Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review
Abstract
:1. Introduction
2. Electrochemical Detection Principles
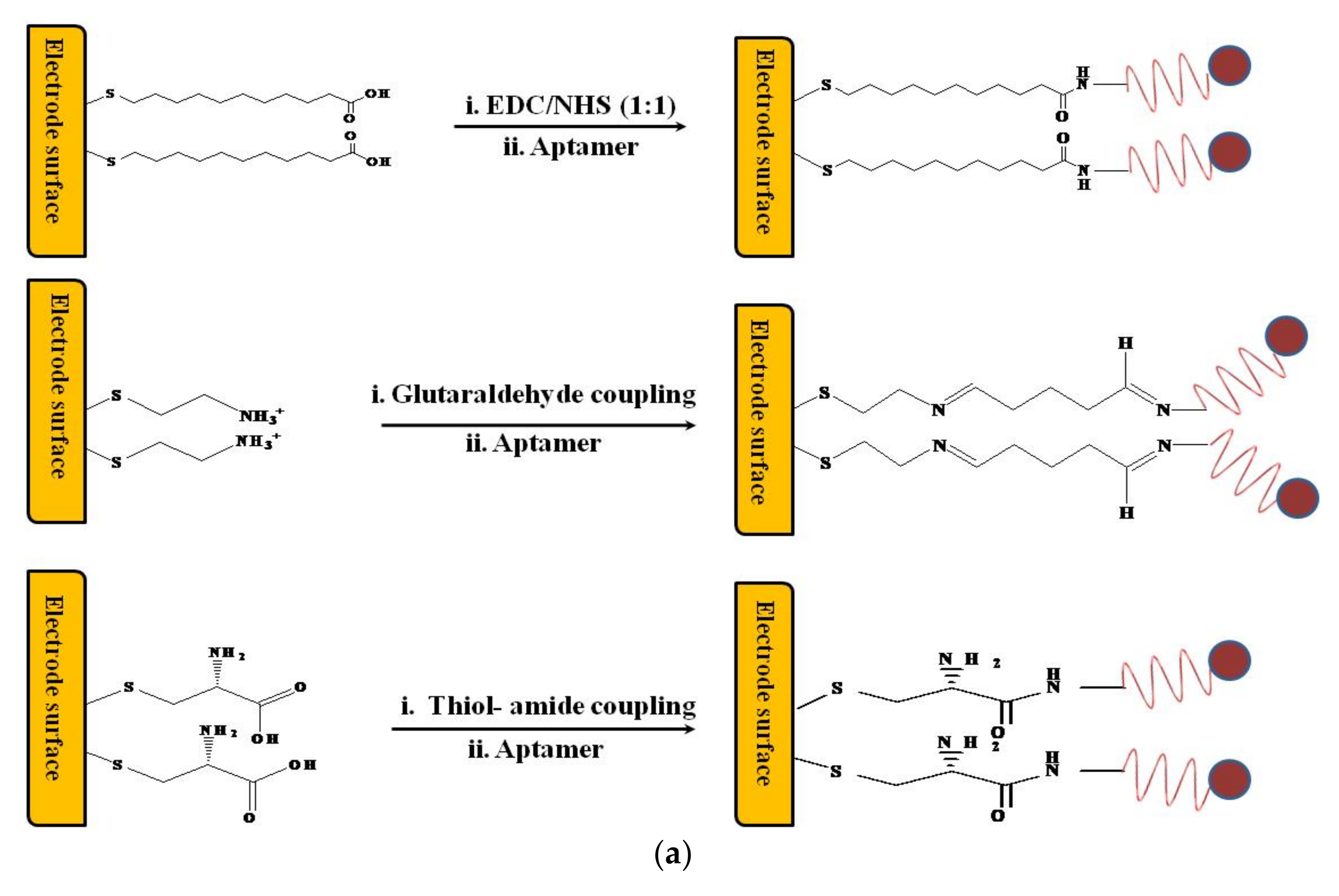
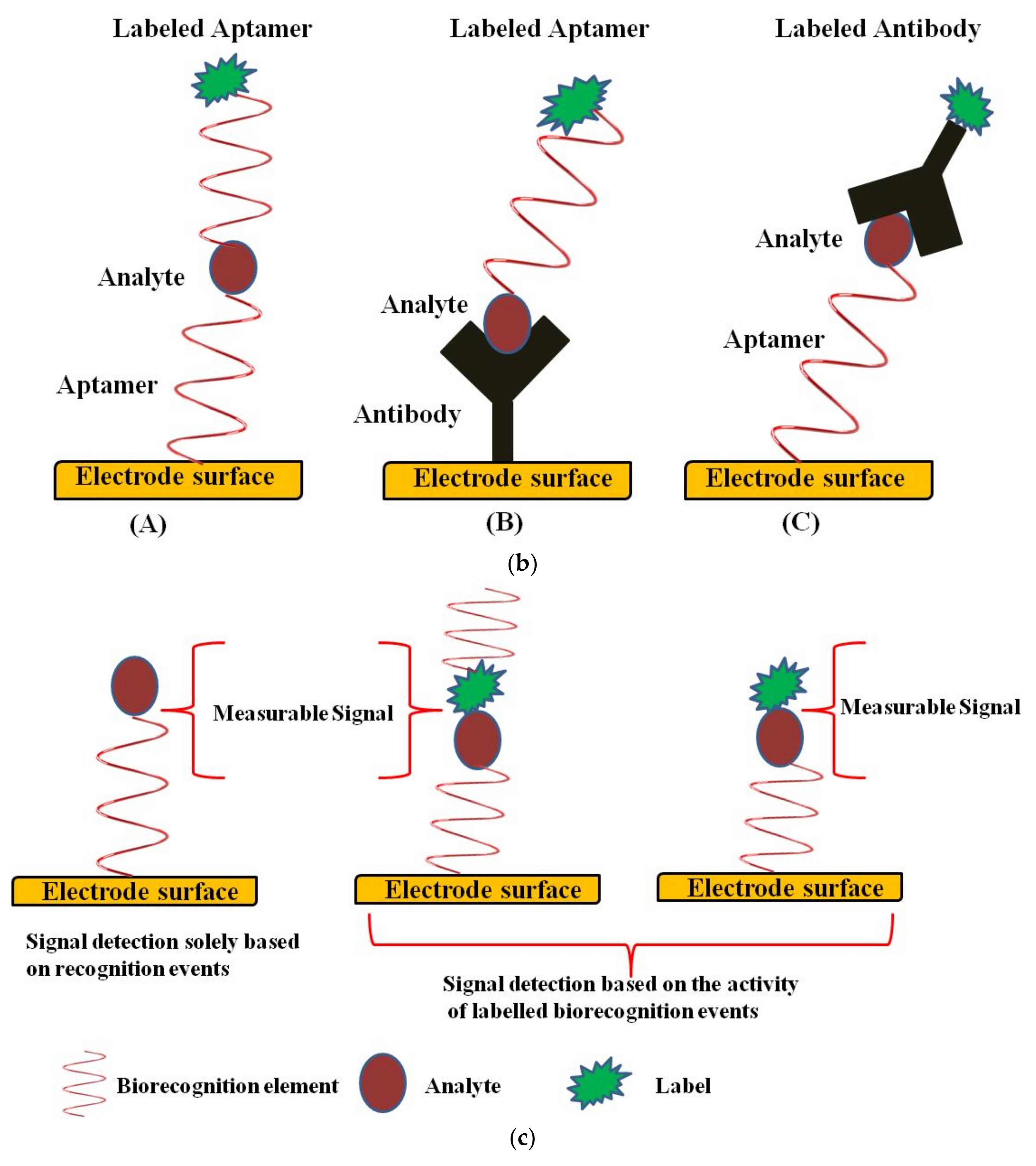
Design of an Electrochemical Aptasensor
3. Electrochemical Aptasensors for Food Safeguarding
3.1. Electrochemical Aptasensors for Microorganisms
3.2. Electrochemical Aptasensors for Food Allergens
3.3. Electrochemical Aptasensors for Fungal Toxins
3.4. Electrochemical Aptasensors for Antibiotics
4. Electrochemical Aptasensors for Environmental Safeguarding
4.1. Electrochemical Aptasensors for Heavy Metals
4.2. Electrochemical Aptasensors for Pesticide Detection
5. Conclusions and Future Perceptions
Acknowledgments
Conflicts of Interest
References
- Tombelli, S.; Minunni, M.; Mascini, M. Aptamers-based assays for diagnostics, environmental and food analysis. Biomol. Eng. 2007, 24, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: Rna ligands to bacteriophage t4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of rna molecules that bind specific ligands. Nature 1990, 346, 818. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Marty, J.-L. Electrochemical aptasensors for the assessment of food quality and safety. TrAC Trends Anal. Chem. 2016, 79, 60–70. [Google Scholar] [CrossRef]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin b1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Gold, L.; Janjic, N.; Jarvis, T.; Schneider, D.; Walker, J.J.; Wilcox, S.K.; Zichi, D. Aptamers and the rna world, past and present. Cold Spring Harb. Perspect. Biol. 2012, 4, a003582. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ragavan, K.; Thakur, M.; Raghavarao, K. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, I.; Mascini, M. Electrochemical nanomaterial-based nucleic acid aptasensors. Anal. Bioanal. Chem. 2012, 402, 3103–3114. [Google Scholar] [CrossRef] [PubMed]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Andreescu, S.; Marty, J.-L. Design of peg-aptamer two piece macromolecules as convenient and integrated sensing platform: Application to the label free detection of small size molecules. Biosens. Bioelectron. 2013, 45, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.L.; Sooter, L.J. Single-stranded DNA aptamers against pathogens and toxins: Identification and biosensing applications. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Morris, M.D.; Macazo, F.C.; Schoukroun-Barnes, L.R.; White, R.J. The current and future role of aptamers in electroanalysis. J. Electrochem. Soc. 2014, 161, H301–H313. [Google Scholar] [CrossRef]
- Hianik, T.; Wang, J. Electrochemical aptasensors–recent achievements and perspectives. Electroanalysis 2009, 21, 1223–1235. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical aptamer-based biosensors: Recent advances and perspectives. Int. J. Electrochem. 2011, 2011. [Google Scholar] [CrossRef]
- Velasco-Garcia, M.; Missailidis, S. New trends in aptamer-based electrochemical biosensors. Gene Ther. Mol. Biol. 2009, 13, 1–10. [Google Scholar]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An aptamer-based electrochemical biosensor for the detection of salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zelada-Guillén, G.A.; Sebastián-Avila, J.L.; Blondeau, P.; Riu, J.; Rius, F.X. Label-free detection of staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosens. Bioelectron. 2012, 31, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Sheikh-Zeinoddin, M.; Ensafi, A.A.; Soleimanian-Zad, S. Fabrication of an electrochemical DNA-based biosensor for bacillus cereus detection in milk and infant formula. Biosens. Bioelectron. 2016, 80, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ligaj, M.; Tichoniuk, M.; Gwiazdowska, D.; Filipiak, M. Electrochemical DNA biosensor for the detection of pathogenic bacteria aeromonas hydrophila. Electrochim. Acta 2014, 128, 67–74. [Google Scholar] [CrossRef]
- Sheikhzadeh, E.; CHamsaz, M.; Turner, A.; Jager, E.; Beni, V. Label-free impedimetric biosensor for salmonella typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016, 80, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, S.J.; Hefle, S.L. Detecting Allergens in Food; Woodhead Publishing Ltd.: Cambridge, UK, 2006; ISBN 9781855737280. [Google Scholar]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; Del Valle, M.; Marty, J.-L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Ocaña, C.; Marty, J.-L. A label free aptasensor for ochratoxin a detection in cocoa beans: An application to chocolate industries. Anal. Chim. Acta 2015, 889, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin m1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Catanante, G.; Mishra, R.K.; Hayat, A.; Marty, J.-L. Sensitive analytical performance of folding based biosensor using methylene blue tagged aptamers. Talanta 2016, 153, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Istamboulie, G.; Marty, J.-L. Sensitive quantitation of ochratoxin a in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem. 2016, 192, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Sharma, A.; Bhand, S. Ultrasensitive detection of streptomycin using flow injection analysis-electrochemical quartz crystal nanobalance (fia-eqcn) biosensor. Biosens. Bioelectron. 2015, 67, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Istamboulie, G.; Hayat, A.; Catanante, G.; Bhand, S.; Marty, J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B Chem. 2017, 245, 507–515. [Google Scholar] [CrossRef]
- Zhou, N.; Luo, J.; Zhang, J.; You, Y.; Tian, Y. A label-free electrochemical aptasensor for the detection of kanamycin in milk. Anal. Methods 2015, 7, 1991–1996. [Google Scholar] [CrossRef]
- Qin, X.; Yin, Y.; Yu, H.; Guo, W.; Pei, M. A novel signal amplification strategy of an electrochemical aptasensor for kanamycin, based on thionine functionalized graphene and hierarchical nanoporous ptcu. Biosens. Bioelectron. 2016, 77, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Hu, G.; Wagberg, T.; Zhan, S.; Xu, H.; Zhou, P. Electrochemical aptasensor for tetracycline using a screen-printed carbon electrode modified with an alginate film containing reduced graphene oxide and magnetite (fe 3 o 4) nanoparticles. Microchim. Acta 2016, 183, 723–729. [Google Scholar] [CrossRef]
- Shen, G.; Guo, Y.; Sun, X.; Wang, X. Electrochemical aptasensor based on prussian blue-chitosan-glutaraldehyde for the sensitive determination of tetracycline. Nano-Micro Lett. 2014, 6, 143–152. [Google Scholar] [CrossRef]
- Zhou, L.; Li, D.-J.; Gai, L.; Wang, J.-P.; Li, Y.-B. Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification. Sens. Actuators B Chem. 2012, 162, 201–208. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.-L.; Sekhon, S.S.; Ahn, J.-Y.; Ko, J.H.; Lee, L.; Cho, S.-J.; Min, J.; Kim, Y.-H. Aptasensor for environmental monitoring. Toxicol. Environ. Health Sci. 2017, 9, 89–101. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zhang, C.; Huang, D.; Lai, C.; Tang, L.; Zhou, Y.; Xu, P.; Wang, H.; Qin, L.; Cheng, M. Practical and regenerable electrochemical aptasensor based on nanoporous gold and thymine-hg2+-thymine base pairs for hg2+ detection. Biosens. Bioelectron. 2017, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Navaee, A.; Salimi, A.; Ahmadi, R. Zeptomolar detection of hg2+ based on label-free electrochemical aptasensor: One step closer to the dream of single atom detection. Electrochem. Commun. 2017, 78, 21–25. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Label-free signal-on aptasensor for sensitive electrochemical detection of arsenite. Biosens. Bioelectron. 2016, 79, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, X.; Gao, J.; Xue, S.; Zhao, J. Label-free and enzyme-free strategy for sensitive electrochemical lead aptasensor by using metal-organic frameworks loaded with agpt nanoparticles as signal probes and electrocatalytic enhancers. Electrochim. Acta 2017, 251, 25–31. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, L.; Wei, G.B.; He, L.L.; Chen, C.D.; kong De, R.; Peng, H.; Fan, H. Reagentless, electrochemical aptasensor for lead (ii) detection. J. New Mater. Electrochem. Syst. 2017, 20, 001–005. [Google Scholar]
- Jiao, Y.; Jia, H.; Guo, Y.; Zhang, H.; Wang, Z.; Sun, X.; Zhao, J. An ultrasensitive aptasensor for chlorpyrifos based on ordered mesoporous carbon/ferrocene hybrid multiwalled carbon nanotubes. RSC Adv. 2016, 6, 58541–58548. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Zhang, Q.; Zhang, C.; Liu, Y.; Tu, K.; Tu, J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol. Lett. 2012, 34, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Selection and characterization of DNA aptamers for electrochemical biosensing of carbendazim. Anal. Chem. 2017, 89, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A regenerative and selective electrochemical aptasensor based on copper oxide nanoflowers-single walled carbon nanotubes nanocomposite for chlorpyrifos detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. Electrochemical aptamer based assay for the neonicotinoid insecticide acetamiprid based on the use of an unmodified gold electrode. Microchim. Acta 2017, 184, 499–505. [Google Scholar] [CrossRef]
- Jiao, Y.; Hou, W.; Fu, J.; Guo, Y.; Sun, X.; Wang, X.; Zhao, J. A nanostructured electrochemical aptasensor for highly sensitive detection of chlorpyrifos. Sens. Actuators B Chem. 2017, 243, 1164–1170. [Google Scholar] [CrossRef]
| Electrochemical Aptasensors Applied for Food Safeguard | ||||
|---|---|---|---|---|
| Analyte | Matrix | Method details | Linear range and LOD | Reference |
| Salmonella Typhimurium | Pork | EIS based on AuNPs and GO | 2.4–2.4 × 103 CFU/mL, 3 CFU/mL | [17] |
| Staphylococcus aureus | Pig Skin | Potentiometry using SWCNT | 800 CFU/mL | [18] |
| Lysozyme | wine | Aptamer–antibody diazonium coupling, DPV | 5 fM–5 nM, 4.3 fM | [21] |
| Ochratoxin A | cocoa beans | Anti-OTA-aptamer on SPCE, EIS | 0.15–2.5 ng/mL, 0.15 ng/mL | [22] |
| Aflatoxin M1 | Milk | Hexaethyleneglycol modified aptamers on SPE, EIS | 2–150 ng/L, 1.15 ng/mL | [23] |
| Aflatoxin B1 | Alcoholic beverage | MB-tagged aptamer on SPCE, hexamethylenediamine and carbodiimide, DPV | 0.05–6.0 ng/mL, 0.05 ng/mL | [6] |
| Kanamycin | Milk | Aptamer on SPCE, EIS | 1.2–75 ng/mL, 0.11 ng/mL | [25] |
| Electrochemical Aptasensors Applied for Environmental Safeguard | ||||
|---|---|---|---|---|
| Analyte | Matrix | Method details | Linear range and LOD | Reference |
| Hg2+ | Drinking water | Aptamer, thymine-Hg2+-thymine nanoporous gold, DPV | 0.01–5000 nM, 0.0036 nM | [29] |
| Hg2+ | Environmental samples | dsDNA on Au electrode, Fe(CN)63−/4−, DPV | 5 zM–55 pM, 0.6 zM | [30] |
| Pb2+ | Herbs | ferrocene-labeled thiolated aptamer, amperometry | 5.0 × 10−10–1.0×10–7 M, 1.2 × 10−10 M | [31] |
| Chlorpyriphos | Vegetables and fruits | Aptamer on mesoporous carbon, chitosan and MWCNTs-CS, CV | 1–105 ng/mL, 0.33 ng/mL | [32] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, G.K.; Sharma, V.; Mishra, R.K. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors 2018, 8, 28. https://doi.org/10.3390/bios8020028
Mishra GK, Sharma V, Mishra RK. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors. 2018; 8(2):28. https://doi.org/10.3390/bios8020028
Chicago/Turabian StyleMishra, Geetesh Kumar, Vinay Sharma, and Rupesh K. Mishra. 2018. "Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review" Biosensors 8, no. 2: 28. https://doi.org/10.3390/bios8020028
APA StyleMishra, G. K., Sharma, V., & Mishra, R. K. (2018). Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors, 8(2), 28. https://doi.org/10.3390/bios8020028





