Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection
Abstract
:1. Introduction
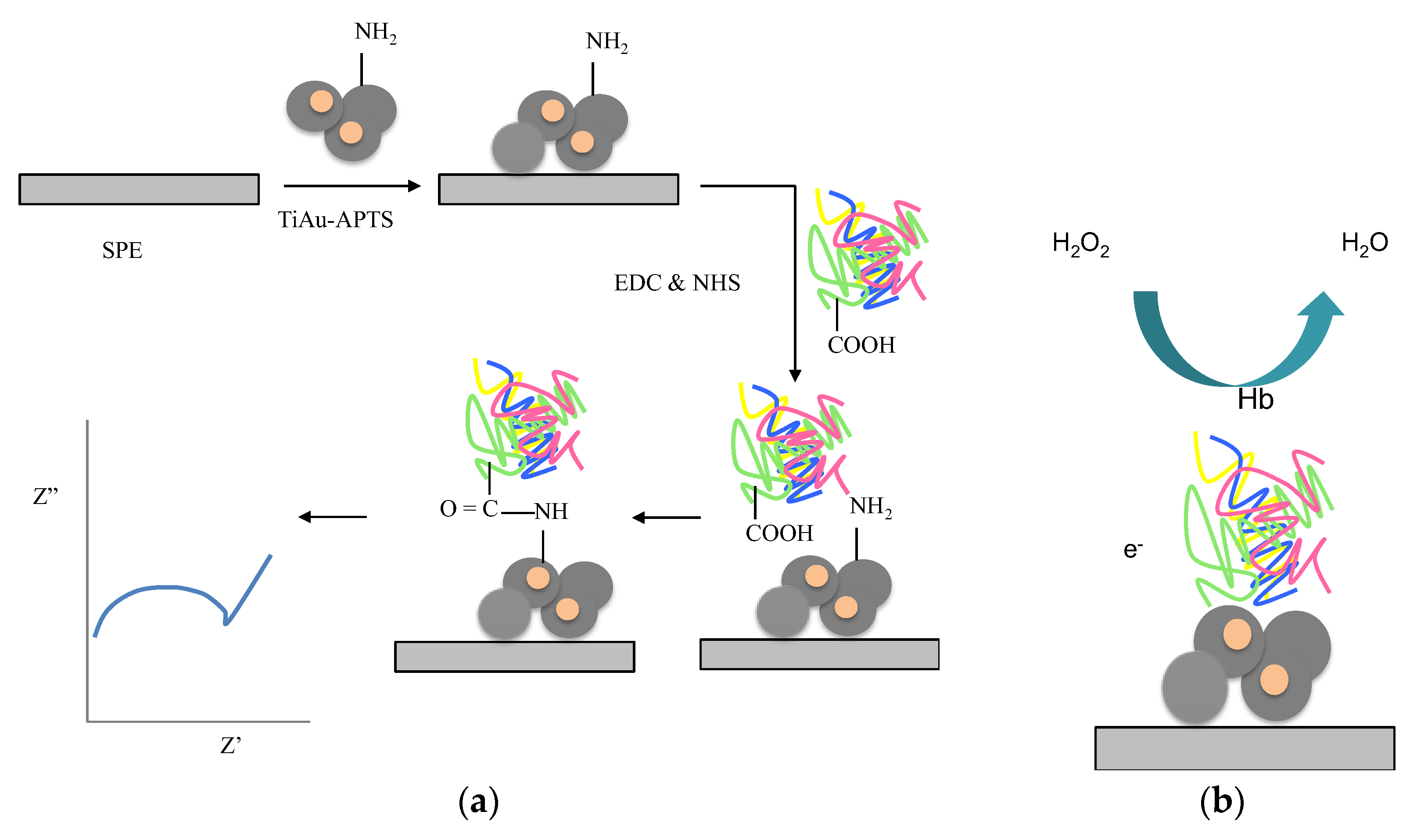
2. Materials and Methods
3. Results and Discussion
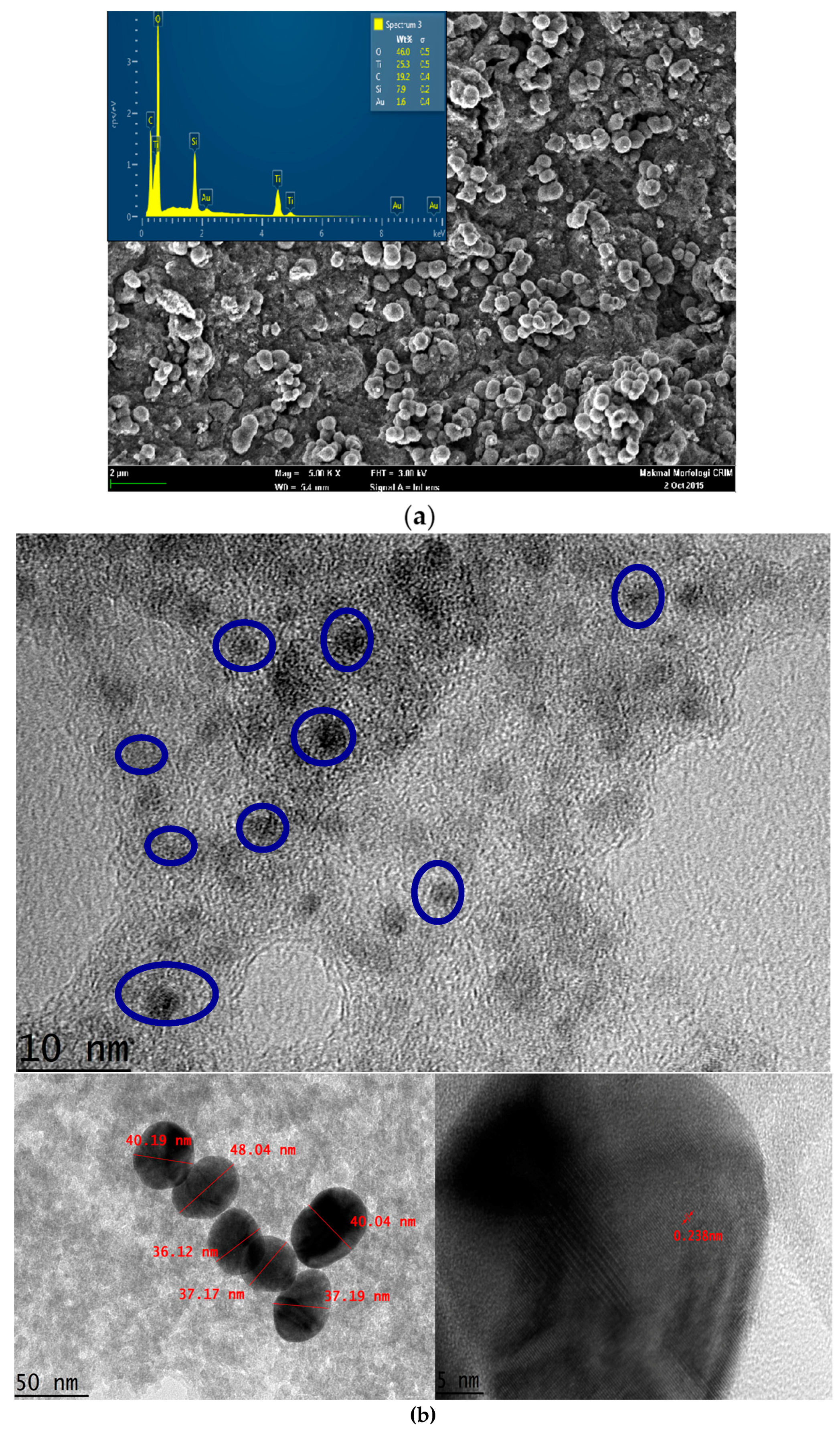
3.1. Characterization of TiAu-APTS
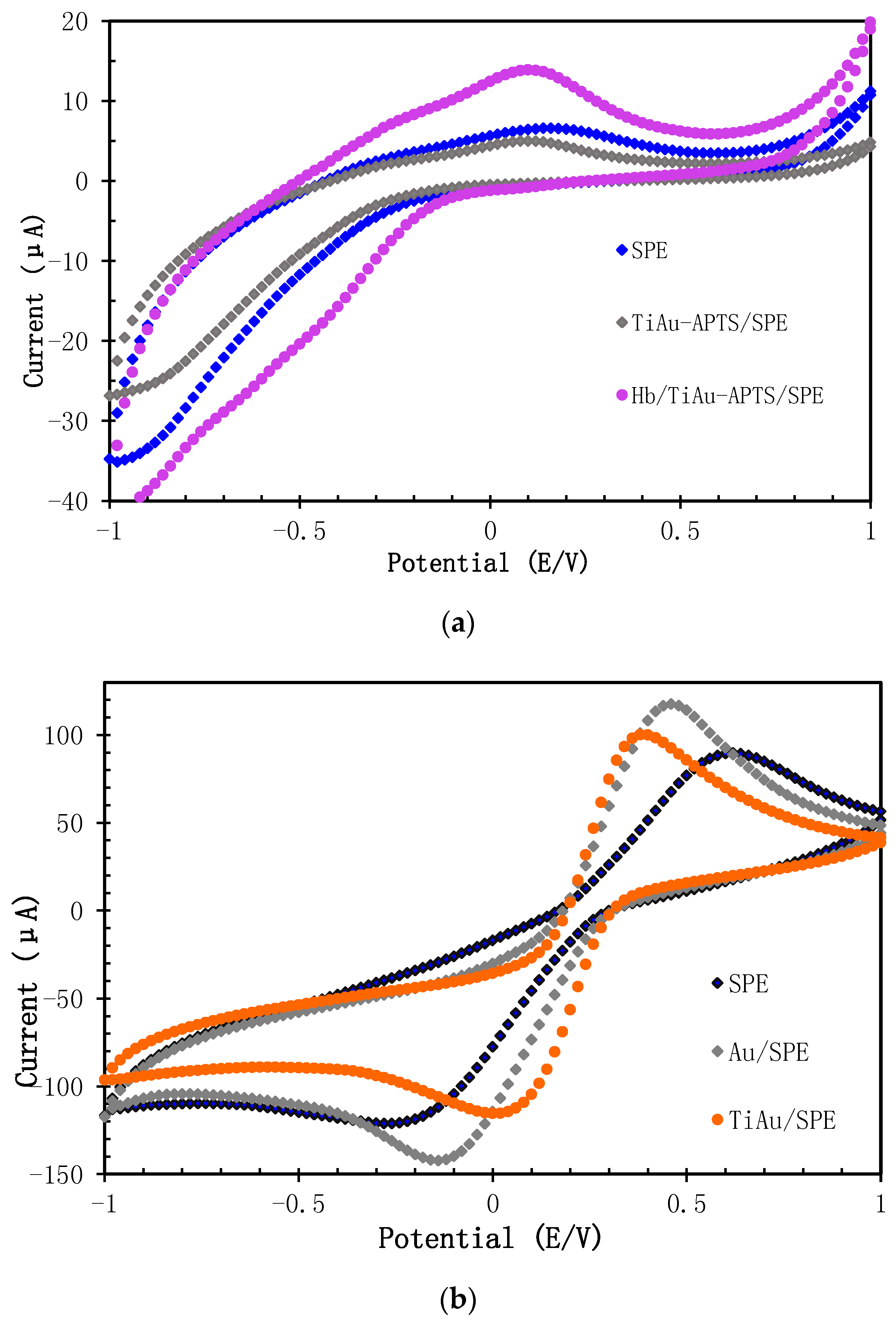
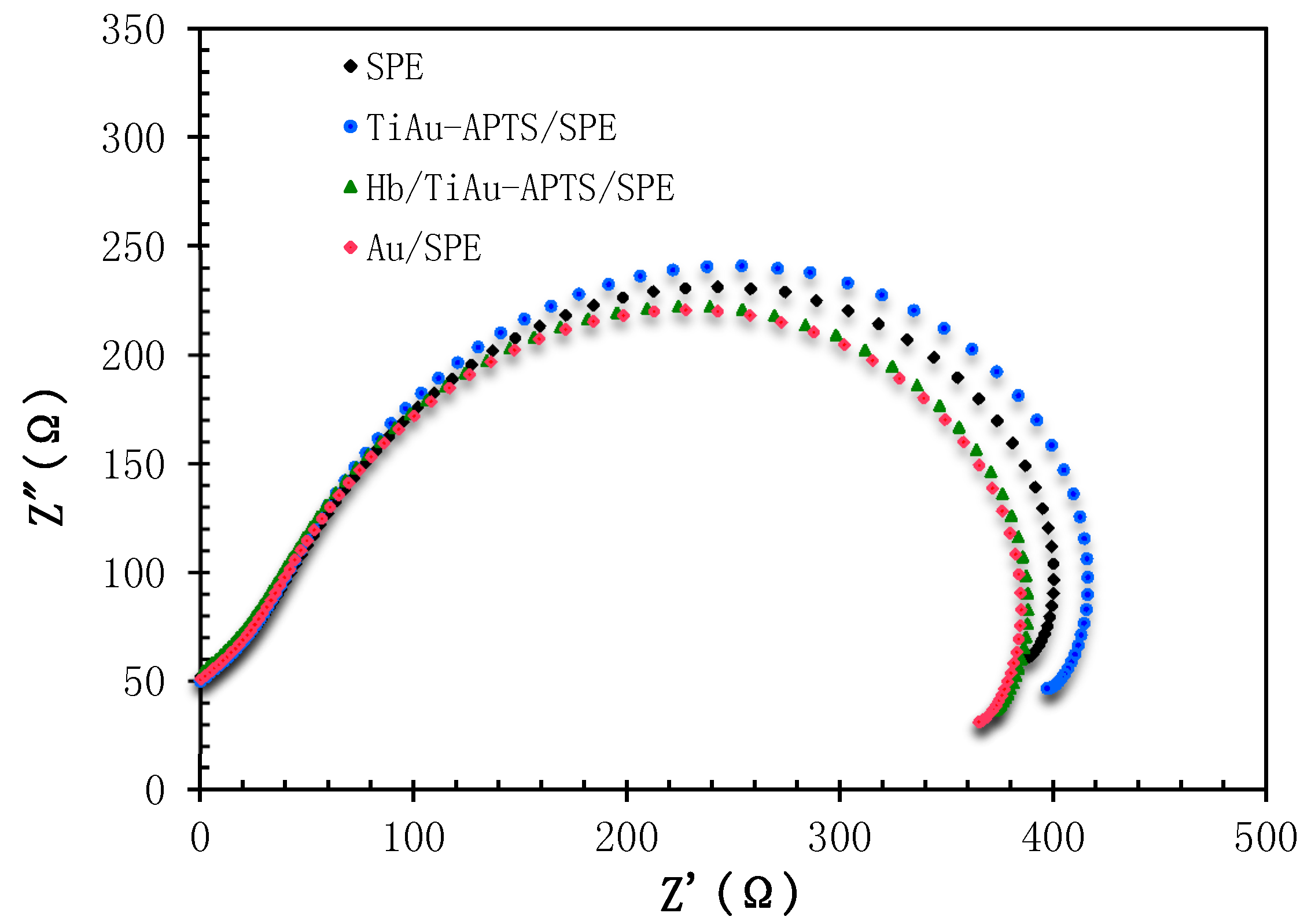
3.2. Characterization of AuNPs/SPE and TiAu-APTS/SPE Electrode
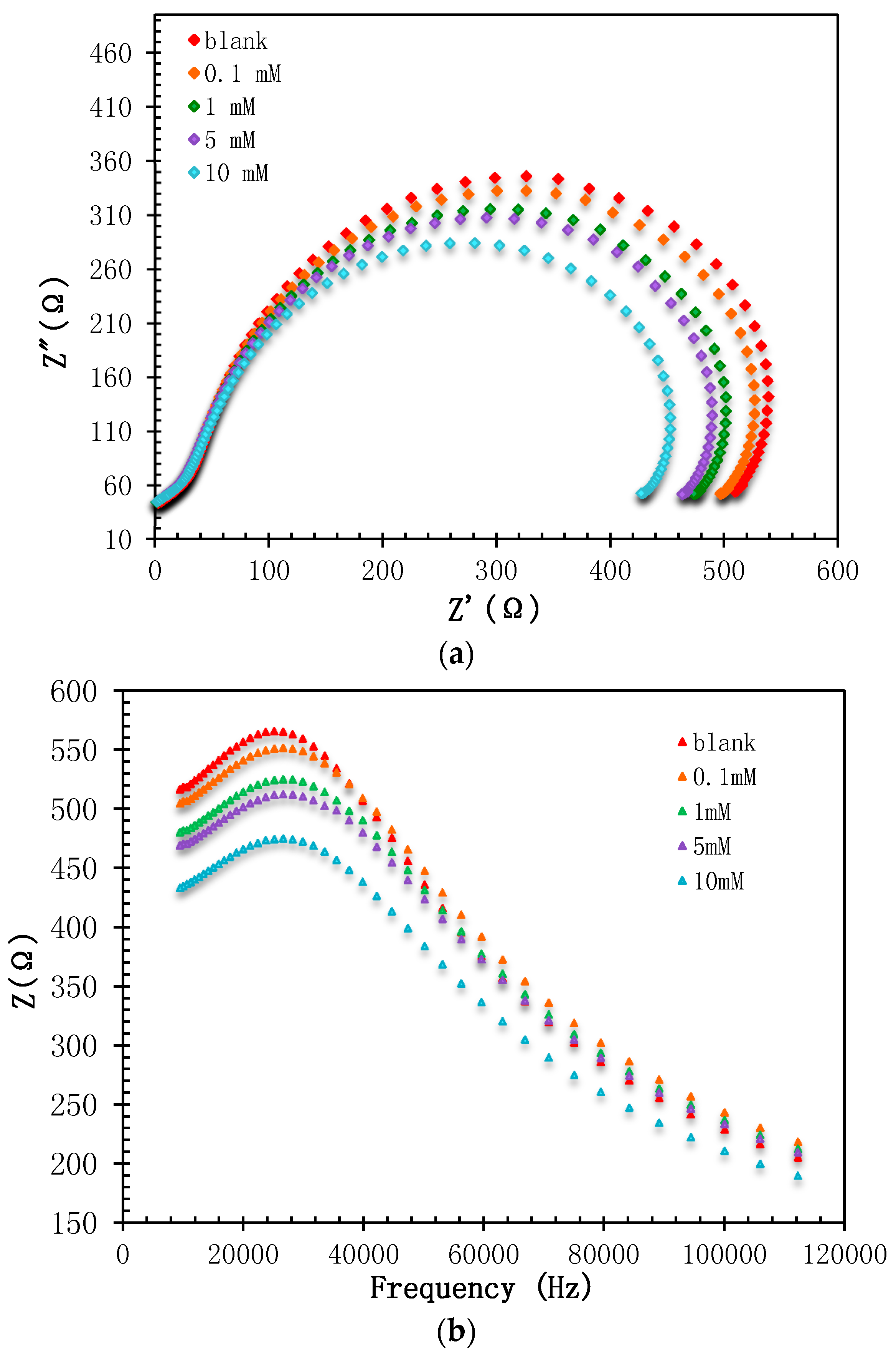
3.3. Performances of Biosensor
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, C.; Liu, C.; Xie, Z. Preparation of a fluorescent silver nanoprism–dye complex for detection of hydrogen peroxide in milk. Anal. Methods 2015, 7, 9749–9752. [Google Scholar] [CrossRef]
- Dong, X.-X.; Li, M.-Y.; Feng, N.-N.; Sun, Y.-M.; Yang, C.; Xu, Z.-L. A nanoporous MgO based nonenzymatic electrochemical sensor for rapid screening of hydrogen peroxide in milk. RSC Adv. 2015, 5, 86485–86489. [Google Scholar] [CrossRef]
- Thandavan, K.; Gandhi, S.; Nesakumar, N.; Sethuraman, S.; Rayappan, J.B.B.; Krishnan, U.M. Hydrogen peroxide biosensor utilizing a hybrid nano-interface of iron oxide nanoparticles and carbon nanotubes to assess the quality of milk. Sens. Actuators 2015, 215, 166–173. [Google Scholar] [CrossRef]
- Chen, C.; Hong, X.; Xu, T.; Chen, A.; Lu, L.; Gao, Y. Synthetic Metals. Synth. Met. 2016, 212, 123–130. [Google Scholar] [CrossRef]
- Hussain, M.; Tariq, S.; Ahmad, M.; Sun, H.; Maaz, K.; Ali, G. Ag-TiO2 nanocomposite for environmental and sensing applications. Mater. Chem. Phys. 2016, 181, 194–203. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2011, 43, 29–41. [Google Scholar] [CrossRef]
- Huang, S.; Si, Z.; Li, X.; Zou, J.; Yao, Y.; Weng, D. A novel Au/r-GO/TNTs electrode for H2O2, O2 and nitrite detection. Sens. Actuators B Chem. 2016, 234, 264–272. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Yu, X.D.; Xu, J.J.; Chen, H.Y. Colorimetric detection of quaternary ammonium surfactants using citrate-stabilized gold nanoparticles (Au NPs). Anal. Methods 2014, 6, 2031–2033. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, J.; Cao, F.; Lu, Y.; Li, H. In situ encapsulation of Au nanoparticles in mesoporous core–shell TiO2 microspheres with enhanced activity and durability. Chem. Commun. 2009, 25, 3789–3791. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Liu, S.; Han, M.-Y. Titania-Coated Metal Nanostructures. Chem. Asian J. 2012, 7, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Solanki, P.R.; Kaneto, K.; Kim, C.G.; Ahmad, S.; Malhotra, B.D. Nanostructured iron oxide platform for impedimetric cholesterol detection. Electroanalysis 2010, 22, 1045–1055. [Google Scholar] [CrossRef]
- Wang, J.; Carmon, K.S.; Luck, L.A.; Suni, I.I. Electrochemical impedance biosensor for glucose detection utilizing a periplasmic E. coli receptor protein. Electrochem. Solid-State Lett. 2005, 8, H61–H64. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Ying, Y. New Trends in impedimetric biosensors for the detection of foodborne Pathogenic bacteria. Sensors 2012, 12, 3449–3471. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance biosensors: Applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Jimenez, G.C.; Mahvash, F.; Guermoune, A.; Tlili, C.; Szkopek, T. Functionalized CVD monolayer graphene for label-free impedimetric biosensing. Nano Res. 2014, 8, 1698–1709. [Google Scholar] [CrossRef]
- Hu, Y.; Zuo, P.; Ye, B.C. Label-free electrochemical impedance spectroscopy biosensor for direct detection of cancer cells based on the interaction between carbohydrate and lectin. Biosens. Bioelectron. 2013, 43, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, X.; Liu, X.; Gao, Q.; Qi, H.; Zhang, C. Signal-on impedimetric electrochemical DNA sensor using dithiothreitol modified gold nanoparticle tag for highly sensitive DNA detection. Anal. Chim. Acta 2013, 799, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Z.O.; Uygun, H.D.E. A short footnote: Circuit design for faradaic impedimetric sensors and biosensors. Sens. Actuators B Chem. 2014, 202, 448–453. [Google Scholar] [CrossRef]
- Voitechovič, E.; Bratov, A.; Abramova, N.; Razumienė, J.; Kirsanov, D.; Legin, A. Development of label-free impedimetric platform based on new conductive polyaniline polymer and three-dimensional interdigitated electrode array for biosensor applications. Electrochim. Acta 2015, 173, 59–66. [Google Scholar] [CrossRef]
- Craine, J.E.; Connely, J.L. Effect of Hemoglobin on Ferricyanide-Dependent Assays. Anal. Biochem. 1970, 38, 539–546. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Ji, X.; Yang, W. Preparation of uniform Au@SiO2 particles by direct silica coating on citrate-capped Au nanoparticles. Colloids Surf. A 2011, 392, 220–224. [Google Scholar] [CrossRef]
- Liu, S.H.; Han, M.Y. Synthesis, functionalization, and bioconjugation of monodisperse, silica-coated gold nanoparticles: Robust bioprobes. Adv. Funct. Mater. 2005, 15, 961–967. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.; Zhang, S.-Y.; Shah, K.W.; Han, M.-Y. Synthesis and Multiple Reuse of Eccentric Au@TiO2 Nanostructures as Catalyst. Chem. Commun. 2011, 47, 6689–6691. [Google Scholar]
- Briñas, R.P.; Hu, M.; Qian, L.; Lymar, E.S.; Hainfeld, J.F. Gold Nanoparticle Size Controlled by Polymeric Au(I) Thiolate Precursor Size. J. Am. Chem. Soc. 2008, 130, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Peng, W.K.; Han, J.; Pumera, M. Direct in vivo electrochemical detection of haemoglobin in red blood cells. Sci. Rep. 2014, 4, 6209. [Google Scholar] [CrossRef] [PubMed]
- Zainiharyati, M.Z.; Norazreen, Z. Hydrogen peroxide impedimetric detection on poly-ortho-phenylenediamine modified platinum disk microelectrode. Malays. J. Anal. Sci. 2014, 18, 107–115. [Google Scholar]
- Zhang, L.; Yin, H.-B.; Luo, J.-J.; Yang, P.-H.; Cai, J.Y. Construction of electrochemical impedance sensor basedon poly dopamine-hyaluronic acid composite membranefor detection of hydrogen peroxide. Chin. J. Anal. Chem. 2013, 41, 534–539. [Google Scholar] [CrossRef]
- Dhand, C.; Solanki, P.R.; Sood, K.N.; Datta, M.; Malhotra, B.D. Polyaniline nanotubes for impedimetric triglyceride detection. Electrochem. Commun. 2009, 11, 1482–1486. [Google Scholar] [CrossRef]
- Zheng, W.; Zheng, Y.F.; Jin, K.W.; Wang, N. Direct electrochemistry and electrocatalysis of hemoglobin immobilized in TiO2 nanotube films. Talanta 2008, 74, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.V.; Huynh, C.D.; Tran, H.V.; Piro, B. Cyclic voltammetry, square wave voltammetry, electrochemical impedance spectroscopy and colorimetric method for hydrogen peroxide detection based on chitosan/silver nanocomposite. Arab. J. Chem. 2016, 1–7. [Google Scholar] [CrossRef]
- Nikkhah, E.; Khaiamy, M.; Heidary, R.; Azar, A.S. The effect of ascorbic acid and H2O2 treatment on the stability of anthocyanin pigments in berries. Turk. J. Biol. 2010, 34, 47–53. [Google Scholar]


| Ratio of Interference to Analyte | Glucose Impedance Value (RCT) | % Change | Ascorbic Acid Impedance Value (RCT) | % Change |
|---|---|---|---|---|
| High (10:1) | 433.67 | −1.66 | 408.00 | −4.90 |
| Medium (1:1) | 419.67 | −2.18 | 414.67 | −3.34 |
| Low (0.1:1) | 417.33 | −2.72 | 438.33 | 2.18 |
| Added H2O2 (mM) | Found in Milk Sample | Recovery % |
|---|---|---|
| 1 | 1.14 | 114 |
| 5 | 4.94 | 98 |
| 10 | 9.02 | 90 |
| 15 | 15.63 | 104 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Halim, N.H.; Lee, Y.H.; Marugan, R.S.P.M.; Hashim, U. Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection. Biosensors 2017, 7, 38. https://doi.org/10.3390/bios7030038
Abdul Halim NH, Lee YH, Marugan RSPM, Hashim U. Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection. Biosensors. 2017; 7(3):38. https://doi.org/10.3390/bios7030038
Chicago/Turabian StyleAbdul Halim, Nur Hamidah, Yook Heng Lee, Radha Swathe Priya Malon Marugan, and Uda Hashim. 2017. "Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection" Biosensors 7, no. 3: 38. https://doi.org/10.3390/bios7030038
APA StyleAbdul Halim, N. H., Lee, Y. H., Marugan, R. S. P. M., & Hashim, U. (2017). Mediatorless Impedance Studies with Titanium Dioxide Conjugated Gold Nanoparticles for Hydrogen Peroxide Detection. Biosensors, 7(3), 38. https://doi.org/10.3390/bios7030038




