Abstract
The capability of alternating current (AC) dielectrophoresis (DEP) for on-chip capture and chaining of the three species representative of freshwater phytoplankton was evaluated. The effects of the AC field intensity, frequency and duration on the chaining efficiency and chain lengths of green alga Chlamydomonas reinhardtii, cyanobacterium Synechocystis sp. and diatom Cyclotella meneghiniana were characterized systematically. C. reinhardtii showed an increase of the chaining efficiency from 100 Hz to 500 kHz at all field intensities; C. meneghiniana presented a decrease of chaining efficiency from 100 Hz to 1 kHz followed by a significant increase from 1 kHz to 500 kHz, while Synechocystis sp. exhibited low chaining tendency at all frequencies and all field intensities. The experimentally-determined DEP response and cell alignment of each microorganism were in agreement with their effective polarizability. Mixtures of cells in equal proportion or 10-times excess of Synechocystis sp. showed important differences in terms of chaining efficiency and length of the chains compared with the results obtained when the cells were alone in suspension. While a constant degree of chaining was observed with the mixture of C. reinhardtii and C. meneghiniana, the presence of Synechocystis sp. in each mixture suppressed the formation of chains for the two other phytoplankton species. All of these results prove the potential of DEP to discriminate different phytoplankton species depending on their effective polarizability and to enable their manipulation, such as specific collection or separation in freshwater.
1. Introduction
The phytoplankton in aquatic systems are highly complex and heterogeneous. Phytoplankton includes an assembly of diverse photoautotrophic species, such as eukaryotic algae, diatoms and cyanobacteria developing in the euphotic zone of natural waters [1,2]. They have an important ecological role, being central for primary productivity in surface water, as well as being essential food sources for zooplankton, fish and mammals [1,2]. Because of their capability to rapidly respond to environmental changes due to their small size and fast metabolic processes, phytoplankton are considered good indicators of water quality [3,4]. In recent years, phytoplankton cells were widely used as biological components in biosensors for water monitoring and demonstrated their sensitivity to a large range of aquatic pollutants, including herbicides, pesticides and toxic metals [5,6,7]. Phytoplankton from the aquatic environment combined with dielectrophoresis (DEP) could offer new means for the development of biosensors for water quality assessment. Previous studies showed the capability of DEP to electrically control the trapping and focusing of bioparticles [8,9,10]. However, despite the recent introduction of DEP-based devices using bioparticles for water quality assessment [11,12,13,14,15], only a few studies have focused on DEP manipulation of live cells and their mixtures [15], and no studies have explored the DEP behavior of representative species from the phytoplankton apart for the green microalga Chlamydomonas reinhardtii [16]. The DEP response of freshwater microorganisms in natural water is thus largely unexplored, and its potential as a tool for the manipulation of complex cell systems in realistic environments has not been fully assessed. Alternating current (AC)-DEP-driven collection and chaining of cells or particles is also an important process in the operation of whole cell biosensors based on 2D arrays of cells [17], as the chaining process represents the first step in the formation of a 2D array [18,19].
The formation of one-dimensional arrays or “pearl-chains” in the direction of the applied electric field involves cell-cell DEP chaining force Fchain [8,19,20,21,22]:
where is the permittivity of the surrounding media, r is the radius of the cell, E is the electric field intensity, the coefficient C depends on the number and distance between the cells within the growing chain (3 < C < 103) and is the Clausius–Mossotti factor, whose real part Re|| corresponds to the polarizability function of a bioparticle and depends on the complex permittivity of the medium and the cell [8,19,23] (Equation (2)):
Examples of microorganisms captured in one-dimensional arrays include viable yeast using castellated microelectrodes [21], coplanar gold electrodes [19,24] or interdigitated electrodes [25], different strains of bacteria using curved microelectrodes [26], polynomial and castellated electrodes [27] or interdigitated electrodes [15,28], as well as microalgae with coplanar gold electrodes [16] and protozoan parasite with interdigitated electrodes [15]. However, the chaining process of phytoplankton cells under realistic conditions, such as in complex mixtures of representative phytoplankton cells, has not been assessed yet despite its high environmental relevance.
The major goal of the present study is to understand the DEP behavior of representative phytoplankton species and to determine to what extent and under what conditions the mixture of these phytoplankton cells could affect the dielectrophoretic response of individual cells and thus influence the phytoplankton cell trapping and chaining in microfluidic on-chip devices. Green microalga C. reinhardtii, cyanobacterium Synechocystis sp. and diatom Cyclotella meneghiniana were chosen as representative model organisms for the freshwater phytoplankton. These three phytoplankton species were hypothesized to show differing DEP behaviors under specific AC field intensities and frequencies due to the differences in their size and cell wall composition, which are expected to affect their DEP behavior, allowing their separation when mixed in suspension. Indeed, Synechocystis sp. has a size 5 times smaller than C. reinhardtii or C. meneghiniana, while C. meneghiniana was chosen because of the specific composition of the cell wall, including silicate, compared to C. reinhardtii and Synechocystis sp. The influence on the chaining efficiency of the AC field intensity, frequency and duration for individual phytoplankton species and their mixtures was explored.
2. Materials and Methods
2.1. Cell Cultures and Test Medium
C. reinhardtii (CPCC 11, Canadian Phycological Culture Centre, Waterloo, ON, Canada) and C. meneghiniana (1020-1a, Experimental Phycology and Culture Collection of Algae at the University of Goettingen, Goettingen, Germany) were cultured at 20 °C under rotary shaking at 115 rpm and continuous illumination of 6000 lux (INFORS HT, Basel, Switzerland) in a four-times diluted Tris-acetate-phosphate medium (Sigma-Aldrich, Buchs, Switzerland) and Talaquil medium, respectively. Synechocystis sp. (PCC 6803, Canadian Phycological Culture Centre, Waterloo, ON, Canada) was grown under the same temperature and shaking conditions, but under day-night illumination of 6000 lux (INFORS HT, Basel, Switzerland) in BG-11 BlueGreen medium. The cells were collected at the mid-exponential growth phase and isolated from each growth medium by centrifugation at 3000 rpm for 10 min (Omnifuge 2.0 RS, Heraeus Sepatech GmbH, Osterode/Harz, Germany). The supernatant was removed, and the cells were re-suspended in Geneva Lake water with physico-chemical composition detailed in Table S1 of the Supplementary Materials and filtered through 0.45-µm pore size filters (Millipore, Billerica, MA, USA). The final cell concentration was 5 × 106 cells·mL−1 for C. reinhardtii and C. meneghiniana and of 5 × 107 cells·mL−1 for Synechocystis sp. (if not specified otherwise).
2.2. DEP Experimental Setup and Parameter Optimization
DEP assembly experiments were performed with coplanar gold electrodes separated by a 2-mm gap enclosed in a 350-μm thick transparent microfluidic chamber as described elsewhere [16,24,29] and chosen based on several advantages, including their simple and robust use [16,19]. The gold electrodes were vapor-deposited onto 25 × 75 mm microscope glass slides. The fabrication and the electrical actuation of these electrodes were the same as previously described [16]. The applied frequencies were limited to a maximum of 500 kHz to avoid distortion of the AC signal created by the amplifier connected to the coplanar electrodes.
2.3. DEP Behavior of Representative Phytoplankton Species
DEP behavior was explored for each phytoplankton species individually and in mixtures. Experiments were performed to understand the DEP behavior of representative phytoplankton species. The electrical field intensity, frequency and duration, as well as the phytoplankton concentration were systematically varied for each phytoplankton species to find the optimal combination of these parameters allowing the formation of the cell chains. AC field intensities of 15 V·mm−1, 20 V·mm−1 and 25 V·mm−1 for frequencies increasing from 100 Hz to 500 kHz were tested. The effect of AC field duration from 5 min to 30 min on the chaining formation was also explored.
To explore the DEP behaviors of phytoplankton under environmentally-realistic conditions, mixtures of these phytoplankton species forming artificial communities were also tested; first, binary mixtures containing equal proportions of cells (1:1): C. reinhardtii + C. meneghiniana, C. reinhardtii + Synechocystis sp. and C. meneghiniana + Synechocystis sp. Cell concentrations were kept at 2.5 × 106 cells·mL−1. Then, mixtures containing C. reinhardtii or C. meneghiniana and 10-times excess of Synechocystis sp. were tested: the concentration of C. reinhardtii and C. meneghiniana was fixed at 2.5 × 106 cells·mL−1, while the concentration of Synechocystis sp. was fixed at 2.5 × 107 cells·mL−1. For all experiments with cell mixtures, 2 AC field intensities of 15 and 25 V·mm−1 and 3 frequencies of 100 Hz, 1 kHz and 500 kHz were applied for 5 min. Each experiment was repeated 3 times.
2.4. Effect of AC Field on Membrane Permeability of the Cells
The possible effect of the AC field on the membrane permeability of the cells was investigated by flow cytometry (FCM) using propidium iodide (PI, Life Technologies Europe B.V, Zug, Switzerland) stain. FCM analyses were performed using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) with an Accuri CSampler (BD Biosciences, San Jose, CA, USA). The number of cells and PI fluorescence emitted at 585 nm after excitation with a 488-nm argon laser were acquired and analyzed using the BD Accuri C6 Software 264.15 (BD Biosciences, San Jose, CA, USA). Data were collected to 10,000 events for each sample. C. reinhardtii and C. meneghiniana treated with 50 µM hydrogen peroxide (Life Technologies Europe B.V, Zug, Switzerland) were used as positive controls, whereas for Synechocystis sp., the cell suspension was heated at 70 °C for 20 min. For all phytoplankton species, the negative control corresponds to the cell suspension with no AC field treatment. PI at 7 µM was added and incubated for 30 min in negative and positive controls, as well as in all samples prior to FCM analysis.
2.5. Microscopy and Image Analysis
The DEP behavior of the cells in the microfluidic chamber was observed at the bottom of the microfluidic channel, in the center between the electrodes on an area of approximately 250 μm × 250 μm with an optical microscope (BX61, OLYMPUS, Volketswil, Switzerland) using a digital camera (XC30, OLYMPUS, Volketswil, Switzerland) and the Cellsens software (Cellsens dimension OLYMPUS, Volketswil, Switzerland) provided. For each combination of tested parameters (field intensity, frequency, duration, concentration of phytoplankton and mixtures of phytoplankton), microscopic images were collected every 5 min for 30 min after AC field application.
2.6. Cell Chaining Efficiency Determination
The efficiency of chain formation was characterized by the percentage of cells in chains and the length of the chains determined using the image processing program ImageJ (National Institute of Mental Health, Bethesda, MD, USA). The percentage of cells in chains was calculated for each image according to:
For each condition, three replicate measurements were performed. The mean of the percentage of cells in the chain and the standard deviation were calculated and compared. In addition, the length of the chains was also determined. For each condition, the fraction of cells forming chains in the range of [0 to 5], [6 to 10], [11 to 15], [16 to 20], [21 to 25], [26 to 30] and [>30] cells per chains are reported as follows:
2.7. Measurements of Cell Size and Zeta Potential
To calculate the effective polarizability of C. reinhardtii, C. meneghiniana and Synechocystis sp., the values of the zeta-potential and cell size in Geneva Lake water of each phytoplankton species are needed. Measurements of phytoplankton hydrodynamic size and zeta-potential were carried out by a Zetasizer Nano-ZS (Malvern, Renens, Switzerland). Three replicates of 7 measurements each were performed for both phytoplankton species, and the means are reported in Table 1.

Table 1.
Numerical parameters used to calculate the effective polarizability of green microalga C. reinhardtii, cyanobacterium Synechocystis sp. and diatom C. meneghiniana.
2.8. Modelling of Chaining Efficiency
The chaining efficiency of the different phytoplankton species was modeled by using the common multishell model [19]. Numerical parameters used to determine the real part of the Clausius–Mossotti factor (Equation (2)) are listed in Table 1. The parameters used to calculate the effective polarizability for C. reinhardtii were assumed to be equivalent to those of Chlorella vulgaris [30] and those for Synechocystis sp. to Escherichia coli [31]. Since no data were available for C. meneghiniana, C. reinhardtii parameters were taken except for the numerical parameters related to the cell wall. Cell wall-related parameters for C. meneghiniana were approximated by the values known for SiO2 [32], since its frustule is made by silica [33].
For multishell particles, such as the green microalgae, bacteria or diatoms used in this study, complex cell permittivity is given by [8,9]:
where is the complex permittivity of the cell wall, is the effective complex permittivity of the cytoplasm, is the counter-ionic layer dielectric constant and Ro and R are the outer radius and the inner radius of the cell [8,20]. The complex permittivity for the different shells was expressed as follows [8,19]:
Cytoplasm:
Cell membrane:
Cell wall:
Counter-ionic layer:
where:
with medium permittivity:
2.9. Statistical Analysis
Statistical differences of chaining efficiency under different AC field treatments were determined using one-way ANOVA, the Student–Neumann–Keuls method for all pairwise multiple comparisons and performed in Sigma Plot 11 (Systat Software Inc., San Jose, CA, USA). The statistically significant differences (p < 0.05) are indicated by different letters.
3. Results
3.1. Collection and “Pearl” Chain Formation by the Individual Microorganisms
To understand the DEP phenotypes of individual cells representative of the phytoplankton, the chaining efficiency and chain length were explored at different AC field intensities and frequencies, durations, as well as different cell concentrations.
3.1.1. Effect of AC Field Frequency and Intensity on Chaining Efficiency and Chain Length
For low frequencies from 100 Hz to 1 kHz, no change in chaining efficiency, as the percentage of cells in chains, was observed for C. reinhardtii at 15 V·mm−1 with increasing frequency (around 3% at 100 Hz and 1 kHz), while an increase of the chaining efficiency was obtained from 0.8% ± 0.2% and 9.1% ± 1.9% at 100 Hz to 18.8% ± 1.0% and 62.7% ± 3.2% at 1 kHz at 20 and 25 V·mm−1, respectively (Figure 1a).
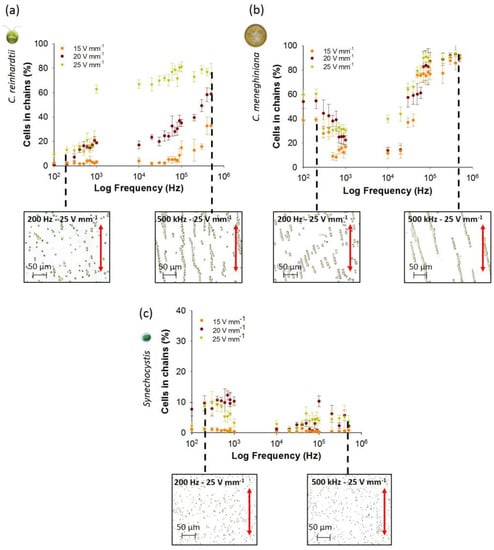
Figure 1.
Effect of AC field intensity and low frequency on the chaining efficiency obtained with the coplanar electrode after 5 min of electric field application for: (a) C. reinhardtii; (b) C. meneghiniana; (c) Synechocystis sp. Micrographs illustrate the chaining pattern, parallel to the electric field direction given by the red arrow, for each phytoplankton species at 200 Hz and 500 kHz, field intensity of 25 V·mm−1. Initial concentration of phytoplankton species: 5 × 106 cells·mL−1 C. reinhardtii and C. meneghiniana; 5 × 107 cells·mL−1 Synechocystis sp.
For diatom C. meneghiniana, a decrease of the chaining efficiency was observed with the increase of the frequency from 100 Hz to 1 kHz for the three AC field intensities (from 59.7% ± 4.4% at 100 Hz to 30.4% ± 1.8% at 1 kHz and 25 V·mm−1) (Figure 1b). Synechocystis sp. showed lower chaining efficiency than diatom and green alga at all AC field intensities and frequencies (Figure 1c). At frequencies below 900 Hz and AC field intensities higher than 20 V·mm−1, the highest chaining efficiency was observed for C. meneghiniana as compared with C. reinhardtii and Synechocystis sp. (Figure 1). When comparing the chaining behavior of C. meneghiniana and C. reinhardtii at low frequencies, the opposite trend was found. Increasing the frequencies from 100 Hz to 1 kHz induced an increase of the chaining efficiency in C. reinhardtii and a decrease in C. meneghiniana. This phenomenon of high chaining efficiency at very low frequency of 100 Hz for C. meneghiniana could be caused by augmentation of the particle collection by fluid AC electroosmosis (ACEO). The ACEO is a complex phenomenon of coupling field gradients and fluid fluxes, where the gradient of the applied electric field between the electrodes induces a fluid motion away from the electrodes, which drags and concentrates the cells in the middle of the experimental chamber [34], as reported earlier with latex particles at low electric field frequencies [20,35]. It is likely that it enhances the chaining, and the larger cell type (C. meneghiniana) will be more susceptible to such fluid drag.
During the characterization of chaining at higher frequencies (Figure 1) for C. reinhardtii, an augmentation of the chaining efficiency was obtained by increasing the frequencies from 10 kHz to 500 kHz and the AC field intensities from 15 to 25 V·mm−1. A maximum of around 80% of cells in chains was reached from 50 kHz to 500 kHz at 25 V·mm−1, while lower chaining efficiency was reached at lower AC field intensities of 15 and 25 V·mm−1 (Figure 1a). These results are consistent with data reported earlier for yeast cells and bacteria [9,11,19,34], where an increase of the cell collection was observed at higher AC field intensity. For example, the increase of the capture efficiency of E. coli, Salmonella and Pseudomonas sp. from 90% to 99% was observed by increasing the AC field intensity from 67 V·cm−1 to 84 V·cm−1 [11]. Similarly, an increase from 50 to 200 V·cm−1 of the electric field intensity enhanced the chain length of yeast cells at all tested frequencies [19]. Unlike C. reinhardtii, C. meneghiniana showed no significant differences in chaining efficiency between the three tested AC field intensities at all frequencies (Figure 1b). An increase of the percentage of cells in chains occurred by increasing the frequency from 10 kHz to 100 kHz to reach a plateau of 90% of cells in chains from frequencies above 100 kHz. Chaining efficiencies for Synechocystis sp. were below 5% at all AC field intensities and frequencies from 10 kHz to 500 kHz (Figure 1c), as observed at low frequencies.
The number of cells in chains was also compared to outline the differences in the distribution of the chain lengths of all three phytoplankton species for a field intensity of 15 and 25 V·mm−1 and frequencies of 100 Hz, 1 kHz and 500 kHz (Figure 2). C. reinhardtii formed long chains of more than 30 cells only at 500 kHz and 25 V·mm−1. At all other AC field intensity/frequency combinations, C. reinhardtii chains contained maximum 10 cells per chain (Figure 2a). At the highest frequency of 500 kHz, C. meneghiniana formed chains of 20 cells per chain at 15 V·mm−1 and chains of 30 cells and more at 25 V·mm−1 (Figure 2b), while chains of less than five cells per chain were observed at low frequencies for both field intensities. C. meneghiniana formed the longest chains, as they also showed the highest chaining efficiency as the percentage of cells in chains. By contrast, Synechocystis sp. formed short chains no longer than five to 10 cells at different AC field intensities and frequencies (Figure 2c). Taken together, the results demonstrate that the chaining efficiency and the length of the chains in freshwater phytoplankton decreased in the order: C. meneghiniana > C. reinhardtii >> Synechocystis sp. As both C. reinhardtii and C. meneghiniana exhibited the highest chaining efficiency and longer chain formation at high frequency and high AC field intensity of 500 kHz and 25 V·mm−1, this combination of AC field intensity and frequency was used in the further experiments to follow the effect of the cell concentration and the duration of the AC field on the chaining efficiency, as well as on the cell viability.
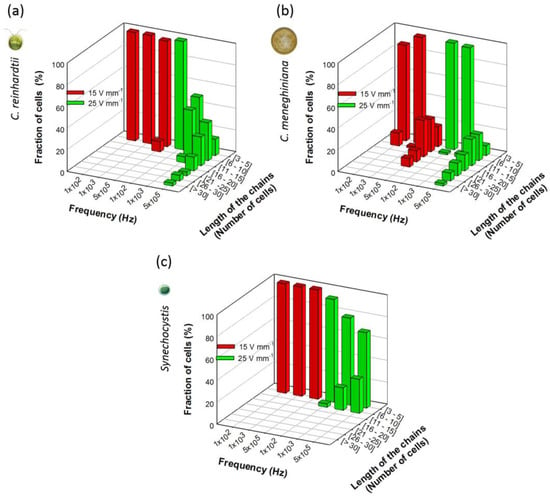
Figure 2.
Length of the chains obtained at 15 and 25 V·mm−1 for 100 Hz, 1 kHz and 500 kHz for: (a) C. reinhardtii; (b) C. meneghiniana; and (c) Synechocystis sp. C. reinhardtii and C. meneghiniana initial concentrations: 5 × 106 cells·mL−1; Synechocystis sp.: 5 × 107 cells·mL−1.
The obtained chaining efficiency results were consistent with the effective polarizability calculated with the multishell model. Negative values of −0.5 and −0.4 for Re|CM(ω)| of C. meneghiniana and C. reinhardtii and a positive value of 0.27 for Synechocystis sp. were determined from 100 Hz to 500 kHz. The obtained values were in accordance with the literature showing the numerical limits of the real part of the Clausius–Mossotti factor: −0.5 < Re|CM(ω)| < 1, independently of the particles’ properties [36]. These results imply that C. meneghiniana and C. reinhardtii might undergo negative DEP as opposed to positive DEP for Synechocystis sp. Indeed, several studies demonstrated the relation between the sign of Re|CM(ω)| and the sign of the DEP [37,38,39]. Positive DEP corresponding to the attraction of the particles to the maxima field regions is observed when Re|CM(ω)| > 0, while negative DEP to the repulsion of the particles from the high-field region is observed when Re|CM(ω)| < 0. For example, calculation of Re|CM(ω)| of E. coli predicted positive values of this factor and, thus, positive dielectrophoresis, p-DEP, at frequencies from 30 kHz to 100 MHz, which was confirmed by the attraction of the bacteria towards the regions of high field strength at the same frequencies [38]. Similarly, calculations of the effective polarizability of latex particles showed positive Re|CM(ω)| at frequencies below 106 Hz, while negative Re|CM(ω)| at frequencies higher than 106 Hz [38]. In experimental observations, low frequencies (down to 10 kHz) induced p-DEP to the particles, while negative DEP, n-DEP, was experienced at high frequencies (up to 20 MHz) [39]. Both can lead to chaining in the direction of the field [35]. Theoretical calculations and experimental observations in the present study were also consistent with those for protozoa Cryptosporidium. Indeed, the previously estimated Re|CM(ω)| of Cryptosporidium changed from positive to negative values at 3 MHz, which was confirmed with experiments where the protozoa showed p-DEP from 100 kHz to 1 MHz and n-DEP above 4 MHz [37].
The differences in Re|CM(ω)| values could therefore explain the difference between the high chaining efficiency of C. meneghiniana and C. reinhardtii and the low chaining efficiency of Synechocystis sp. Indeed the capability of cells to form chains seemed to be clearly correlated to their effective polarizability, as shown previously [18,19,35,38,40].
3.1.2. Effect of Cell Concentrations on Chaining Efficiency
For all of the tested microorganisms, low chaining efficiencies were found at cell concentrations of 106 cell·mL−1 (Figure S1): less than 40% of cells of C. reinhardtii and C. meneghiniana were in chains, while no chain formation was observed for Synechocystis sp. The increase of cell concentration to 3 × 106 cells·mL−1 resulted in the capture of 80% of cells for C. reinhardtii; however, a further increase of the cell concentrations resulted in no significant increase of the percentage of chained cells (Figure S1a). The continuous rise of the percentage of the cells captured in chains was found for C. meneghiniana, reaching almost 100% of cells in chains at 8 × 106 cells·mL−1 (Figure S1b). For Synechocystis sp., a 10× higher concentration (50 × 106 cells·mL−1) was necessary to obtain inclusion of about 10% of cells in chains (Figure S1c). As the distance between the cells enters in the calculation of the DEP chaining force (Equation (1)) through the coefficient C [18,19], the size, as well as the concentration of the cells influence the distance between the cells and indirectly the chaining efficiency, as previously observed with the increase of the assembly rate by increasing the yeast cell concentration [19]. The small cell size (<2 µm) of Synechocystis sp. could explain the necessity to increase their concentration compared to the two other phytoplankton species (C. reinhardtii and C. meneghiniana) and thus to decrease the distance between these weakly polarizable cells.
3.1.3. Effect of AC Field Duration on Chaining Efficiency
The AC field duration was also found to have a cell-specific effect on the efficiency of chaining. A collection time of 5 min resulted in a high percentage of C. reinhardtii (73.6% ± 5.8%) and C. meneghiniana (90.1% ± 1.2%) assembled in chains (Figure 3). However, a further increase of the time to 30 min has no effect on the percentage of cells captured in chains for both phytoplankton species. By contrast, the enhanced duration of AC field from 5 min and 15 min allowed a significant increase on the cell chaining efficiency of Synechocystis sp. from 6.2% ± 2.1% to 42.9% ± 4.8%, keeping steady until 30 min (Figure 3). The above observations were in agreement with the effective polarizability of the phytoplankton species. Rapid chaining formation was observed for the two phytoplankton species with low effective polarizability, while for the cells with high effective polarizability, such as Synechocystis sp., the duration of AC field application had a key role in the formation of chains using coplanar electrodes. Increasing collection time for cells with positive effective polarizability seemed to lead to an increase of the chaining efficiency.
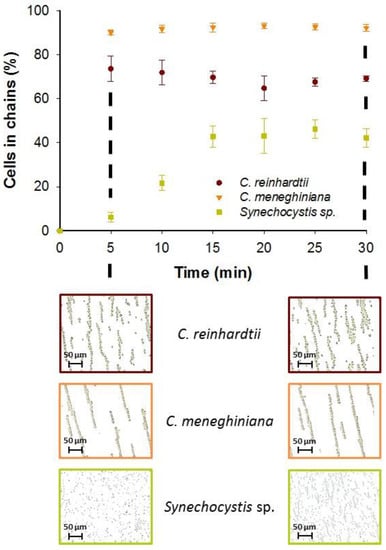
Figure 3.
Effect of AC field duration on the chaining efficiency at 25 V·mm−1 and 500 kHz for C. reinhardtii, C. meneghiniana and Synechocystis sp. Micrographs obtained for each phytoplankton species after 5 min and 30 min of AC field application in Geneva Lake water. Initial concentration of C. reinhardtii and C. meneghiniana: 5 × 106 cells·mL−1; Synechocystis sp.: 5 × 107 cells·mL−1.
Overall, these results confirm the cell-specific “dielectrophoretic phenotype” and show the importance of the optimization of field intensity, frequency and duration for each cell type in order to achieve the best collection conditions.
3.1.4. Effect of the AC Field on the Cell Viability
No DEP-induced damages to the membrane permeability for the studied phytoplankton species was observed at 5 min of AC field duration at 25 V·mm−1 and 500 kHz (Figure S2). However, for the same combination of AC field intensity and frequency, a longer AC field duration of 30 min induced a shift in the PI fluorescence intensity for all phytoplankton species, showing significant membrane permeability damages. The membrane of C. reinhardtii seemed to be more impacted by the AC field after 30 min than the other two phytoplankton species, as their FCM cytograms showed a higher proportion of affected cells (Figure S2a) compared to C. meneghiniana and Synechocystis sp. (Figure S2b). Previous studies showed that the electric field could induce damage to yeast cells or bacteria with a long time AC field application [28,41]. For example, the number of viable yeast cells was reduced by 56.8% to 89.7% with a DEP treatment longer than 4 h [28], and trapping of genetically-modified E. coli was only workable for an AC field application of less than 10 min [41]. These results demonstrate the importance of the careful selection of the AC field duration to avoid damage of cell membrane integrity. Taking into account all of these results, an AC field duration of 5 min was preferred, since it allows one to achieve high chaining efficiency for C. reinhardtii and C. meneghiniana, while avoiding membrane damage.
3.2. Collection and “Pearl” Chain Formation by Artificial Communities
In natural waters, phytoplankton is composed of different species at different concentrations. In this section, mixtures containing equal concentrations of C. reinhardtii, C. meneghiniana or Synechocystis sp. and mixtures of C. reinhardtii or C. meneghiniana and 10-fold excess of Synechocystis sp. were used to evaluate the AC DEP-driven collection and chaining of the simple artificial community of phytoplankton species.
3.2.1. Chaining of Binary Artificial Communities in Equal Concentration
No significant differences in the chaining efficiency between 100 Hz and 1 kHz at 15 and 25 V·mm−1 were observed for artificial communities containing equal concentrations of C. reinhardtii and C. meneghiniana (Figure 4a).
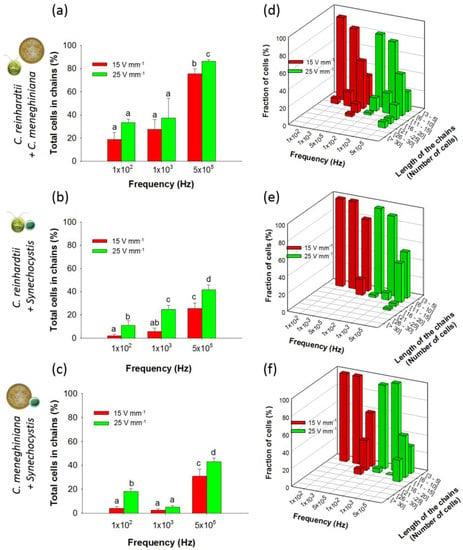
Figure 4.
Chaining efficiency and length of mixed chains (1:1) at 15 and 25 V·mm−1 and at 100 Hz, 1 kHz and 500 kHz after 5 min of AC field application. Histograms represent the total percentage of cells in chains for: (a) C. reinhardtii + C. meneghiniana; (b) C. reinhardtii + Synechocystis sp.; and (c) C. meneghiniana + Synechocystis sp.; 3D histograms represent the fraction of cells in mixed chains of different lengths for: (d) C. reinhardtii + C. meneghiniana; (e) C. reinhardtii + Synechocystis sp.; and (f) C. meneghiniana + Synechocystis sp.; Different letters indicate significant differences between the measurements obtained by one-way ANOVA and the Student–Neumann–Keuls method (p < 0.05). Cell concentrations: 2.5 × 106 cells·mL−1.
Indeed, from 100 Hz to 1 kHz at both field intensities, chaining efficiency was always lower than 40% (Figure 4a). However, at a much higher frequency of 500 kHz, the chaining increased, substantially reaching 86.3% ± 1.5% at 25 V·mm−1 (Figure 4a). The length of the chains formed in the mixture containing C. reinhardtii and C. meneghiniana (Figure 4d) was comparable with those found for the individual species (Figure 2a,b). Furthermore, a higher number of long chains was observed for C. reinhardtii in presence of C. meneghiniana as compared with C. reinhardtii alone.
By contrast, adding Synechocystis sp. to C. reinhardtii or C. meneghiniana resulted in low chaining efficiencies at frequencies below 100 kHz at both AC field intensities (Figure 4a,b). An increase of the frequencies augmented the chaining efficiency to 45% for the community containing equivalent proportions of cyanobacteria and diatom or cyanobacteria and green alga at 500 kHz and 25 V·mm−1 (Figure 4a,b). However, the chains formed in such mixtures of Synechocystis sp. and C. reinhardtii or C. meneghiniana were shorter (Figure 4e,f).
Mixing C. meneghiniana and C. reinhardtii showed an increase of the percentage of C. reinhardtii in chains compared with the results obtained for C. reinhardtii alone, while a comparable percentage of C. meneghiniana in chains was obtained except at low frequencies (Figure S3). At 1 kHz, most of the chains were formed by C. reinhardtii cells (Figure S4a), which is consistent with the low chaining efficiency obtained for C. meneghiniana alone at this frequency (Figure 1b). However, for the other combination of AC field parameters, the chains were formed by the same proportion of C. meneghiniana and C. reinhardtii (Figure S4a). When mixed with Synechocystis sp., a decrease of the percentage of C. reinhardtii or C. meneghiniana in chains was observed (Figure S3c), and the chains were only formed by C. reinhardtii or C. meneghiniana cells (Figure S4b).
The above results showed that the presence of C. meneghiniana in the suspension increased the capability of C. reinhardtii to form chains at low frequencies and AC field intensities, while the presence of Synechocystis sp. decreased the capacity of C. reinhardtii and C. meneghiniana to form chains at all tested frequencies and AC field intensities. For the mixture of C. reinhardtii and C. meneghiniana, no significant changes in chaining were observed. Because of their similar polarizability, C. reinhardtii and C. meneghiniana aligned together in the direction of the electric field in the tested frequency range. Despite the difference in polarizability between Synechocystis sp. and C. reinhardtii or C. meneghiniana, no alternating chains were formed for mixtures containing Synechocystis sp., as previously observed with mixtures of yeast cells and polystyrene beads of lower and higher polarizability than the media using 2-mm gap coplanar copper electrodes [22]. The lack of Synechocystis sp. chaining, in parallel or in the transversal direction to the AC field, could be due to its low polarizability compared to the two other phytoplankton species, as well as a low cell density in Synechocystis sp., as cells’ concentration and size are important parameters affecting in the chaining efficiency, as mentioned above.
3.2.2. Chaining of Binary Artificial Communities Containing an Excess of Cyanobacteria
Suspensions with cyanobacteria excess were tested in this section to simulate episodically cyanobacteria blooms occurring in natural freshwaters. Under a 10-fold excess of cyanobacteria (2.5 × 106 cells·mL−1 of C. reinhardtii or C. meneghiniana and 2.5 × 107 cells·mL−1 of Synechocystis sp.), lower chaining efficiency at all frequencies and AC field intensities (Figure 5a,b) was observed as compared with the results found for artificial communities containing equal concentrations of two phytoplankton species. While previously, the chaining efficiencies at 25 V·mm−1 and 500 kHz reached 45% with 1:1 community containing Synechocystis sp. (Figure 4b,c), here, the chaining efficiencies was found to be only 26.1% ± 4.3% for C. reinhardtii and 17.5% ± 3.1% for C. meneghiniana in the presence of 2.5 × 107 cells·mL−1 of Synechocystis sp. (Figure 5a,b). Under comparable DEP conditions, C. reinhardtii and Synechocystis sp. formed shorter chains at high AC field intensity and high frequency than C. reinhardtii alone (Figure 5c). Identical conclusions were made with the artificial community consisting of C. meneghiniana and Synechocystis sp. (Figure 5d). A decrease of the percentages of C. reinhardtii and C. meneghiniana in chains in the community containing cyanobacteria was observed (Figure S5a,c) compared to each phytoplankton alone, while the percentage of Synechocystis sp. increased when mixed with both C. reinhardtii and C. meneghiniana (Figure S5b,d). At most frequencies and AC field intensities, chains of phytoplankton mixtures containing Synechocystis sp. were only formed by C. reinhardtii, except at 500 kHz (Figure S4d), or by C. meneghiniana, except for 100 Hz and 500 kHz at 25 V·mm−1 (Figure S4e).
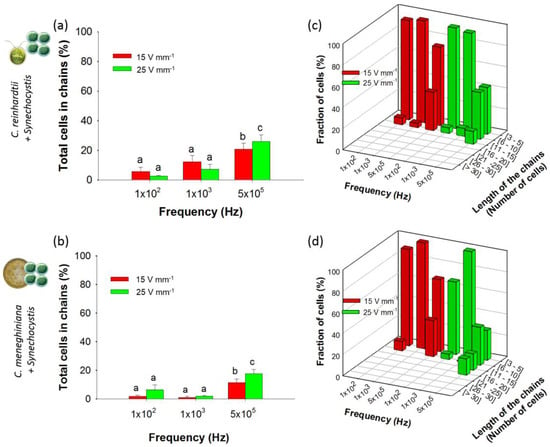
Figure 5.
Chaining efficiency and structure of mixed chains (1:10) at 15 and 25 V·mm−1 and at 100 Hz, 1 kHz and 500 kHz after 5 min of AC field application. Histograms represent the total percentage of cells in chains for: (a) C. reinhardtii + Synechocystis sp.; and (b) C. meneghiniana + Synechocystis sp. 3D histograms represent the fraction of cells in mixed chains of different lengths for: (c) C. reinhardtii + Synechocystis sp.; and (d) C. meneghiniana + Synechocystis sp. Different letters indicate significant differences between the measurements obtained by one-way ANOVA and the Student–Neumann–Keuls method (p < 0.05). Concentrations of C. reinhardtii and C. meneghiniana: 2.5 × 106 cells·mL−1; Synechocystis sp. concentration: 2.5 × 107 cells·mL−1.
Overall, increasing the proportion of Synechocystis sp. in the artificial communities resulted in lowering the chain formation by C. reinhardtii and C. meneghiniana. Furthermore, under these conditions, Synechocystis sp. collection and chaining were observed when most of the cells of cyanobacterium were incorporated into the chains of green alga or diatom. Although we are not aware of previous studies of DEP of live cells mixtures that can be used for direct comparison, the findings of this study are consistent with the previous reports showing that small latex particles become dielectrophoretically-trapped between larger ones [24,42]. Primary chains of large particles were rapidly formed when the small particles were pulled in by DEP into the high-field intensity regions created by the pairs of large particles [36]. Moreover, it was shown that permanent chains of small and large particles of opposite charges only occurred when the small particles were in excess in the suspension [42]. The lack of alternating chains of Synechocystis sp., as observed in mixtures of particles with opposite polarizabilities [22], was attributed to the insufficient concentration or polarizability in Synechocystis sp. However, no alternating chains of Synechocystis sp. were observed even when this species was in excess in mixtures, which seemed to be due to its low polarizability. The results demonstrate how DEP-driven capturing and chaining under realistic conditions could be done while accounting for possible interactions between phytoplankton species existing in aquatic environments. These results highlight the possibility to capture selectively the phytoplankton species and to develop future biosensors based on the artificial communities better targeted to complex environmentally-relevant phytoplankton systems. By using various electrode configurations, DEP manipulation could allow one: (i) to discriminate different phytoplankton species depending on their effective polarizability and to enable their manipulation, such as specific collection or separation in freshwater; and (ii) to immobilize the cells in 1D or 2D arrays. Indeed, most of the present tools for water quality assessment are based on biosensors developed with only one phytoplankton species [7], whereas phytoplankton communities are composed of a large number of different microorganisms [1]. Future DEP-based biosensors could make use of the opportunity to form a 2D array of mixed phytoplankton species from freshwater, while allowing fluorescence microscopic observations, as we previously demonstrated with C. reinhardtii [17].
4. Conclusions
This study compares the DEP behavior of representative phytoplankton species alone and in mixture in order to evaluate the capability of DEP-driven selective collection and chaining on chip. Experimental results revealed different dielectrophoretic behavior for cyanobacteria and green alga or diatom in terms of the percentage of cells in chains and the length of the chains. Chaining efficiency and the length of chains of C. reinhardtii increased from 100 Hz to 500 kHz at all field intensities; C. meneghiniana showed a decrease of chaining efficiency from 100 Hz to 1 kHz followed by a significant increase of both chaining efficiency and chains length from 1 kHz to 500 kHz; while Synechocystis sp. showed low chaining efficiency at all frequency and field intensity combinations. The DEP behavior of these phytoplankton species was in agreement with the calculated values of the effective polarizability for these cells. Higher percentage of cells in chains and longer chains were observed for simple artificial communities containing C. reinhardtii and C. meneghiniana, while shorter chains and a lower percentage of cells in chains were obtained in all mixtures containing Synechocystis sp.
The results with individual cells and their mixtures representing artificial phytoplankton communities demonstrated the potential of DEP to manipulate representative phytoplankton species according to their DEP “phenotype” determined by their effective polarizability. In the future, we foresee the use of similar principles in new generations of devices for precise characterization of algal and microbial species in water. Separation or selective collection of the phytoplankton species from their natural environment could be conveniently achieved due to the differences in their polarizability. The precise control of frequency and fluid flows could allow continuous, on-line, cell species separation, detection and quantification for long-term monitoring of environmental water quality.
Supplementary Materials
The following are available online at www.mdpi.com/2079-6374/7/1/4/s1: Table S1: Major physico-chemical parameters of Geneva Lake water; Figure S1: Effect of cell concentration on the chaining efficiency at 25 V·mm−1 and 500 kHz; Figure S2: Effect of AC-field application on membrane integrity using propidium iodide; Figure S3: Chaining efficiency of individual phytoplankton species in equal proportion mixtures compared to results obtained when alone in freshwater; Figure S4: Composition of mixed chains (1:1 and 1:10) for two voltages, 15 and 25 V·mm−1; Figure S5: Chaining efficiency of individual phytoplankton species in mixtures with predominant concentration of Synechocystis sp. compared to chaining efficiency obtained when alone in freshwater.
Acknowledgments
The authors gratefully acknowledge Bettina Wagner from Swiss Federal Institute of Aquatic Science and Technology, Eawag for providing the C. meneghiniana culture. O. Velev gratefully acknowledges partial support from the NSF Research Triangle Materials Research Science and Engineering Center (DMR-1121107) and from NSF-ASSIST Nanosystems Engineering Research Center (EEC-1160483).
Author Contributions
C.S. carried out the experiments for chaining optimization with all phytoplankton species and manuscript writing. V.I.S. and O.D.V. were involved in the development of the project, co-supervised all experiments and provided scientific manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suthers, I.M.; Rissik, D. Plankton: A Guide to Their Ecology and Monitoring for Water Quality; CSIRO Publishing: Collingwood, Australia, 2008. [Google Scholar]
- Willén, E. Phytoplankton in water quality assessment—An indicator concept. In Water Quality Measurements Series; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 56–80. [Google Scholar]
- Thakur, R.K.; Jindal, R.; Singh, U.B.; Ahluwalia, A.S. Plankton diversity and water quality assessment of three freshwater lakes of mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environ. Monit. Assess. 2013, 185, 8355–8373. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Gopal, K. Biomonitoring of Water and Waste Water; Springer: Heidelberg, Germany, 2013. [Google Scholar]
- Su, L.A.; Jia, W.Z.; Hou, C.J.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Coute, A.; Livage, J.; Perrette, C.; Sicard, C. Micro-algal biosensors. Anal. Bioanal. Chem. 2011, 401, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Pasco, N.F.; Weld, R.J.; Hay, J.M.; Gooneratne, R. Development and applications of whole cell biosensors for ecotoxicity testing. Anal. Bioanal. Chem. 2011, 400, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Review article-dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, K.; Nahavandi, S.; Baratchi, S.; Mitchell, A.; Kalantar-Zadeh, K. Dielectrophoretic platforms for bio-microfluidic systems. Biosens. Bioelectron. 2011, 26, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac electrokinetics: A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 1998, 31, 2338–2353. [Google Scholar] [CrossRef]
- Jesus-Perez, N.M.; Lapizco-Encinas, B.H. Dielectrophoretic monitoring of microorganisms in environmental applications. Electrophoresis 2011, 32, 2331–2357. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Hughes, M.P.; Green, N.G. Separation of submicron bioparticles by dielectrophoresis. Biophys. J. 1999, 77, 516–525. [Google Scholar] [CrossRef]
- Suehiro, J.; Hamada, R.; Noutomi, D.; Shutou, M.; Hara, M. Selective detection of viable bacteria using dielectrophoretic impedance measurement method. J. Electrost. 2003, 57, 157–168. [Google Scholar] [CrossRef]
- Zhu, K.; Kaprelyants, A.S.; Salina, E.G.; Markx, G.H. Separation by dielectrophoresis of dormant and nondormant bacterial cells of mycobacterium smegmatis. Biomicrofluidics 2010, 4, 022809. [Google Scholar] [CrossRef] [PubMed]
- Allahrabbi, N.; Chia, Y.S.; Saifullah, M.S.; Lim, K.M.; Yung, L.Y. A hybrid dielectrophoretic system for trapping of microorganisms from water. Biomicrofluidics 2015, 9, 034110. [Google Scholar] [CrossRef] [PubMed]
- Suscillon, C.; Velev, O.D.; Slaveykova, V.I. Alternating current-dielectrophoresis driven on-chip collection and chaining of green microalgae in freshwaters. Biomicrofluidics 2013, 7, 24109. [Google Scholar] [CrossRef] [PubMed]
- Siebman, C.; Velev, O.D.; Slaveykova, V.I. Two-dimensional algal collection and assembly by combining ac-dielectrophoresis with fluorescence detection for contaminant-induced oxidative stress sensing. Biosensors 2015, 5, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Bhatt, K.H. On-chip micromanipulation and assembly of colloidal particles by electric fields. Soft Matter 2006, 2, 738–750. [Google Scholar] [CrossRef]
- Gupta, S.; Alargova, R.G.; Kilpatrick, P.K.; Velev, O.D. On-chip dielectrophoretic coassembly of live cells and particles into responsive biomaterials. Langmuir 2010, 26, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Lumsdon, S.O.; Kaler, E.W.; Velev, O.D. Two-dimensional crystallization of microspheres by a coplanar ac electric field. Langmuir 2004, 20, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Kadaksham, J.; Singh, P.; Aubry, N. Dielectrophoresis induced clustering regimes of viable yeast cells. Electrophoresis 2005, 26, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Giner, V.; Sancho, M.; Lee, R.S.; Martinez, G.; Pethig, R. Transverse dipolar chaining in binary suspensions induced by rf fields. J. Phys. D Appl. Phys. 1999, 32, 1182–1186. [Google Scholar] [CrossRef]
- Pethig, R.; Markx, G.H. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 1997, 15, 426–432. [Google Scholar] [CrossRef]
- Gupta, S.; Alargova, R.G.; Kilpatrick, P.K.; Velev, O.D. On-chip electric field driven assembly of biocomposites from live cells and functionalized particles. Soft Matter 2008, 4, 726–730. [Google Scholar] [CrossRef]
- Flores-Rodriguez, N.; Markx, G.H. Improved levitation and trapping of particles by negative dielectrophoresis by the addition of amphoteric molecules. J. Phys. D Appl. Phys. 2004, 37, 353–361. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Baratchi, S.; Tovar-Lopez, F.J.; Nahavandi, S.; Wlodkowic, D.; Mitchell, A.; Kalantar-Zadeh, K. On-chip separation of lactobacillus bacteria from yeasts using dielectrophoresis. Microfluid. Nanofluid. 2012, 12, 597–606. [Google Scholar]
- Markx, G.H.; Huang, Y.; Zhou, X.F.; Pethig, R. Dielectrophoretic characterization and separation of microorganisms. Microbiology 1994, 140, 585–591. [Google Scholar] [CrossRef]
- Yang, L.; Banada, P.P.; Bhunia, A.K.; Bashir, R. Effects of dielectrophoresis on growth, viability and immuno-reactivity of listeria monocytogenes. J. Biol. Eng. 2008, 2, 1. [Google Scholar] [CrossRef]
- Bhatt, K.H.; Velev, O.D. Control and modeling of the dielectrophoretic assembly of on-chip nanoparticle wires. Langmuir 2004, 20, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Wanichapichart, P.; Bunthawin, S.; Kaewpaiboon, A.; Kanchanapoom, K. Determination of cell dielectric properties using dielectrophoretic technique. ScienceAsia 2002, 28, 113–119. [Google Scholar] [CrossRef]
- Park, S.; Zhang, Y.; Wang, T.H.; Yang, S. Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab Chip 2011, 11, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.H.; Jones Stephen, H. The General Properties of Si, Ge, SiGe, SiO2 and Si3N4; Virginia Semiconductor: Fredericksburg, VA, USA, 2002. [Google Scholar]
- Kroger, N.; Poulsen, N. Diatoms-from cell wall biogenesis to nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Melvin, E.M.; Moore, B.R.; Gilchrist, K.H.; Grego, S.; Velev, O.D. On-chip collection of particles and cells by ac electroosmotic pumping and dielectrophoresis using asymmetric microelectrodes. Biomicrofluidics 2011, 5, 034113. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.H.; Grego, S.; Velev, O.D. An ac electrokinetic technique for collection and concentration of particles and cells on patterned electrodes. Langmuir 2005, 21, 6603–6612. [Google Scholar] [CrossRef] [PubMed]
- Ermolina, I.; Morgan, H. The electrokinetic properties of latex particles: Comparison of electrophoresis and dielectrophoresis. J. Colloid Interface Sci. 2005, 285, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Unni, H.N.; Hartono, D.; Yung, L.Y.L.; Ng, M.M.L.; Lee, H.P.; Khoo, B.C.; Lim, K.M. Characterization and separation of cryptosporidium and giardia cells using on-chip dielectrophoresis. Biomicrofluidics 2012, 6, 012805. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Morales, F.H.; Duarte, J.E.; Samitier-Marti, J. Bacterial handling under the influence of non-uniform electric fields: Dielectrophoretic and electrohydrodynamic effects. An. Acad. Bras. Cienc. 2008, 80, 627–638. [Google Scholar] [CrossRef]
- Green, N.G.; Morgan, H. Dielectrophoresis of submicrometer latex spheres. 1. Experimental results. J. Phys. Chem. B 1999, 103, 41–50. [Google Scholar] [CrossRef]
- Green, N.G.; Ramos, A.; Morgan, H.; Castellanos, A. Sub-micrometre ac electrokinetics: Particle dynamics under the influence of dielectrophoresis and electrohydrodynamics. Inst. Phys. Conf. Ser. 1999, 163, 89–92. [Google Scholar]
- Donato, S.S.; Chu, V.; Prazeres, D.M.; Conde, J.P. Metabolic viability of Echerichia coli trapped by dielectrophoresis in microfluidics. Electrophoresis 2013, 34, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Findenegg, G.H.; Velev, O.D. Co-assembly of oppositely charged particles into linear clusters and chains of controllable length. Sci. Rep. 2012, 2, 1004. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).