google Scholar] [CrossRef]
- Kozitsina, A.N.; Shalygina, Z.V.; Dedeneva, S.S.; Rusinov, G.L.; Tolshchina, S.G.; Verbitskiy, E.V.; Brainina, K.Z. Catalytic systems based on the organic nickel (II) complexes in chronoamperometric determination of urea and creatinine. Russ. Chem. Bull. 2009, 58, 1119–1125. [Google Scholar] [CrossRef]
- Kozitsina, A.; Dedeneva, S.S.; Shalygina, Z.V.; Okhokhonin, A.; Chizhov, D.; Matern, A.I.; Brainina, K. Determination of urea and creatinine by chronoamperometry. J. Anal. Chim. 2014, 69, 758–762. [Google Scholar] [CrossRef]
- Ferrer, S.J.; Granados, S.G.; Bedioui, F.; Ordaz, A.A. Amperometric Detection of Urea in Aqueous Solution by Poly(Ni-cyclam) Film-Modified Glassy Carbon Electrode. Electroanalysis 2003, 15, 70–73. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Desimoni, E.; Ricciardi, G.; Lelj, F. Study of the nickel-based chemically modified electrode obtained by electrochemical deposition of an NiII-tetramethyl-dibenzo-tetraaza [14] annulene complex. Redox catalysis of carbohydrates in alkaline solutions. II. Electroanalysis 1995, 7, 435–441. [Google Scholar] [CrossRef]
- Manriquez, J.; Bravo, J.L.; Gutierrez-Granados, S.; Sucar Succar, S.; Bied-Charreton, C.; Alatorre Ordaz, A.; Bedioui, F. Electrocatalysis of the oxidation of alcohol and phenol derivative pollutants at vitreous carbon electrode coated by nickel macrocyclic complex-based films. Anal. Chim. Acta 1999, 378, 159–168. [Google Scholar] [CrossRef]
- Elahi, M.Y.; Heli, H.; Bathaie, S.Z.; Mousavi, M.F. Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode. J. Solid State Electrochem. 2007, 11, 273–282. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chim. Interfacial Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Subat, M.; Borovik, A.S.; König, B. Synthetic creatinine receptor: Imprinting of a Lewis acidic zinc(II)cyclen binding site to shape its molecular recognition selectivity. J. Am. Chem. Soc. 2004, 126, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, P.; Saraswathi, R. Enhanced electrochemical growth and redox characteristics of poly(o-phenylenediamine) on a carbon nanotube modified glassy carbon electrode and its application in the electrocatalytic reduction of oxygen. J. Phys. Chem. C 2007, 111, 11320–11328. [Google Scholar] [CrossRef]
- Yang, Y.J.; Guo, L.; Zhang, W. The electropolymerization of CTAB on glassy carbon electrode for simultaneous determination of dopamine, uric acid, tryptophan and theophylline. J. Electroanal. Chim. 2016, 768, 102–109. [Google Scholar] [CrossRef]
- Okhokhonin, A.V.; Saraeva, S.Y.; Matern, A.I.; Kozitsina, A.N. Enzymeless determination of cholesterol using gold and silver nanoparticles as electrocatalysts. J. Anal. Chem. 2017, 72, 354–361. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Fernandes, E.G.R.; Zucolotto, V. A peroxidase biomimetic system based on Fe3O4 nanoparticles in non-enzymatic sensors. Talanta 2015, 141, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fekry, A.M. A new simple electrochemical Moxifloxacin Hydrochloride sensor built on carbon paste modified with silver nanoparticles. Biosens. Bioelectron. 2017, 87, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Musameh, M.; Lin, Y. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Tsai, T.-H.; Chen, S.-M. Performing enzyme-free H2O2 biosensor and simultaneous determination for AA, DA, and UA by MWCNT-PEDOT film. Biosens. Bioelectron. 2010, 26, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. One-step electrochemical synthesis of PtNi nanoparticle-graphene nanocomposites for nonenzymatic amperometric glucose detection. ACS Appl. Mater. Interfaces 2011, 3, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, X.; Wen, Y.; Li, C.; Xiong, Q.; Chen, P. A graphene-cobalt oxide based needle electrode for non-enzymatic glucose detection in micro-droplets. Chem. Commun. (Camb.) 2012, 48, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B. Molecularly imprinted polymers—Potential and challenges in analytical chemistry. Anal. Chim. Acta 2005, 534, 31–39. [Google Scholar] [CrossRef]
- Sharma, P.S.; Iskierko, Z.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Bioinspired intelligent molecularly imprinted polymers for chemosensing: A mini review. Electrochem. Commun. 2015, 50, 81–87. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Гендриксон, О.Д.; Жердев, А.В.; Дзантиев, Б.Б. Молекулярно импринтированные полимеры и их применение в биохимическом анализе. Успехи биологической химии 2006, 46, 149–192. [Google Scholar]
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. (Eds.) Molecularly Imprinted Sensors: Overview and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-56331-6. [Google Scholar]
- Madikizela, L.M.; Tavengwa, N.T.; Tutu, H.; Chimuka, L. Green aspects in molecular imprinting technology: From design to environmental applications. Trends Environ. Anal. Chim. 2018, 17, 14–22. [Google Scholar] [CrossRef]
- Niu, P.; Gich, M.; Fernández-Sánchez, C.; Roig, A. Sol–Gel Nanocomposites for Electrochemical Sensor Applications. In The Sol-Gel Handbook; Levy, D., Zayat, R., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2015; pp. 1413–1434. ISBN 978-3-527-67081-9. [Google Scholar]
- Xu, L.; Shen, Y.; Wang, L.; Ding, Y.; Cai, Z. Preparation of vinyl silica-based organic/inorganic nanocomposites and superhydrophobic polyester surfaces from it. Colloid Polym. Sci. 2015, 293, 2359–2371. [Google Scholar] [CrossRef]
- Zhao, W.; Sheng, N.; Zhu, R.; Wei, F.; Cai, Z.; Zhai, M.; Du, S.; Hu, Q. Preparation of dummy template imprinted polymers at surface of silica microparticles for the selective extraction of trace bisphenol A from water samples. J. Hazard. Mater. 2010, 179, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shi, Y.; Chu, L.; Wang, Y.; Zhang, L.; Liu, J. Preparation and characterization of silica/polypyrrole core-shell colloidal particles in the presence of ethanol as the cosolvent. J. Appl. Polym. Sci. 2012, 123, 3270–3274. [Google Scholar] [CrossRef]
- Feng, X. Synthesis of Ag/Polypyrrole Core-Shell Nanospheres by a Seeding Method. Chin. J. Chem. 2010, 28, 1359–1362. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dadkhah, S.; Baradaran Kayyal, T.; Jabbari, A. Design of a surface-immobilized 4-nitrophenol molecularly imprinted polymer via pre-grafting amino functional materials on magnetic nanoparticles. J. Chromatogr. A 2014, 1364, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Griffete, N.; Lamouri, A.; Felidj, N.; Chehimi, M.M.; Mangeney, C. Nanocomposites of Gold Nanoparticles@Molecularly Imprinted Polymers: Chemistry, Processing, and Applications in Sensors. Chem. Mater. 2015, 27, 5464–5478. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madrakian, T.; Afkhami, A. Molecularly imprinted polymer coated magnetite nanoparticles as an efficient mefenamic acid resonance light scattering nanosensor. Anal. Chim. Acta 2014, 852, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Moghaddam, H.; Toosi, M.; Toosi, M. Synthesis and comparison of new layer-coated silica nanoparticles and bulky molecularly imprinted polymers for the solid-phase extraction of glycine. Anal. Methods 2015, 7, 7488–7495. [Google Scholar] [CrossRef]
- Kitahara, K.; Yoshihama, I.; Hanada, T.; Kokuba, H.; Arai, S. Synthesis of monodispersed molecularly imprinted polymer particles for high-performance liquid chromatographic separation of cholesterol using templating polymerization in porous silica gel bound with cholesterol molecules on its surface. J. Chromatogr. A 2010, 1217, 7249–7254. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.A.; Machunsky, S.; Peuker, U.; Kunz, U.; Turek, T. Magnetite core-shell nano-composites with chlorine functionality: Preparation by miniemulsion polymerization and characterization. J. Polym. Res. 2011, 18, 79–88. [Google Scholar] [CrossRef]
- Hasantabar, V.; Lakouraj, M.M.; Zare, E.N.; Mohseni, M. Innovative magnetic tri-layered nanocomposites based on polyxanthone triazole, polypyrrole and iron oxide: Synthesis, characterization and investigation of the biological activities. RSC Adv. 2015, 5, 70186–70196. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Nien, P.-C.; Hu, C.-W.; Ho, K.-C. Detection of uric acid based on multi-walled carbon nanotubes polymerized with a layer of molecularly imprinted PMAA. Sens. Actuators B Chem. 2010, 146, 466–471. [Google Scholar] [CrossRef]
- Effati, E.; Pourabbas, B. One-pot synthesis of sub-50 nm vinyl- and acrylate-modified silica nanoparticles. Powder Technol. 2012, 219, 276–283. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Bokern, S.; Getze, J.; Agarwal, S.; Greiner, A. Polymer grafted silver and copper nanoparticles with exceptional stability against aggregation by a high yield one-pot synthesis. Polymer 2011, 52, 912–920. [Google Scholar] [CrossRef]
- Meekel, A.A.; Resmini, M.; Pandit, U.K. Regioselectivity and enantioselectivity in an antibody catalyzed hetero Diels-Alder reaction. Bioorg. Med. Chem. 1996, 4, 1051–1057. [Google Scholar] [CrossRef]
- Zhang, H.; Piacham, T.; Drew, M.; Patek, M.; Mosbach, K.; Ye, L. Molecularly Imprinted Nanoreactors for Regioselective Huisgen 1,3-Dipolar Cycloaddition Reaction. J. Am. Chem. Soc. 2006, 128, 4178–4179. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Memar, M.Y.; Moaddab, S.R.; Kafil, H.S. Aptamer-assisted novel technologies for detecting bacterial pathogens. Biomed. Pharmacother. 2017, 93, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Phang, W.-M.; Hashim, U. Aptamer-based ‘point-of-care testing’. Biotechnol. Adv. 2016, 34, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Citartan, M.; Ch’ng, E.-S.; Rozhdestvensky, T.S.; Tang, T.-H. Aptamers as the ‘capturing’ agents in aptamer-based capture assays. MicroChem. J. 2016, 128, 187–197. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.D.; Ali, M.A. Chapter 1—Nanomaterials in Biosensors: Fundamentals and Applications. In Nanomaterials for Biosensors; Micro and Nano Technologies; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–74. ISBN 978-0-323-44923-6. [Google Scholar]
- Morales-Narváez, E.; Naghdi, T.; Zor, E.; Merkoçi, A. Photoluminescent Lateral-Flow Immunoassay Revealed by Graphene Oxide: Highly Sensitive Paper-Based Pathogen Detection. Anal. Chem. 2015, 87, 8573–8577. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Wang, J.; Tang, Z.; Pounds, J.G.; Lin, Y. Rapid and Sensitive Detection of Protein Biomarker Using a Portable Fluorescence Biosensor Based on Quantum Dots and a Lateral Flow Test Strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of toxicity of selenium and cadmium selenium quantum dots: A review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, Y. Surface modifications technology of quantum dots based biosensors and their medical applications. Chin. J. Anal. Chim. 2014, 42, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Ruan, Y.-F.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Quantum-dots-based photoelectrochemical bioanalysis highlighted with recent examples. Biosens. Bioelectron. 2017, 94, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chandan, H.R.; Schiffman, J.D.; Balakrishna, R.G. Quantum dots as fluorescent probes: Synthesis, surface chemistry, energy transfer mechanisms, and applications. Sens. Actuators B Chem. 2018, 258, 1191–1214. [Google Scholar] [CrossRef]
- Akter, R.; Kyun Rhee, C.; Rahman, M.A. A stable and sensitive voltammetric immunosensor based on a new non-enzymatic label. Biosens. Bioelectron. 2013, 50, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jang, J.; Cha, C. Carbon nanomaterials as versatile platforms for theranostic applications. Drug Discov. Today 2017, 22, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chim. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Gopal, P.; Reddy, T.M. Fabrication of carbon-based nanomaterial composite electrochemical sensor for the monitoring of terbutaline in pharmaceutical formulations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 600–609. [Google Scholar] [CrossRef]
- Şenocak, A.; Göl, C.; Basova, T.V.; Demirbaş, E.; Durmuş, M.; Al-Sagur, H.; Kadem, B.; Hassan, A. Preparation of single walled carbon nanotube-pyrene 3D hybrid nanomaterial and its sensor response to ammonia. Sens. Actuators B Chem. 2018, 256, 853–860. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.; Wang, Y.-Q.; Pan, Z.-J. Carbon/graphene composite nanofiber yarns for highly sensitive strain sensors. Mater. Des. 2018, 143, 214–223. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Singhal, C.; Malhotra, N.; Narang, S.; Pn, A.K.; Gupta, R.; Kansal, R.; Pundir, C.S. Impedimetric genosensor for ultratrace detection of hepatitis B virus DNA in patient samples assisted by zeolites and MWCNT nano-composites. Biosens. Bioelectron. 2016, 86, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Malekzad, H.; Zangabad, P.S.; Mohammadi, H.; Sadroddini, M.; Jafari, Z.; Mahlooji, N.; Abbaspour, S.; Gholami, S.; Ghanbarpoor, M.; Pashazadeh, R.; et al. Noble metal nanostructures in optical biosensors: Basics, and their introduction to anti-doping detection. TrAC Trends Anal. Chim. 2018. [Google Scholar] [CrossRef]
- Ma, X.-M.; Sun, M.; Lin, Y.; Liu, Y.-J.; Luo, F.; Guo, L.-H.; Qiu, B.; Lin, Z.-Y.; Chen, G.-N. Progress of visual biosensor based on gold nanoparticles. Chin. J. Anal. Chim. 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, H.J. Electrochemical sandwich-type biosensors for α-1 antitrypsin with carbon nanotubes and alkaline phosphatase labeled antibody-silver nanoparticles. Biosens. Bioelectron. 2017, 89, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, K.; Han, H. Ultrasensitive electrochemical detection of Bacillus thuringiensis transgenic sequence based on in situ Ag nanoparticles aggregates induced by biotin-streptavidin system. Biosens. Bioelectron. 2011, 28, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. Orientational nanoparticle assemblies and biosensors. Biosens. Bioelectron. 2016, 79, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes ‘Meet’ Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends Anal. Chim. 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Leng, C.; Lai, G.; Yan, F.; Ju, H. Gold nanoparticle as an electrochemical label for inherently crosstalk-free multiplexed immunoassay on a disposable chip. Anal. Chim. Acta 2010, 666, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Dai, H.; Pan, X.; Liu, S. Simultaneous detection of dual proteins using quantum dots coated silica nanoparticles as labels. Biosens. Bioelectron. 2011, 28, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, X.; Yu, J.; Wu, Y.; Cheng, S.; Xing, Y.; Liu, L. Design of electrochemical biosensors with peptide probes as the receptors of targets and the inducers of gold nanoparticles assembly on electrode surface. Sens. Actuators B Chem. 2017, 239, 834–840. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Boujakhrout, A.; Díez, P.; Sánchez, A.; Martínez-Ruíz, P.; Pingarrón, J.M.; Villalonga, R. Gold nanoparticles-decorated silver-bipyridine nanobelts for the construction of mediatorless hydrogen peroxide biosensor. J. Colloid Interface Sci. 2016, 482, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Chinnasamy, T.; Veerappan, S.; Senthilkumar, K.; Kannaiyan, D. Dual labeled Ag@SiO2 core–shell nanoparticle based optical immunosensor for sensitive detection of E. coli. Mater. Sci. Eng. C 2014, 45, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rao, V.K.; Kamboj, D.V.; Gaur, R.; Upadhyay, S.; Shaik, M. Relative efficiency of zinc sulfide (ZnS) quantum dots (QDs) based electrochemical and fluorescence immunoassay for the detection of Staphylococcal enterotoxin B (SEB). Biotechnol. Rep. 2015, 6, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zaibudeen, A.W.; Philip, J. Magnetic nanofluid based non-enzymatic sensor for urea detection. Sens. Actuators B Chem. 2018, 255, 720–728. [Google Scholar] [CrossRef]
- De la Rosa-Romo, L.M.; Oropeza-Guzmán, M.T.; Olivas-Sarabia, A.; Pina-Luis, G. Flavone functionalized magnetic nanoparticles: A new fluorescent sensor for Cu2+ ions with nanomolar detection limit. Sens. Actuators B Chem. 2016, 233, 459–468. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chim. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N.; Mokhtarzadeh, A.; Bageri, L.; Sadeghi, S.; Mahboob, S. Graphene quantum dots decorated with magnetic nanoparticles: Synthesis, electrodeposition, characterization and application as an electrochemical sensor towards determination of some amino acids at physiological pH. Mater. Sci. Eng. C 2016, 68, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Amidi, S.; Madrakian, T. Graphene nanoribbon/FePt bimetallic nanoparticles/uric acid as a novel magnetic sensing layer of screen printed electrode for sensitive determination of ampyra. Talanta 2018, 176, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Tajyani, S.; Babaei, A. A new sensing platform based on magnetic Fe3O4@NiO core/shell nanoparticles modified carbon paste electrode for simultaneous voltammetric determination of Quercetin and Tryptophan. J. Electroanal. Chim. 2018, 808, 50–58. [Google Scholar] [CrossRef]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Aysan, A.B.; Knejzlík, Z.; Ulbrich, P.; Šoltys, M.; Zadražil, A.; Štěpánek, F. Effect of surface functionalisation on the interaction of iron oxide nanoparticles with polymerase chain reaction. Colloids Surf. B Biointerfaces 2017, 153, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Nair, R.S.; Ameer, J.M.; Alison, M.R.; Anilkumar, T.V. A gold nanoparticle coated porcine cholecyst-derived bioscaffold for cardiac tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, H.; Liu, M.; Deng, F.; Huang, H.; Wan, Q.; Li, Z.; Wang, K.; He, X.; Zhang, X.; et al. A bioinspired strategy for surface modification of silica nanoparticles. Appl. Surf. Sci. 2015, 357, 1996–2003. [Google Scholar] [CrossRef]
- Kozitsina, A.N.; Malysheva, N.N.; Verbitsky, E.V.; Utepova, I.A.; Glazyrina, Y.A.; Mitrofanova, T.S.; Rusinov, G.L.; Matern, A.I.; Chupakhin, O.N.; Brainina, K.Z. Synthesis and research of electrochemical behavior of magnetic nanocomposites based on Fe3O4. Russ. Chem. Bull. 2013, 62, 2327–2336. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xu, D.; Ho, S.-L.; Wang, K.; Wong, M.S.; Li, H.-W. Magnetically controlled immunosensor for highly sensitive detection of carcinoembryonic antigen based on an efficient “turn-on” cyanine fluorophore. Sens. Actuators B Chem. 2018, 258, 133–140. [Google Scholar] [CrossRef]
- Kozitsina, A.; Svalova, T.; Malysheva, N.; Glazyrina, Y.; Matern, A. A New enzyme-free electrochemical immunoassay for Escherichia coli detection using magnetic nanoparticles. Anal. Lett. 2016, 49, 245–257. [Google Scholar] [CrossRef]
- Kozitsina, A.; Svalova, T.; Malysheva, N.; Glazyrina, Y.; Matern, A.; Rusinov, V. Determination of Staphylococcus aureus B-1266 by an enzyme-free electrochemical immunosensor incorporating magnetite nanoparticles. Anal. Lett. 2017, 50, 924–935. [Google Scholar] [CrossRef]
- Malysheva, N.N.; Glazyrina, Y.A.; Zhdanovskikh, V.O.; Svalova, T.S.; Matern, A.I.; Kozitsina, A.N. Nonenzymatic electrochemical method for determination of the measles virus antigen using the synthesized IgG-(Fe3O4-SiO2) conjugate as the signal label. Russ. Chem. Bull. 2014, 63, 1633–1638. [Google Scholar] [CrossRef]
- Kozitsina, A.N.; Malysheva, N.N.; Utepova, I.A.; Glazyrina, Y.A.; Matern, A.I.; Brainina, K.Z.; Chupakhin, O.N. An enzyme-free electrochemical method for the determination of E. coli using Fe3O4 nanocomposites with a SiO2 shell modified by ferrocene. J. Anal. Chem. 2015, 70, 540–545. [Google Scholar] [CrossRef]
- Ríos, Á.; Zougagh, M.; Avila, M. Miniaturization through lab-on-a-chip: Utopia or reality for routine laboratories? A review. Anal. Chim. Acta 2012, 740, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luong, J.H.T. Chapter 15—Lab-on-a-Chip (LOC) Immunoassays. In Handbook of Immunoassay Technologies; Academic Press: Cambridge, MA, USA, 2018; pp. 415–431. ISBN 978-0-12-811762-0. [Google Scholar]
- Hernández-Neuta, I.; Pereiro, I.; Ahlford, A.; Ferraro, D.; Zhang, Q.; Viovy, J.-L.; Descroix, S.; Nilsson, M. Microfluidic magnetic fluidized bed for DNA analysis in continuous flow mode. Biosens. Bioelectron. 2018, 102, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yeo, L. Electrokinetically Driven Microfluidics and Nanofluidics; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-86025-3. [Google Scholar]
- Zhang, Y.; Jiang, H.-R. A review on continuous-flow microfluidic PCR in droplets: Advances, challenges and future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Chudy, M.; Tokarska, K.; Jastrzębska, E.; Bułka, M.; Drozdek, S.; Lamch, Ł.; Wilk, K.A.; Brzózka, Z. Lab-on-a-chip systems for photodynamic therapy investigations. Biosens. Bioelectron. 2018, 101, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, E.; Flis, S.; Rakowska, A.; Chudy, M.; Jastrzebski, Z.; Dybko, A.; Brzozka, Z. A microfluidic system to study the cytotoxic effect of drugs: The combined effect of celecoxib and 5-fluorouracil on normal and cancer cells. Microchim. Acta 2013, 180, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2018, 99, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-plastic hybrid microfluidic device for smartphone-based colorimetric analysis of urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An overview of the development of flexible sensors. Adv. Mater. Weinheim 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Camacho, E.; Abraham, M.E.; Bruce, S.S.; Dumont, E.L.; Zacharia, B.E.; D’Amico, R.; Slomian, J.; Reginster, J.Y.; Bruyère, O.; Connolly, E.S. Smart wearable body sensors for patient self-assessment and monitoring. Arch. Public Health 2014, 72, 28. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Long, Y. Wearable Chemosensors: A Review of Recent Progress. Chim. Open 2017, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present challenges and future prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Libanori, R.; Erb, R.M.; Reiser, A.; Le Ferrand, H.; Süess, M.J.; Spolenak, R.; Studart, A.R. Stretchable heterogeneous composites with extreme mechanical gradients. Nat. Commun. 2012, 3, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Song, J.; Choi, W.M.; Kim, H.-S.; Kim, R.-H.; Liu, Z.; Huang, Y.Y.; Hwang, K.-C.; Zhang, Y.; Rogers, J.A. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl. Acad. Sci. USA 2008, 105, 18675–18680. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Yeo, W.-H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.-Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pharr, M.; Wang, L.; Kim, J.; Liu, Y.; Xue, Y.; Ning, R.; Wang, X.; Chung, H.U.; Feng, X.; et al. Soft Elastomers with ionic liquid-filled cavities as strain isolating substrates for wearable electronics. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Xue, Y.; Luan, H.; Pharr, M.; Feng, X.; Rogers, J.A.; Huang, Y. Collapse of liquid-overfilled strain-isolation substrates in wearable electronics. Int. J. Solids Struct. 2017, 117, 137–142. [Google Scholar] [CrossRef]
- Lee, C.H.; Ma, Y.; Jang, K.-I.; Banks, A.; Pan, T.; Feng, X.; Kim, J.S.; Kang, D.; Raj, M.S.; McGrane, B.L.; et al. Soft core/shell packages for stretchable electronics. Adv. Funct. Mater. 2015, 25, 3698–3704. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Ultrasensitive and Selective Organic FET-type Nonenzymatic Dopamine Sensor Based on Platinum Nanoparticles-Decorated Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 39526–39533. [Google Scholar] [CrossRef] [PubMed]
- Munje, R.D.; Muthukumar, S.; Panneer Selvam, A.; Prasad, S. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Sci. Rep. 2015, 5, 14586. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, J.; Ying, Y.; Liu, Y. Writing sensors on solid agricultural products for in situ detection. Anal. Chem. 2015, 87, 10703–10707. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.-L.; Han, J.-W.; Moon, D.-I.; Yoon, K.J.; Hwang, C.S.; Meyyappan, M. All-printed triboelectric nanogenerator. Nano Energy 2018, 44, 82–88. [Google Scholar] [CrossRef]
- Xing, F.; Jie, Y.; Cao, X.; Li, T.; Wang, N. Natural triboelectric nanogenerator based on soles for harvesting low-frequency walking energy. Nano Energy 2017, 42, 138–142. [Google Scholar] [CrossRef]
- Zhang, R.; Örtegren, J.; Hummelgård, M.; Olsen, M.; Andersson, H.; Olin, H. Harvesting triboelectricity from the human body using non-electrode triboelectric nanogenerators. Nano Energy 2018, 45, 298–303. [Google Scholar] [CrossRef]
- Kammoun, M.; Berg, S.; Ardebili, H. Stretchable spiral thin-film battery capable of out-of-plane deformation. J. Power Sources 2016, 332, 406–412. [Google Scholar] [CrossRef]
- Pang, S.; Gao, Y.; Choi, S. Flexible and stretchable microbial fuel cells with modified conductive and hydrophilic textile. Biosens. Bioelectron. 2018, 100, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Kim, J.; Wan An, B.; Park, J.; Ji, S.; Kim, S.-Y.; Jang, J.; Lee, Y.; Park, Y.-G.; Cho, E.; et al. Stretchable electronic devices using graphene and its hybrid nanostructures. FlatChem 2017, 3, 71–91. [Google Scholar] [CrossRef]
- Madej, E.; Espig, M.; Baumann, R.R.; Schuhmann, W.; La Mantia, F. Optimization of primary printed batteries based on Zn/MnO2. J. Power Sources 2014, 261, 356–362. [Google Scholar] [CrossRef]
- Huebner, G.; Krebs, M. 18—Printed, flexible thin-film-batteries and other power storage devices. In Handbook of Flexible Organic Electronics; Logothetidis, S., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 429–447. ISBN 978-1-78242-035-4. [Google Scholar]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Grattieri, M.; Minteer, S.D. Self-powered biosensors. ACS Sens. 2018, 3, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sekretaryova, A.N.; Beni, V.; Eriksson, M.; Karyakin, A.A.; Turner, A.P.F.; Vagin, M.Y. Cholesterol self-powered biosensor. Anal. Chem. 2014, 86, 9540–9547. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based mitochondrial electrochemical biosensor for pesticide detection. Electroanalysis 2016, 28, 854–859. [Google Scholar] [CrossRef]
- Rasmussen, M.; Minteer, S.D. Self-powered herbicide biosensor utilizing thylakoid membranes. Anal. Methods 2013, 5, 1140–1144. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, I.S.; Gil, G.C.; Park, H.S.; Kim, H.J. Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol. Lett. 2003, 25, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Karube, I.; Matsunaga, T.; Mitsuda, S.; Suzuki, S. Microbial electrode BOD sensors. Biotechnol. Bioeng. 1977, 19, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Thomson, A.R.; Schneider, K.; Cameron, P.J.; Ieropoulos, I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens. Bioelectron. 2014, 62, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Dharmalingam, S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta 2014, 818, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chouler, J.; Bentley, I.; Vaz, F.; O’Fee, A.; Cameron, P.J.; Di Lorenzo, M. Exploring the use of cost-effective membrane materials for Microbial Fuel Cell based sensors. Electrochimica Acta 2017, 231, 319–326. [Google Scholar] [CrossRef]
- Roustazadeh Sheikhyousefi, P.; Nasr Esfahany, M.; Colombo, A.; Franzetti, A.; Trasatti, S.P.; Cristiani, P. Investigation of different configurations of microbial fuel cells for the treatment of oilfield produced water. Appl. Energy 2017, 192, 457–465. [Google Scholar] [CrossRef]
- Kim, M.; Sik Hyun, M.; Gadd, G.M.; Joo Kim, H. A novel biomonitoring system using microbial fuel cells. J. Environ. Monit. 2007, 9, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Curtis, T.P.; Head, I.M.; Scott, K. A single-chamber microbial fuel cell as a biosensor for wastewaters. Water Res. 2009, 43, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Wallace, J.F.; Pardo, S.; Parkes, J.L. Performance of the CONTOUR® TS Blood Glucose Monitoring System. J. Diabetes Sci. Technol. 2011, 5, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.R.; Hashim, U.; Gopinath, S.C.B.; Poopalan, P.; Ramayya, H.R.; Iqbal Omar, M.; Haarindraprasad, R.; Veeradasan, P. A Point-of-care immunosensor for human chorionic gonadotropin in clinical urine samples using a cuneated polysilicon nanogap lab-on-chip. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lactose Sensor. Available online: http://www.directsens.com/lactose-sensor/ (accessed on 31 January 2018).
- Rajendran, V.; Irudayaraj, J. Detection of glucose, galactose, and lactose in milk with a microdialysis-coupled flow injection amperometric sensor. J. Dairy Sci. 2002, 85, 1357–1361. [Google Scholar] [CrossRef]
- Zhdanov, A.; Keefe, J.; Franco-Waite, L.; Konnaiyan, K.R.; Pyayt, A. Mobile phone based ELISA (MELISA). Biosens. Bioelectron. 2018, 103, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Morioka, K.; Shimojima, M.; Van An, L.; Nakajima, H.; Hemmi, A.; Uchiyama, K.; Loong, S.K.; AbuBakar, S.; Yang, M.; et al. A Handy field-portable ELISA system for rapid onsite diagnosis of infectious diseases. Jpn. J. Infect. Dis. 2016, 69, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Nan, H.; Lee, M.-J.; Kang, S.H. Fast on-site diagnosis of influenza A virus by Palm PCR and portable capillary electrophoresis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 963, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.V.; Cho, S.; Harshman, D.K.; Song, J.-Y.; Yoon, J.-Y. A portable, shock-proof, surface-heated droplet PCR system for Escherichia coli detection. Biosens. Bioelectron. 2015, 74, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, W.; Lai, R.Y. A folding-based electrochemical aptasensor for detection of vascular endothelial growth factor in human whole blood. Biosens. Bioelectron. 2011, 26, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tian, D.; Zhang, L.; Guo, Q.; Cui, Y.; Yang, M. Dual signal amplification strategy for amperometric aptasensing using hydroxyapatite nanoparticles. Application to the sensitive detection of the cancer biomarker platelet-derived growth factor BB. Microchim. Acta 2017, 184, 4375–4381. [Google Scholar] [CrossRef]
- Kumar, V.; Shorie, M.; Ganguli, A.K.; Sabherwal, P. Graphene-CNT nanohybrid aptasensor for label free detection of cardiac biomarker myoglobin. Biosens. Bioelectron. 2015, 72, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Chebl, M.; Moussa, Z.; Peurla, M.; Patra, D. Polyelectrolyte mediated nano hybrid particle as a nano-sensor with outstandingly amplified specificity and sensitivity for enzyme free estimation of cholesterol. Talanta 2017, 169, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kozitsina, A.N.; Okhokhonin, A.V.; Matern, A.I. Amperometric detection of cholesterol using cobalt (II) chloride as an electrocatalyst in aprotic media. J. Electroanal. Chim. 2016, 772, 89–95. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Liu, X.; Yuen, M.M.F.; Fu, X.-Z.; Yang, Y.; Sun, R.; Wong, C.-P. 3D porous Cu@Cu2O films supported Pd nanoparticles for glucose electrocatalytic oxidation. Electrochimica Acta 2017, 248, 299–306. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, C.; Li, X.; Ding, Y.; Liang, H.; Zhao, G.; Wang, Y. A CuNi/C Nanosheet Array Based on a Metal-Organic Framework Derivate as a Supersensitive Non-Enzymatic Glucose Sensor. Nano-Micro Lett. 2018, 10, 28. [Google Scholar] [CrossRef]
- Xu, L.; Ma, J.; Zhou, N.; Guo, P.; Wang, G.; Su, C. Well-dispersed poly(m-phenylenediamine)/silver composite for non-enzymatic amperometric glucose sensor applied in a special alkaline environment. Ionics 2017, 1–11. [Google Scholar] [CrossRef]
- Golabi, M.; Kuralay, F.; Jager, E.W.H.; Beni, V.; Turner, A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.; Patra, S.; Tiwari, A.; Madhuri, R.; Sharma, P.K. Single cell imprinting on the surface of Ag-ZnO bimetallic nanoparticle modified graphene oxide sheets for targeted detection, removal and photothermal killing of E. coli. Biosens. Bioelectron. 2017, 89, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Rong, Z.; Wang, J.; Xiao, R.; Wang, S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF). Biosens. Bioelectron. 2015, 66, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, S.H.; Karimabadi, N.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Electrochemical and optical aptamer-based sensors for detection of tetracyclines. Trends Food Sci. Technol. 2018, 73, 45–57. [Google Scholar] [CrossRef]
- Verdian, A. Apta-nanosensors for detection and quantitative determination of acetamiprid—A pesticide residue in food and environment. Talanta 2018, 176, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Hayat, A.; Gáspár, S.; Marty, J.-L. Advantages of carbon nanomaterials in electrochemical aptasensors for food analysis. Electroanalysis 2018, 30, 2–19. [Google Scholar] [CrossRef]
- Feng, X.; Gan, N.; Zhang, H.; Yan, Q.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A novel “dual-potential” electrochemiluminescence aptasensor array using CdS quantum dots and luminol-gold nanoparticles as labels for simultaneous detection of malachite green and chloramphenicol. Biosens. Bioelectron. 2015, 74, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Zhang, H.; Yan, Z.; Li, T.; Chen, Y.; Xu, Q.; Jiang, Q. Electrochemical simultaneous assay of chloramphenicol and PCB72 using magnetic and aptamer-modified quantum dot-encoded dendritic nanotracers for signal amplification. Microchim. Acta 2016, 183, 1099–1106. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Lynnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of Aflatoxin B1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Kutyreva, M.; Gataulina, A.; Ulakhovich, N.; Evtugyn, V.; Hianik, T. Impedimetric Aptasensor for Ochratoxin A Determination Based on Au Nanoparticles Stabilized with Hyper-Branched Polymer. Sensors 2013, 13, 16129–16145. [Google Scholar] [CrossRef] [PubMed]
- Vikas, A.; Pundir, C.S. Biosensors: Future Analytical Tools. Sens. Transducers 2007, 76, 935–944. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).