A Review on Passive and Integrated Near-Field Microwave Biosensors
Abstract
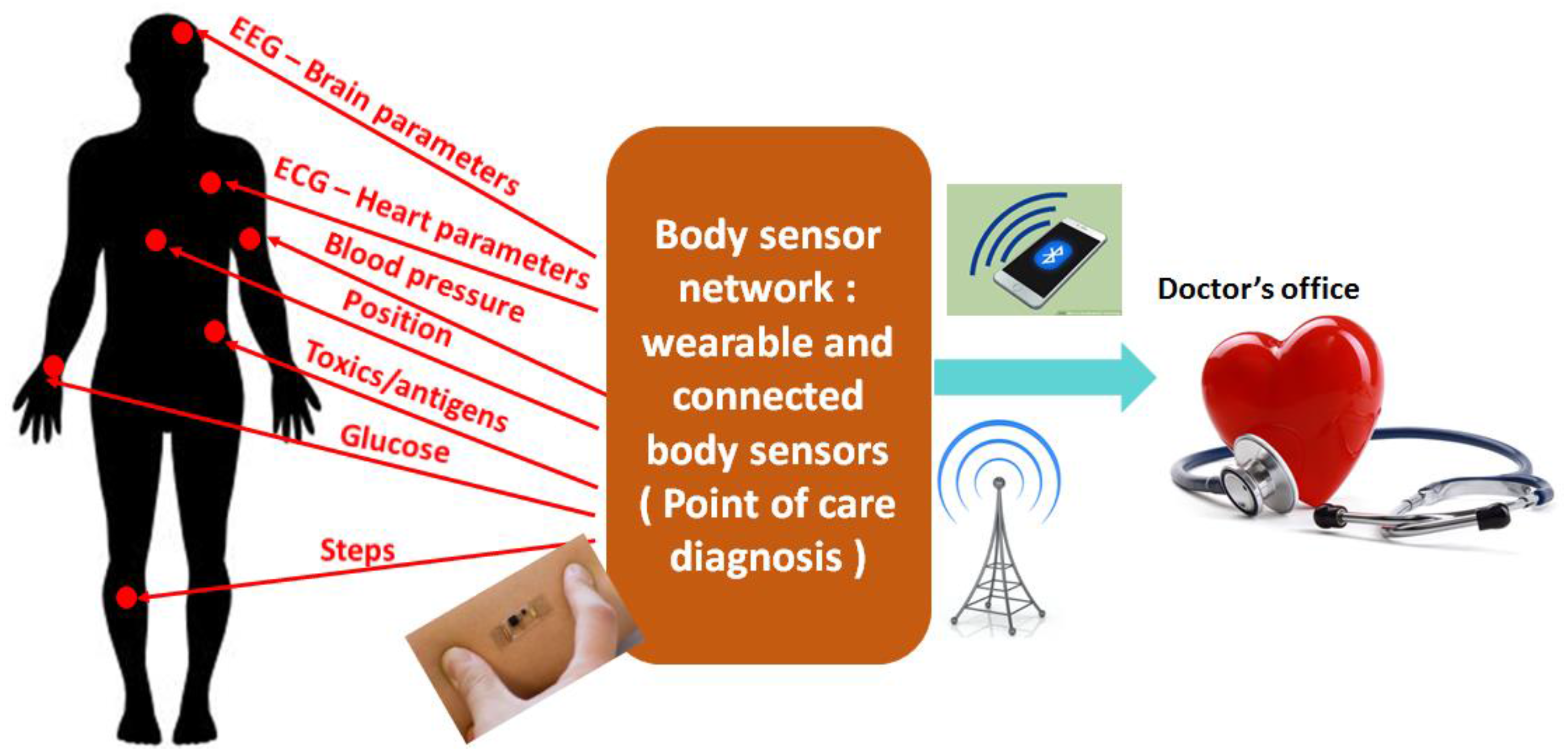
:1. Introduction
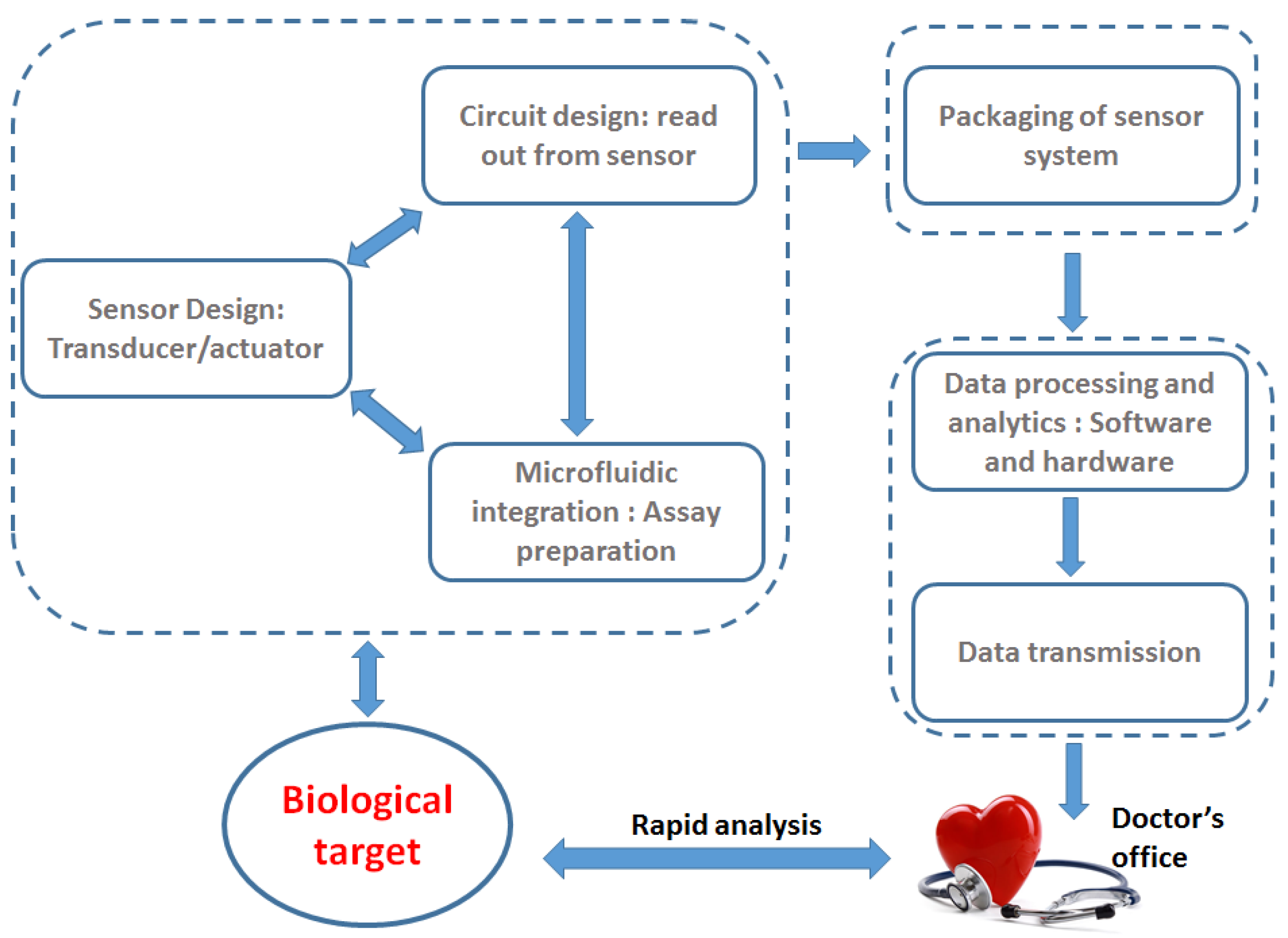
2. Microwave Biosensors
Microwave Interaction with Biomaterial
3. Passive Biosensors
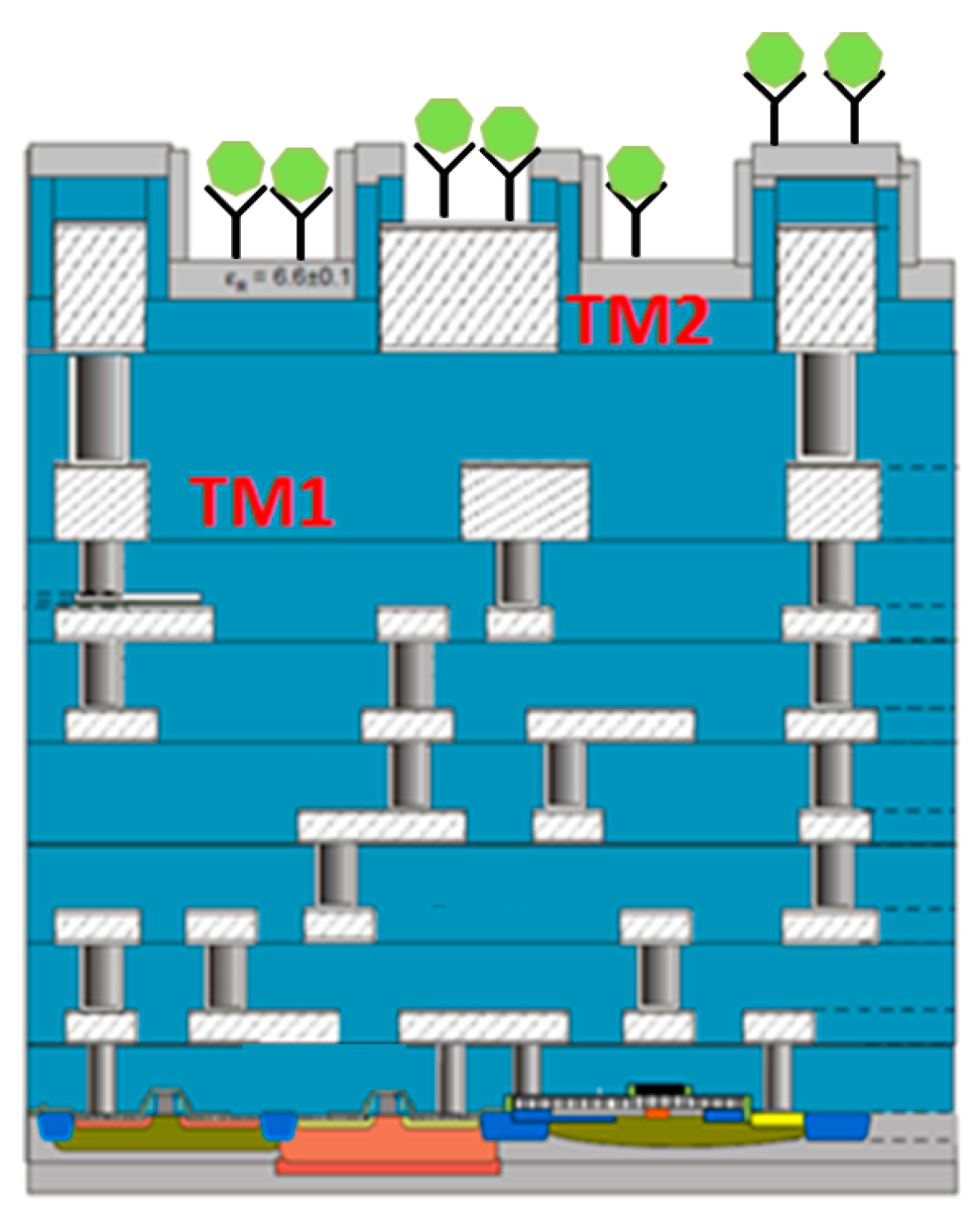
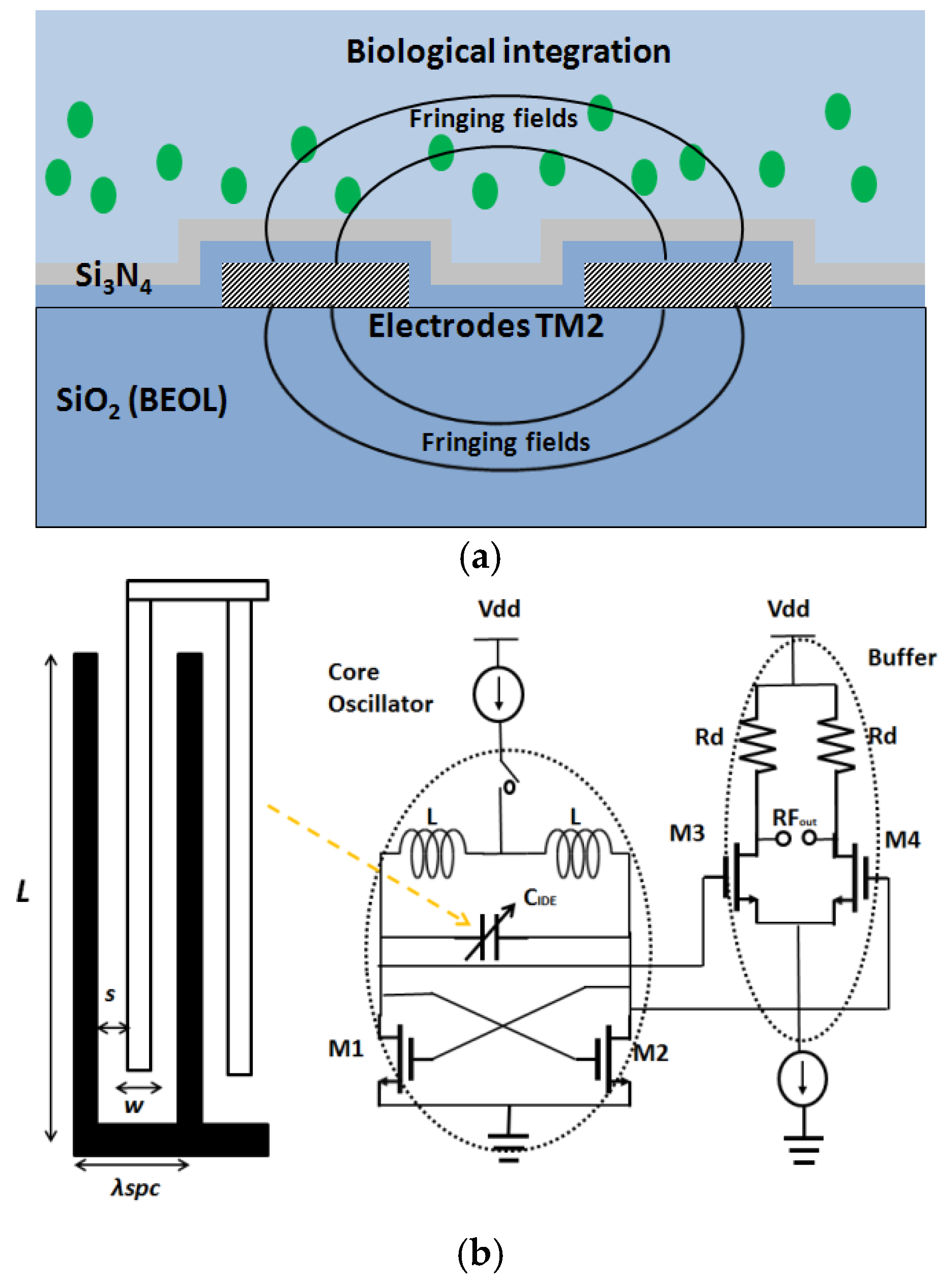
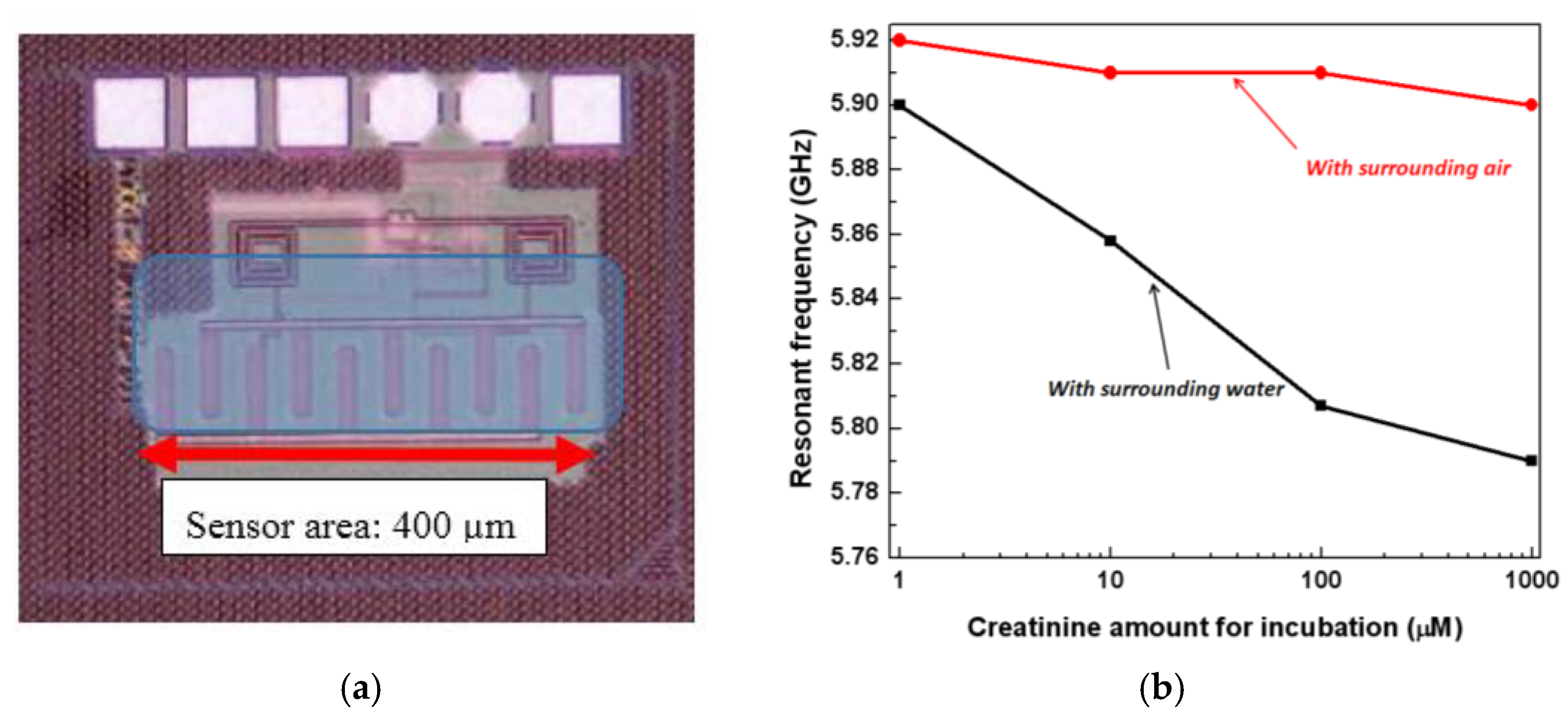
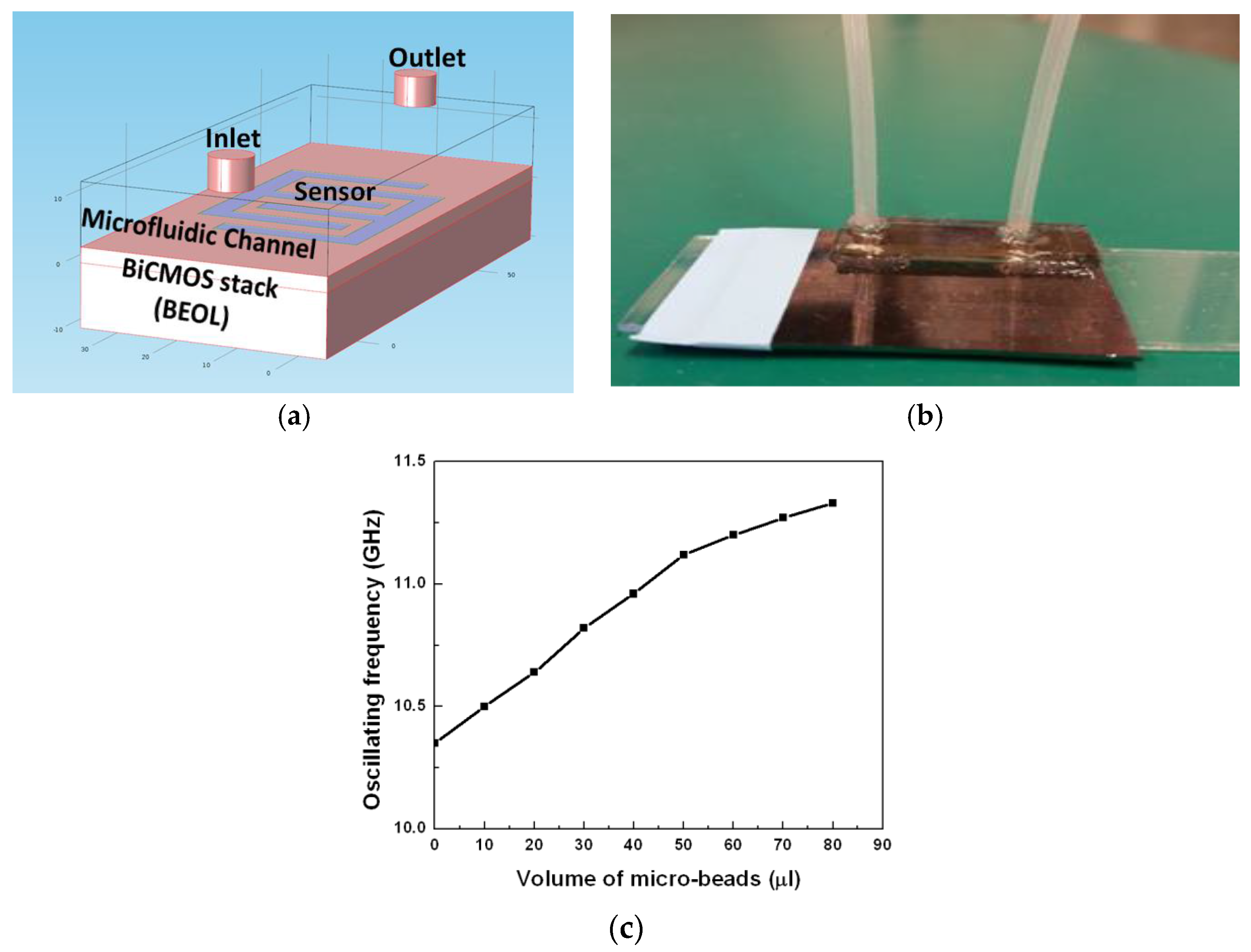
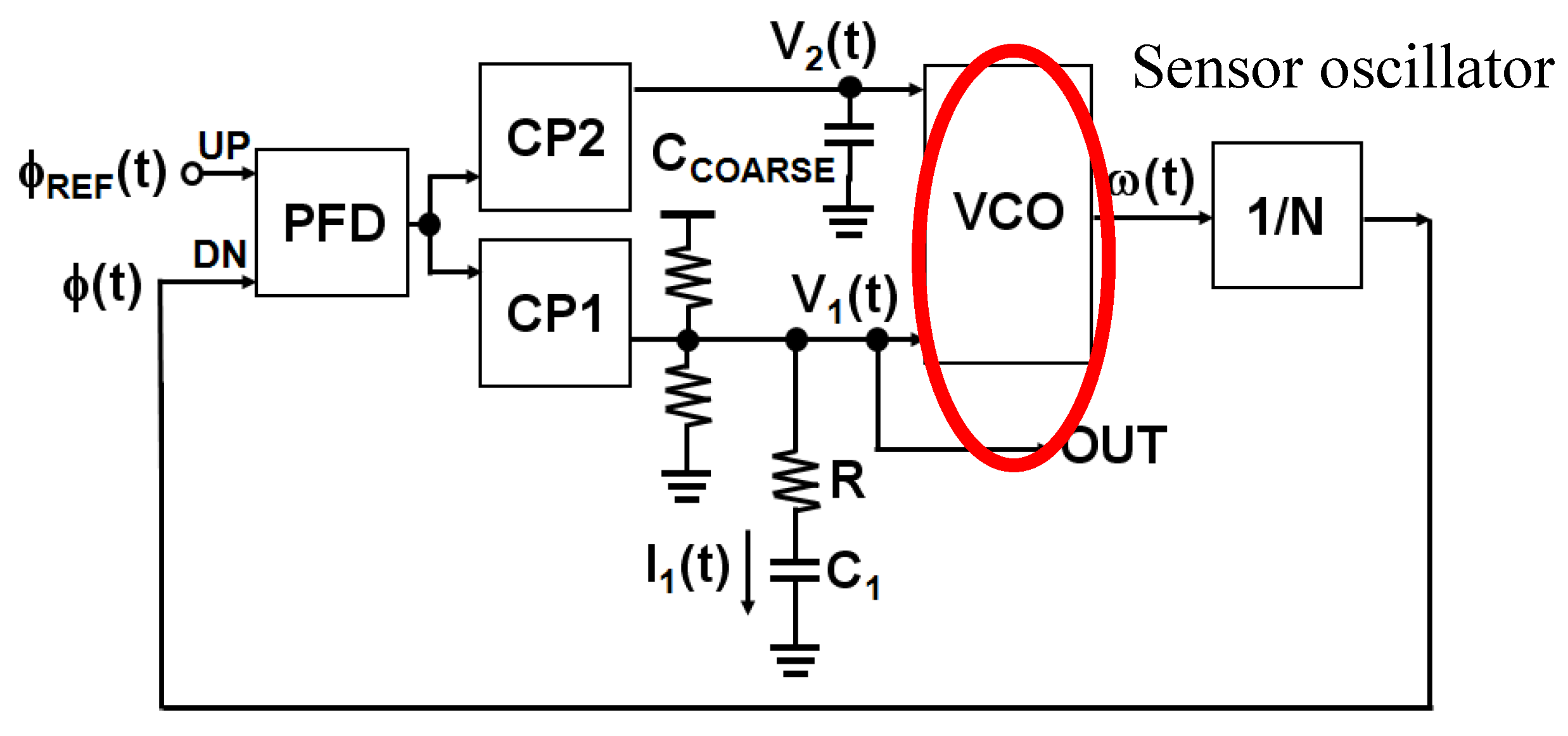
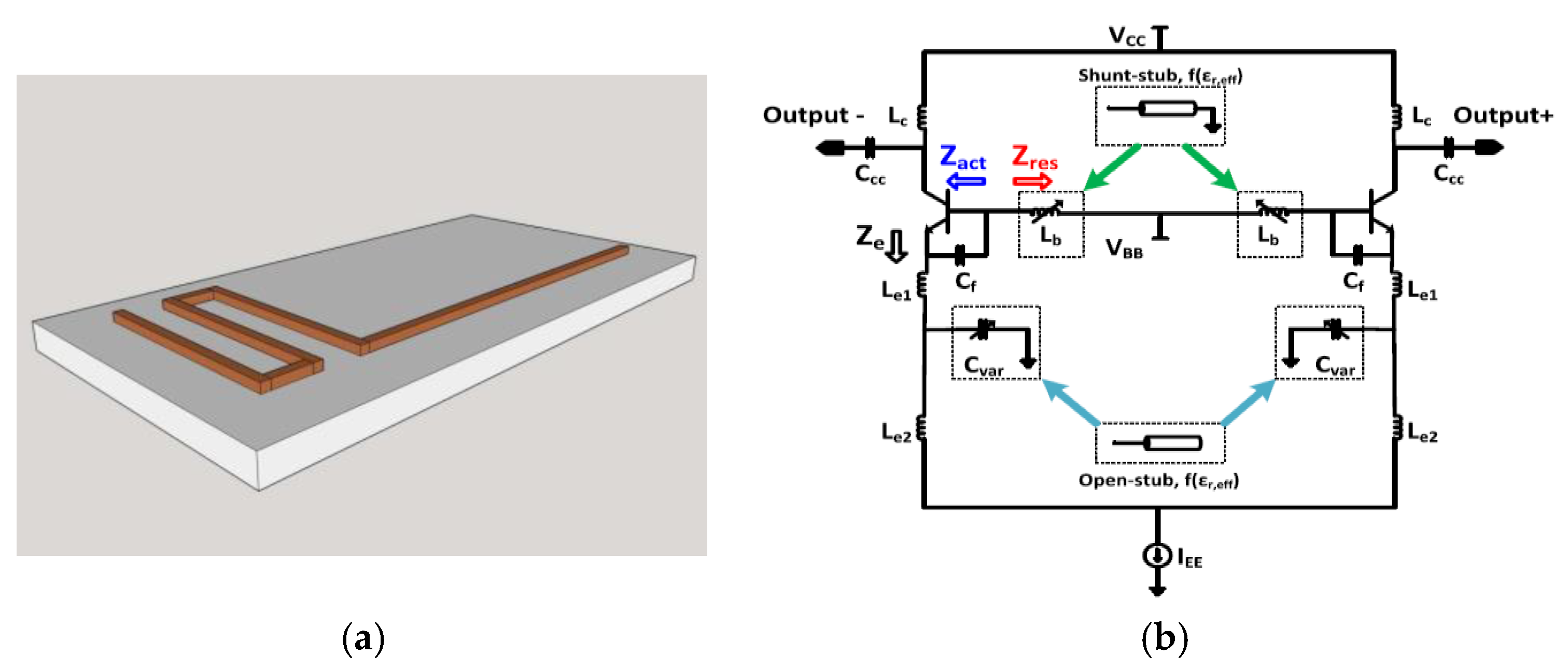
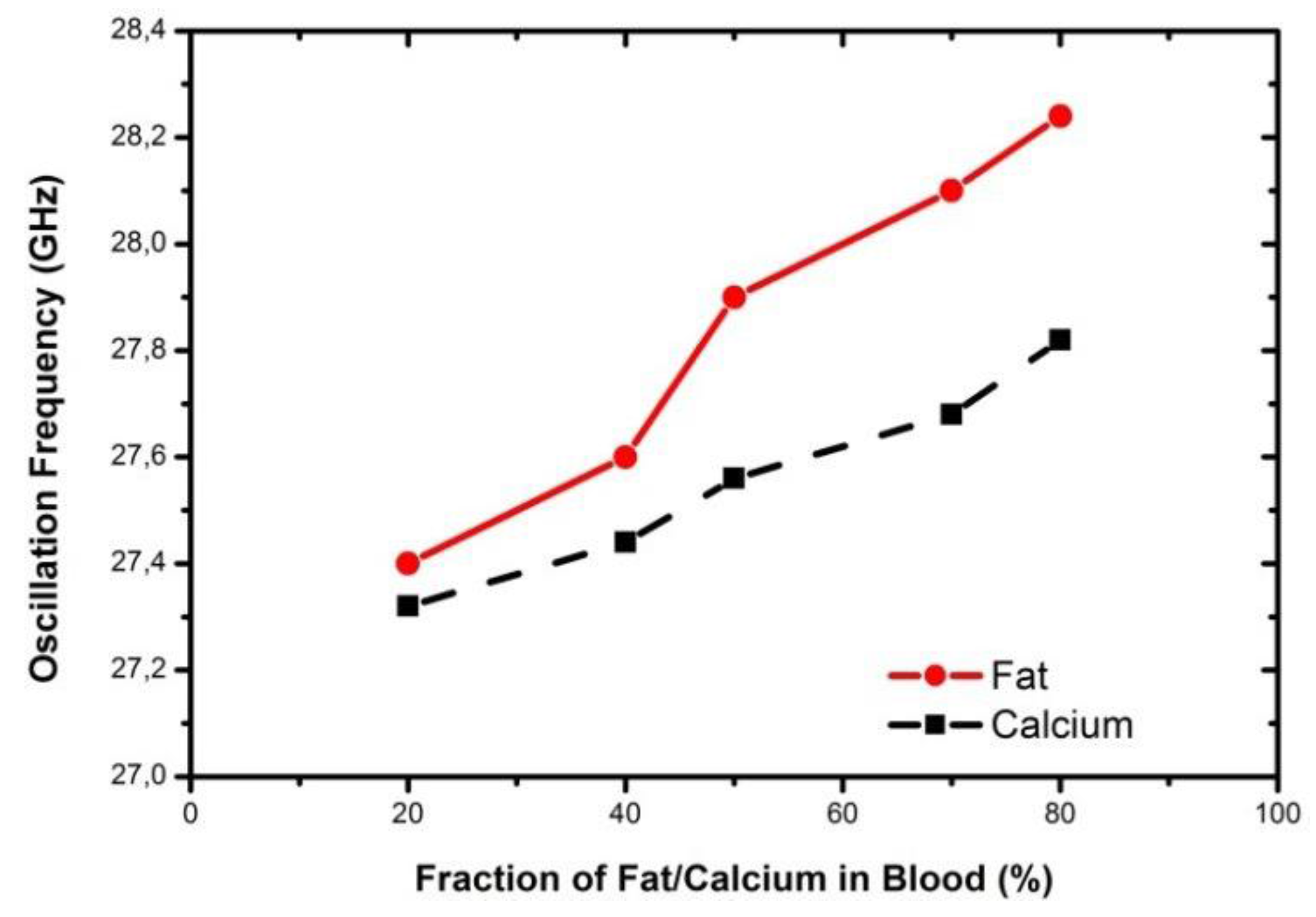
4. Integrated Microwave Biosensor
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations. World Population Ageing 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- United States Census Bureau. An Aging World: 2015; International Population Reports; United States Census Bureau: Suitland, MD, USA, 2016.
- OECD. Health at a Glance: Europe 2012; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Cavenaghi, S. Demographic transformations and inequalities in Latin America. Ser. Investig. Lat. Am. Popul. Assoc. 2009, 8, 13–14. [Google Scholar]
- Otsu, K.; Shibayama, K. Population ageing and potential growth in Asia. Asian Dev. Rev. 2016, 33, 56–73. [Google Scholar] [CrossRef]
- Wilmott, C.; Arrowsmith, J.E. Point-of-care testing. Surgery 2010, 28, 159–160. [Google Scholar] [CrossRef]
- Petryayeva, E.; Russ Algar, W. Toward point-of-care diagnostics with consumer electronic devices: The expanding role of nanoparticles. RSC Adv. 2015, 5, 22256–22282. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Cui, X.; Yang, C. The application of on-chip optofluidic microscopy for imaging Giardia lamblia trophozoites and cysts. Biomed. Microdevices 2009, 11, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, N.; Rui, Z.; Liu, H.B.; Dai, C.C.; Kaushik, R.; Ratnaharika, B.M.; Gong, H.Q. Real-time PCR-based microfluidic array chip for simultaneous detection of multiple waterborne pathogens. Sens. Actuators B Chem. 2010, 145, 543–552. [Google Scholar] [CrossRef]
- Gervais, L.; Delmarche, E. Toward one step point of care immunodiagnostics using capillary-driven microfluidics and PDMS substrates. Lab Chip 2009, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with luminescent semiconductor quantum dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Yang, M.; Kostov, Y.; Bruck, H.A.; Rasooly, A. Carbon nanotubes with enhanced chemiluminescence immunoassay for CCD-based detection of staphylococcal enterotoxin B in food. Anal. Chem. 2008, 80, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; de Cesare, G.; Dolci, L.S.; Mirasoli, M.; Nascetti, A.; Roda, A.; Scipinotti, R. Microfluidic chip with integrated a-Si.H photodiodes for chemiluminescence-based bioassays. IEEE Sens. J. 2013, 13, 2595–2602. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S. Pulse Oximeters: Fundamentals and Design; NXP, FreeScale Semiconductors Document; Freescale Semiconductor, Inc.: Austin, TX, USA, 2012. [Google Scholar]
- Mohammed, M.-I.; Desmulliez, M.P.Y. Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: A review. Lab Chip 2011, 11, 569–595. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef] [PubMed]
- Rusling, F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A.M.; Didar, T.F.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.-H. Label-free SERS selective detection of dopamine and serotonin using graphene-Au nanopyramid heterostructure. ACS Anal. Chem. 2015, 87, 10255. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; He, P.; Kurth, V.; Surareungchai, W.; Baemner, A.J. Multi-channel PMMA microfluidic biosensor with integrated IDUAs for electrochemical detection. Anal. Bional. Chem. 2013, 405, 5965–5974. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Fygenson, D.K. Improving fluorescence detection in lab on chip devices. Lab Chip 2008, 8, 649–652. [Google Scholar] [PubMed]
- Abbott Laboratories. Abott Point of Care; Abbott Laboratories: Chicago, IL, USA, 2008. [Google Scholar]
- Kageyama, K.; Onoyama, Y.; Kogawa, H.; Goto, E.; Tanabe, K. The maximum and minimum water content and cell volume of human erythrocytes in vitro. Biophys. Chem. 1989, 34, 79–82. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of body tissues. Phys. Med. Biol. 1996, 41, 2231–2293. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaka, T.; Sato, D.; Ashida, N.; Saito, A.; Iizuka, K.; Suzuki, T.; Ogawa, Y.; Fujishima, M. CMOS biosensor IC focusing on dielectric relaxations of biological water with 120 and 60 GHz oscillator arrays. IEEE J. Solid State Circuits 2016, 51, 2534–2544. [Google Scholar] [CrossRef]
- Pozar, D. Microwave Engineering, 4th ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Barba, A.; D’Amore, M. Chapter 6: Relevance of dielectric properties in microwave assisted processes. In Microwave Materials Characterization; Costanzo, S., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Belrhiti, M.D.; Bri, S.; Nakheli, A.; Haddad, M.; Mamouni, A. Dielectric constant determination of liquid using regular waveguide structure combined with EM simulation. J. Mater. Environ. Sci. 2012, 3, 575–584. [Google Scholar]
- Goh, S.; Ram, R.J. Impedance spectroscopy for in situ biomass measurements in microbioreactor. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Science, Groningen, The Netherlands, 3–7 October 2010. [Google Scholar]
- Kommenhoek, E.E.; Gardeniers, J.G.E.; Bomer, J.G.; Van den Berg, A.; Li, X.; Ottens, M.; van der Wielen, L.A.M.; van Dedem, G.W.K.; Van Leeuwen, M.; van Gulik, W.M.; et al. Monitoring of yeast cell concentration using micromachined impedance sensor. Sens. Actuators B Chem. 2006, 115, 384–389. [Google Scholar] [CrossRef]
- Faenza, A.; Bocchi, M.; Pecorari, N.; Franchi, E.; Guerrieri, R. Impedance measurement technique for high sensitivity cell detection in micro structures with non-uniform conductivity distribution. Lab Chip 2012, 12, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Micronit Microtechnologies. Electrochemical Impedance Spectroscopy Chips (EIS Chips); Micronit Microtechnologies: Enschede, The Netherlands, 2014. [Google Scholar]
- Gamry Instruments. Electrochemical Impedance Spectroscopy; Gamry Instruments: Warminster, PA, USA, 2005. [Google Scholar]
- Stuchly, S.S.; Bassey, C.E. Microwave coplanar sensors for dielectric measurements. Meas. Sci. Technol. 1998, 9, 1324–1329. [Google Scholar] [CrossRef]
- Shaforost, E.; Klein, N.; Vitusevich, S.; Offenhäuser, A.; Barannik, A. Nanoliter liquid characterization by open whispering-gallery mode dielectric resonators at millimeter wave frequencies. J. Appl. Phys. 2008, 104, 074111. [Google Scholar] [CrossRef]
- Rowe, D.J.; Porch, A.; Barrow, D.A.; Allender, C.J. Microfluidic device for compositional analysis of solvent systems at microwave frequencies. Sens. Actuators B Chem. 2012, 169, 213–221. [Google Scholar] [CrossRef]
- Booth, J.C.; Mateu, J.; Janezic, M.; Baker-Jarvis, J.; Beall, J.A. Broadband permittivity measurements of liquid and biological samples using microfluidic channels. In Proceedings of the IEEE/MTT-S International Microwave Symposium, San Francisco, CA, USA, 11–16 June 2006; pp. 1750–1753. [Google Scholar]
- Zhang, L.Y.; Bounaix Morand du Puch, C.; Dalmay, C.; Lacroix, A.; Landoulsi, A.; Leroy, J.; Melin, C.; Lalloue, F.; Battu, S.; Lautrette, C.; et al. Discrimination of colorectal cancer cell lines using microwave biosensors. Sens. Actuators A Phys. 2014, 2016, 405–416. [Google Scholar] [CrossRef]
- Grenier, K.; Dubuc, D.; Chen, T.; Artis, F.; Chretiennot, T.; Poupot, M.; Fournie, J.-J. Recent advances in microwave-based dielectric spectroscopy at the cellular level for cancer investigation. IEEE Trans. Microw. Theory Techniques 2013, 61, 2023–2030. [Google Scholar] [CrossRef]
- Nikolic-Jaric, M.; Romanuik, S.F.; Ferrier, G.A.; Bridges, G.E.; Butler, M.; Sunley, K.; Thomson, D.J.; Freeman, M.R. Microwave frequency sensor for detection of biological cells in microfluidic channels. Biomicrofluidics 2009, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
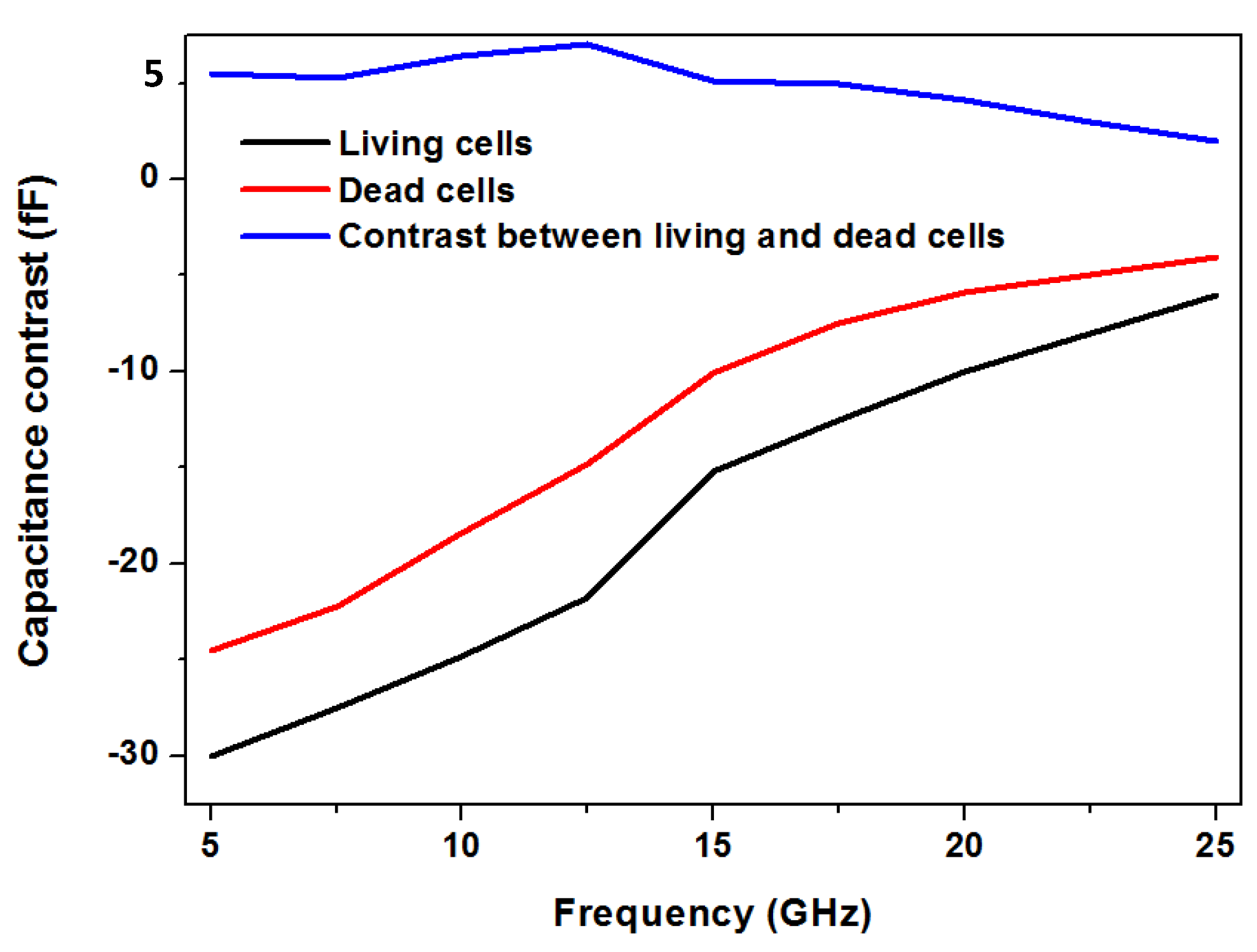
- Chen, T.; Dubuc, D.; Poupot, M.; Fournié, J.-J.; Grenier, K. Broadband discrimination of living and dead lymphomas cells with a microwave interdigitated capacitor. In Proceedings of the 2013 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Austin, TX, USA, 20–23 January 2013; pp. 64–66. [Google Scholar]
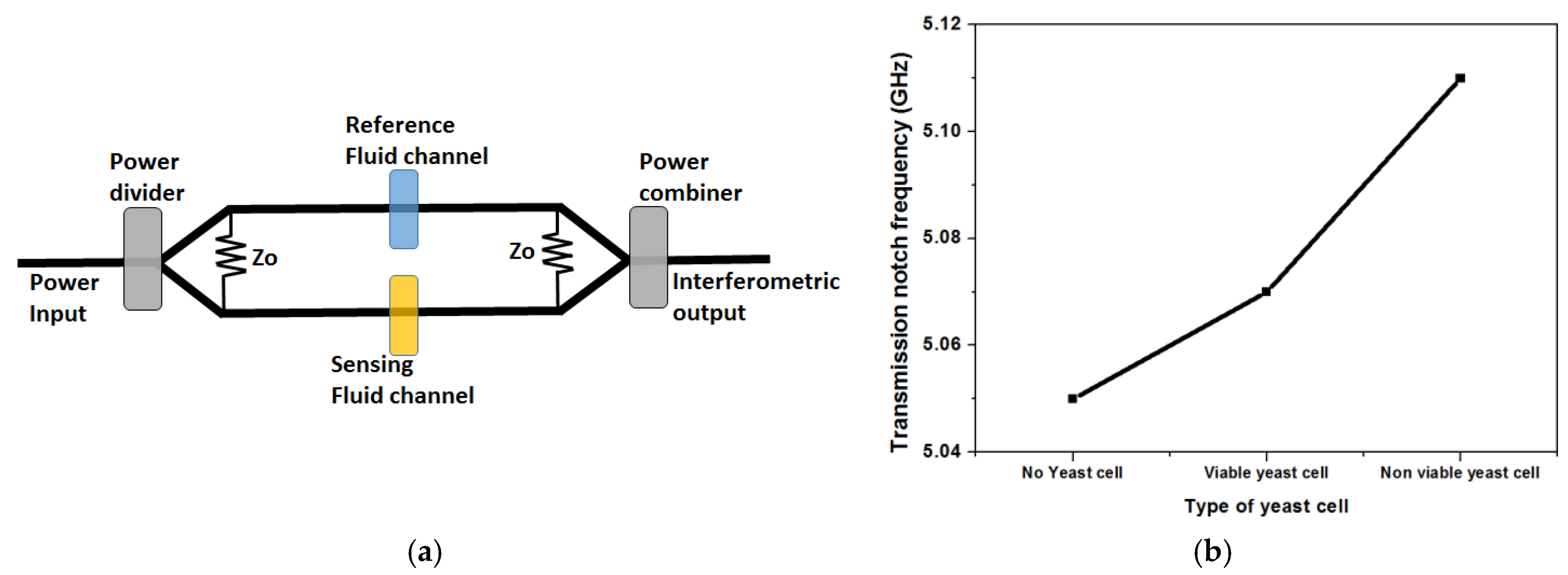
- Yang, Y.; Zhang, H.; Zhu, J.; Wang, G.; Tzeng, T.R.; Xuan, X.; Huang, K.; Wang, P. Distinguishing the viability of a single yeast cell with an ultra-sensitive radio frequency sensor. Lab Chip 2010, 10, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Gubin, A.I.; Barannik, A.A.; Cherpak, N.T.; Vitusevich, S.; Offenhaeusser, A.; Klein, N. Whispering-Gallery mode resonator technique for Characterization of small volumes of Biochemical liquids in microfluidic channel. In Proceedings of the 41st European Microwave conference, Manchester, UK, 10–13 October 2011. [Google Scholar]
- Shaforost, O.N.; Klein, N.; Vitusevich, S.A.; Barannik, A.A.; Cherpak, N.T. High-sensitive microwave characterisation of organic molecule solutions of nanolitre volume. Appl. Phys. Lett. 2009, 94, 112901. [Google Scholar] [CrossRef]
- Moore, G.E. Cramming more components onto integrated circuits. Electronics 1965, 38, 16. [Google Scholar] [CrossRef]
- Arden, W.; Brillouet, M.; Cogez, P.; Graef, M.; Huizing, B.; Mahnkopf, R. More-Than-Moore: White Paper. 2010. Version 2. Available online: http://www.itrs2.net/uploads/4/9/7/7/49775221/irc-itrs-mtm-v2_3.pdf (accessed on 23 September 2017).
- Lee, K.H.; Lee, J.O.; Choi, S.; Yoon, J.B.; Cho, G.H. A CMOS label-free DNA sensor using electrostatic induction of molecular charges. Biosens. Bioelectron. 2012, 31, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Samori, B.; Paulus, C.; Schienle, M.; Augustyniak, M.; Thewes, R. CMOS DNA sensor array with integrated A/D conversion based on label-free capacitance measurement. IEEE J. Solid State Circuits 2006, 41, 2956–2964. [Google Scholar] [CrossRef]
- Anderson, E.; Daniels, J.; Yu, H.; Lee, T.; Pourmand, N. A label-free CMOS DNA microarray based on charge sensing. In Proceedings of the IEEE Instrumentation and Measurement Technology Conference, Victoria, BC, Canada, 12–15 May 2008. [Google Scholar]
- Hassibi, A.; Lee, T.H. A programmable 0.18 µm CMOS electrochemical sensor microarray for biomolecular detection. IEEE Sens. J. 2006, 6, 1380–1388. [Google Scholar] [CrossRef]
- Lee, K.; Nam, J.; Choi, S.; Lim, H.; Shin, S.; Cho, G. A CMOS impedance cytometer for 3D flowing single-cell real time analysis with ΔΣ error correction. In Proceedings of the IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC), San Francisco, CA, USA, 19–23 February 2012; pp. 304–306. [Google Scholar]
- Adiguzel, Y.; Kulah, H. CMOS cell sensors for point-of-care diagnostics. Sensors 2012, 12, 10042–10066. [Google Scholar] [CrossRef] [PubMed]
- Ghafar-Zadeh, E.; Sawan, M. CMOS based capacitive detection lab-on-chip: Design and experimental results. In Proceedings of the IEEE Custom Integrated Circuits Conference (CICC), New Orleans, LA, USA, 27–30 May 2007. [Google Scholar]
- Chien, J.; Anwar, M.; Yeh, E.; Lee, L.P.; Niknejad, A.M. A 6.5/17.5 GHz dual-channel interferometer-based capacitive sensor in 65 nm CMOS for high-speed flow cytometry. In Proceedings of the IEEE/MTT-S International Microwave Symposium, Tampa, FL, USA, 1–6 June 2014. [Google Scholar]
- Wang, H.; Sideris, C.; Hajimiri, A. A frequency-shift based CMOS magnetic biosensor with spatially uniform sensor transducer gain. In Proceedings of the IEEE Custom Integrated Circuits Conference (CICC), San Jose, CA, USA, 19–22 September 2010. [Google Scholar]
- Guha, S.; Warsinke, A.; Tientcheu, C.M.; Schamlz, K.; Meliani, C.; Wenger, C. Label free sensing of creatinine using 6 GHz CMOS near-field dielectric immunosensor. Analyst 2015, 140, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Entesari, K. A 1–8 GHz miniaturized spectroscopy system for permittivity detection and mixture characterization of organic chemicals. IEEE Trans. Microw. Theory Tech. 2012, 80, 4157–4170. [Google Scholar] [CrossRef]
- Guha, S.; Lisker, M.; Trusch, A.; Wolf, A.; Meliani, C.; Wenger, C. 12 GHz CMOS MEMS lab-on-chip system for detection of concentration of suspended particles in bio-suspensions. In Proceedings of the Biodevice 2015, Lisbon, Portugal, 12–15 January 2015. [Google Scholar]
- Guha, S.; Schmalz, K.; Wenger, C.; Herzel, F. Self-calibrating highly sensitive dynamic capacitance sensor towards rapid sensing and counting of particles in laminar flow systems. Analyst 2015, 140, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Wenger, C. Radio frequency CMOS chem-bio Viscosity sensors based on dielectric spectroscopy. In Proceedings of the Biodevice 2017, Porto, Portugal, 21–23 February 2017. [Google Scholar]
- Jamal, F.I.; Guha, S.; Eissa, M.; Borngräaber, J.; Meliani, C.; Ng, H.J.; Kissinger, D.; Wessel, J. Low power miniature K-Band sensors for dielectric characterization of biomaterials. IEEE Trans. Microw. Theory Tech. 2017, 65, 1012–1023. [Google Scholar] [CrossRef]
- Jamal, F.I.; Guha, S.; Eissa, M.; Borngräaber, J.; Meliani, C.; Ng, H.J.; Kissinger, D.; Wessel, J. A fully integrated low powe K-Band chem-bio sensor with on chip DC read-out in SiGe BiCMOS technology. In Proceedings of the European Microwave Conference, London, UK, 3–7 October 2016. [Google Scholar]
- Jamal, F.I.; Guha, S.; Eissa, M.; Vehring, S.; Kissinger, D.; Meliani, C. K-band BiCMOS based near filed biomedical dielectric sensor for detection of fat and calcium in blood. In Proceedings of the European Microwave Conference, Rome, Italy, 7–10 September 2015. [Google Scholar]
- Guha, S.; Wagner, D.; Jamal, F.I.; Schumann, U.; Meliani, C.; Schmidt, B.; Wenger, C.; Wessel, J.; Detert, M. Integrated high-frequency sensors in catheters for minimally invasive plaque characterization. In Proceedings of the European Microelectronics and Packaging Conference, Friedrischagen, Germany, 14–16 September 2015. [Google Scholar]
- Razavi, B. RF Microelectronics, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Wang, H.; Weng, C.C.; Hajimiri, A. Phase noise and fundamental sensitivity of oscillator-based reactance sensors. IEEE Trans. Microw. Theory Tech. 2013, 61, 2215–2229. [Google Scholar] [CrossRef]
- Nehring, J.; Bartels, M.; Weigel, R.; Kissinger, D. A permittivity sensitive phase locked loop based on a silicon-integrated capacitive sensor for microwave biosensing application. In Proceedings of the 2015 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), San Diego, CA, USA, 25–28 January 2015. [Google Scholar]
- Helmy, A.; Jeon, H.; Lo, Y.-C.; Larson, A.J.; Kulkarni, R.; Kim, J.; Silva-Martinez, J.; Entesari, K. A self-sustained CMOS microwave chemical sensor using a frequency synthesizer. IEEE J. Solid State Circuits 2012, 47, 2467–2483. [Google Scholar] [CrossRef]
- Chien, J.-C.; Niknejad, A. Oscillator based reactance sensors with injection locking for high-throughput flow cytometry using microwave dielectric spectroscopy. IEEE J. Solid State Circuits 2016, 51, 457–472. [Google Scholar] [CrossRef]
- Chien, J.-C.; Niknejad, A. Design and analysis of chopper stabilized injection locked oscillator sensors employing near-field modulation. IEEE J. Solid State Circuits 2016, 51, 1851–1865. [Google Scholar] [CrossRef]
- Elkholy, M.; Entesari, K. A wideband low-power LC-DCO-based complex dielectric spectroscopy system in 0.18 µm CMOS. IEEE Trans. Microw. Theory Tech. 2017. [Google Scholar] [CrossRef]
- Bajestan, M.M.; Helmy, A.A.; Hedayari, H.; Entesari, K. A 0.62–10 GHz complex dielectric spectroscopy system in 0.18 µm CMOS. IEEE Trans. Microw. Theory Tech. 2014, 62, 3522–3537. [Google Scholar] [CrossRef]
- Bakhshiani, M.; Suster, M.A.; Mohseni, P. A 9 MHz–2.4 GHz fully integrated transceiver IC for a microfluidic-CMOS platform dedicated to miniaturized dielectric spectroscopy. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 849–861. [Google Scholar] [PubMed]
- Nasr, I.; Nehring, J.; Aufinger, K.; Fischer, G.; Weigel, R.; Kissinger, D. Single and dual port 50–100 GHz integrated vector network analyzers with on-chip dielectric sensors. IEEE Trans. Microw. Theory Tech. 2014, 62, 2168–2179. [Google Scholar] [CrossRef]
- Nehring, J.; Nasr, I.; Borutta, K.; Weigel, R.; Kissinger, D. A silicon integrated microwave vector network analyzer for biomedical sensor read-out applications. In Proceedings of the IEEE MTT-S International Microwave Symposium (IMS), Tampa, FL, USA, 1–6 June 2014. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guha, S.; Jamal, F.I.; Wenger, C. A Review on Passive and Integrated Near-Field Microwave Biosensors. Biosensors 2017, 7, 42. https://doi.org/10.3390/bios7040042
Guha S, Jamal FI, Wenger C. A Review on Passive and Integrated Near-Field Microwave Biosensors. Biosensors. 2017; 7(4):42. https://doi.org/10.3390/bios7040042
Chicago/Turabian StyleGuha, Subhajit, Farabi Ibne Jamal, and Christian Wenger. 2017. "A Review on Passive and Integrated Near-Field Microwave Biosensors" Biosensors 7, no. 4: 42. https://doi.org/10.3390/bios7040042
APA StyleGuha, S., Jamal, F. I., & Wenger, C. (2017). A Review on Passive and Integrated Near-Field Microwave Biosensors. Biosensors, 7(4), 42. https://doi.org/10.3390/bios7040042




