Implementing Silicon Nanoribbon Field-Effect Transistors as Arrays for Multiple Ion Detection
Abstract
:1. Introduction
2. Context and Basic Operation Principles
3. Materials and Methods
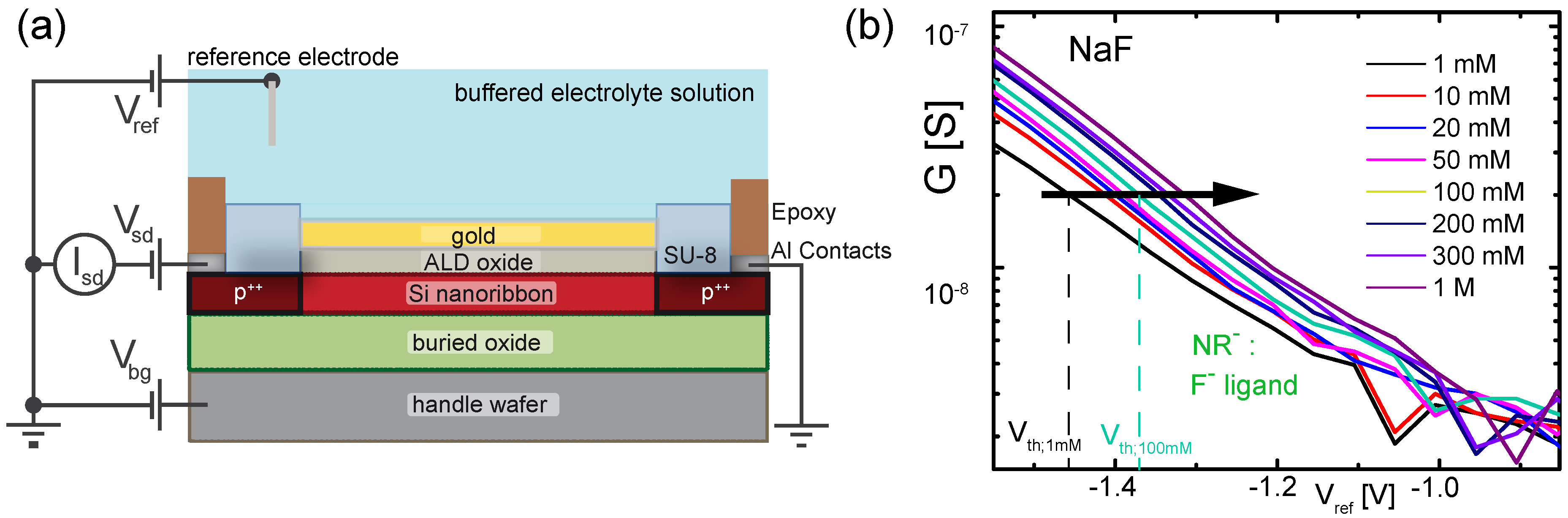
3.1. Device Fabrication
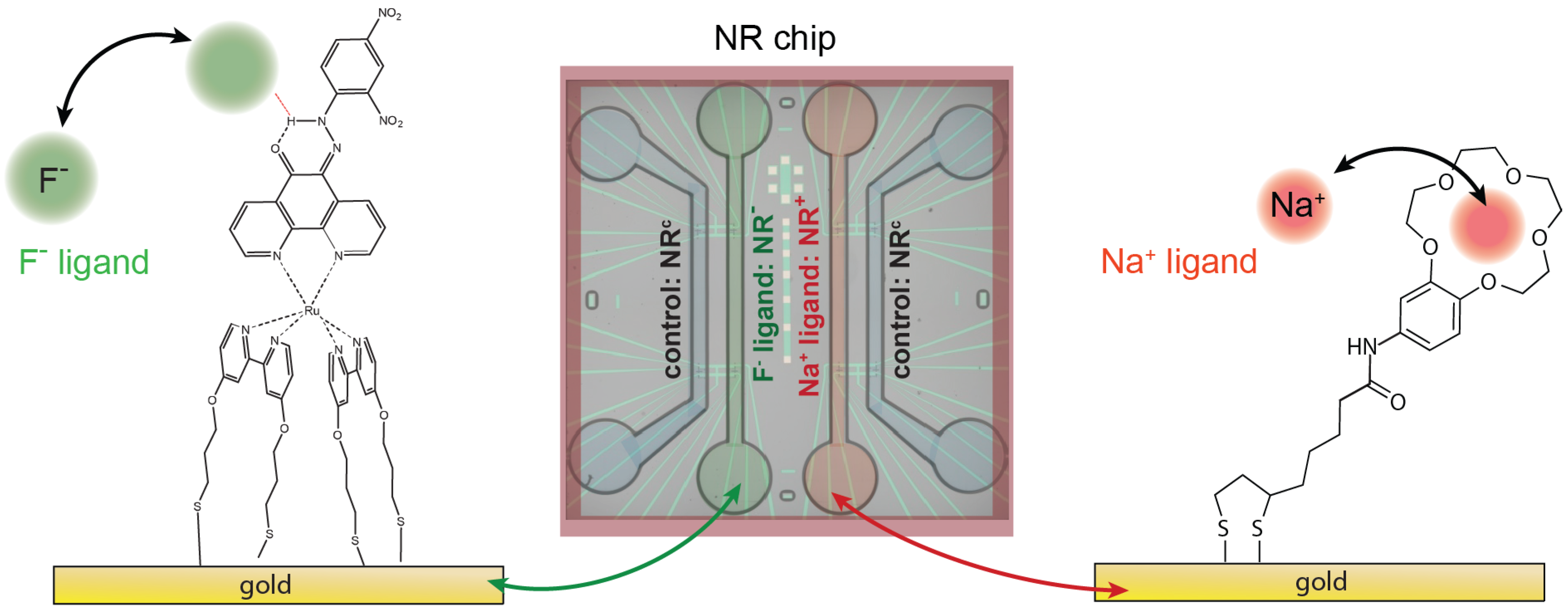
3.2. Surface Functionalization
3.3. Buffer Solutions
3.4. Measurement Setup
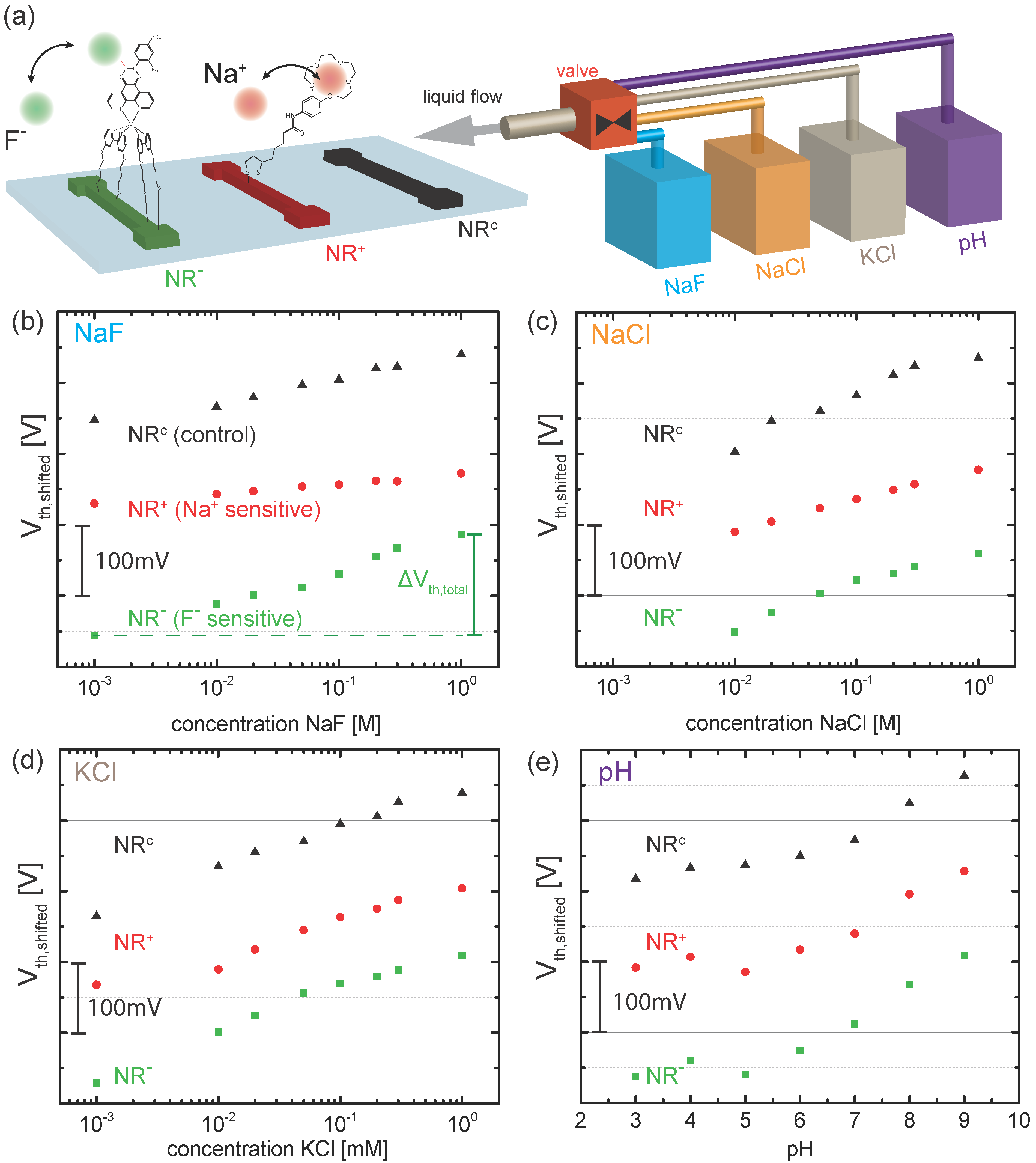
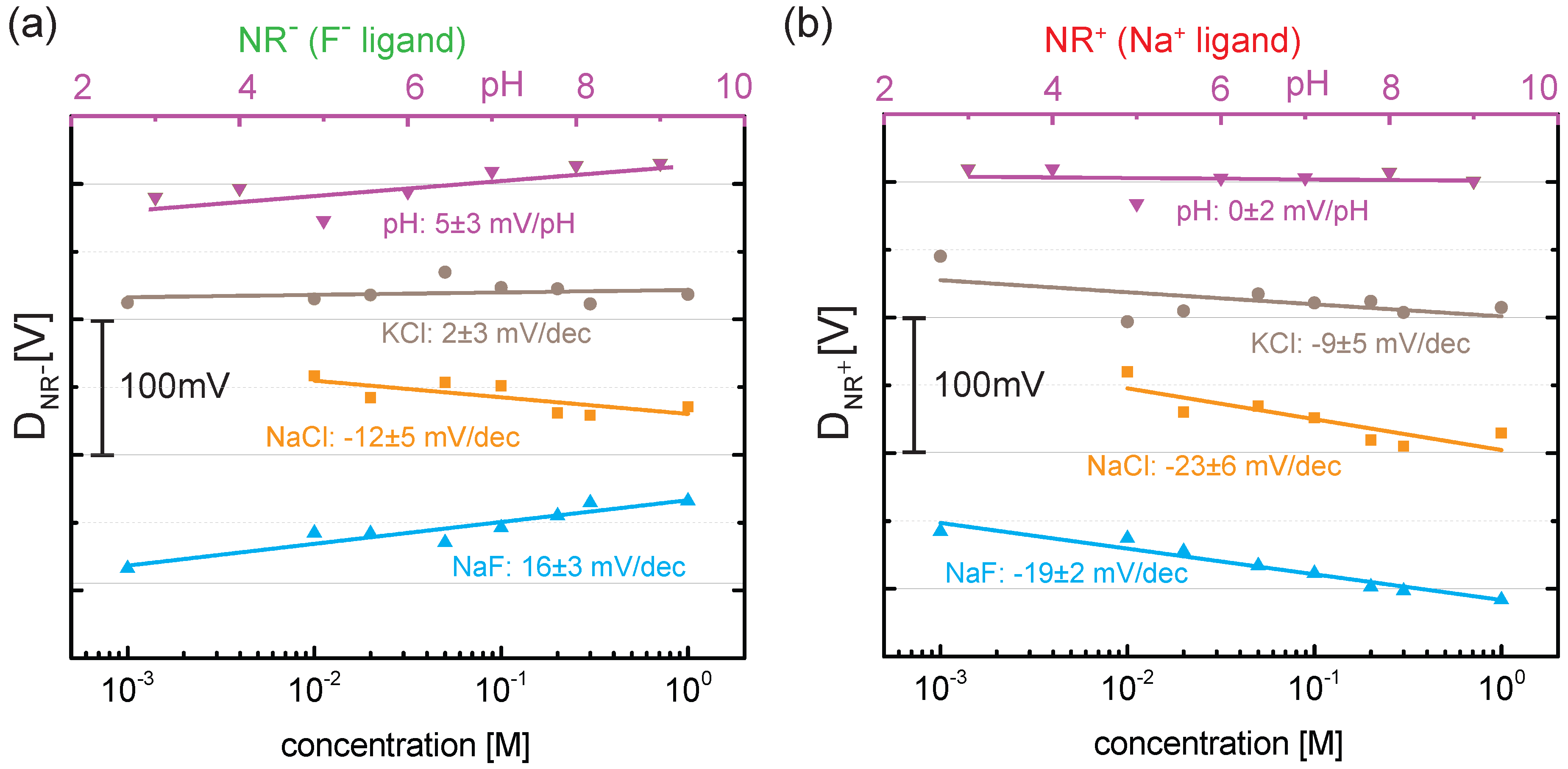
4. Results and Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FETs | field-effect transistor |
| Si | silicon |
| NRs | nanoribbons |
| PDMS | polydimethylsiloxane |
| ISFET | ion-sensitive field-effect transistor |
| ALD | atomic layer deposition |
| PTFE | polytetrafluoroethylene |
References
- Lerche, H.; Jurkat-Rott, K.; Lehmann-Horn, F. Ion channels and epilepsy. Am. J. Med. Genet. 2001, 106, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.F.; Green, K.N.; LaFerla, F.M. Calcium dysregulation in Alzheimer’s disease: Recent advances gained from genetically modified animals. Cell Calcium 2005, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Jouny, C.C.; Franaszczuk, P.J.; Bergey, G.K. Improving Early Seizure Detection. Epilepsy Behav. 2011, 22, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.M.; Garg, S.K.; Keep, R.F.; Albin, R.L.; Banerjee, R. Na+ and K+ ion imbalances in Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Meier, W. NCCR Molecular Systems Engineering. Available online: http://www.nccr-mse.ch (accessed on 12 February 2016).
- Wipf, M.; Stoop, R.L.; Tarasov, A.; Bedner, K.; Fu, W.; Wright, I.A.; Martin, C.J.; Constable, E.C.; Calame, M.; Schönenberger, C. Selective Sodium Sensing with Gold-Coated Silicon Nanowire Field-Effect Transistors in a Differential Setup. ACS Nano 2013, 7, 5978–5983. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.L.; Wipf, M.; Müller, S.; Bedner, K.; Wright, I.A.; Martin, C.J.; Constable, E.C.; Fu, W.; Tarasov, A.; Calame, M.; et al. Competing surface reactions limiting the performance of ion-sensitive field-effect transistors. Sens. Actuators B Chem. 2015, 220, 500–507. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bomer, J.G.; Carlen, E.T.; van den Berg, A. Al2O3/Silicon NanoISFET with Near Ideal Nernstian Response. Nano Lett. 2011, 11, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, J.M.; Hinz, W.; Rearick, T.M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J.H.; Johnson, K.; Milgrew, M.J.; Edwards, M.; et al. An Integrated Semiconductor Device Enabling Non-Optical Genome Sequencing. Nature 2011, 475, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Wipf, M.; Stoop, R.L.; Bedner, K.; Fu, W.; Guzenko, V.A.; Knopfmacher, O.; Calame, M.; Schönenberger, C. Understanding the Electrolyte Background for Biochemical Sensing with Ion-Sensitive Field-Effect Transistors. ACS Nano 2012, 6, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Janata, J. Chemical Sensors. Anal. Chem. 1992, 64, R196–R219. [Google Scholar] [CrossRef]
- Sudhölter, E.J.R.; van der Wal, P.D.; Skowronska-Ptasinska, M.; van den Berg, A.; Reinhoudt, D.N. Ion-Sensing Using Chemically-Modified ISFETs. Sens. Actuators 1989, 17, 189–194. [Google Scholar] [CrossRef]
- Rocher, V.; Jaffrezic-Renault, N.; Perrot, H.; Chevalier, Y.; Perchec, P.L. Nitrate-Sensitive Field-Effect Transistor with Silica Gate Insulator Modified by Chemical Grafting. Anal. Chim. Acta 1992, 256, 251–255. [Google Scholar] [CrossRef]
- Reinhoudt, D.N.; Engbersen, J.F.J.; Brzozka, Z.; van der Vlekkert, H.H.; Honig, G.W.N.; Holterman, H.A.J.; Verkerk, U.H. Development of Durable K+-Selective Chemically Modified Field Effect Transistors with Functionalized Polysiloxane Membranes. Anal. Chem. 1994, 66, 3618–3623. [Google Scholar] [CrossRef]
- Park, L.; Hur, Y.; Sohn, B. Effect of Membrane Structure on the Performance of Field-Effect Transistor Potassium-Sensitive Sensor. Sens. Actuators A Phys. 1996, 57, 239–243. [Google Scholar] [CrossRef]
- Luo, L.; Jie, J.; Zhang, W.; He, Z.; Wang, J.; Yuan, G.; Zhang, W.; Wu, L.C.M.; Lee, S.T. Silicon Nanowire Sensors for Hg2+ and Cd2+ Ions. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Clément, N.; Nishiguchi, K.; Dufreche, J.F.; Guerin, D.; Fujiwara, A.; Vuillaume, D. A silicon nanowire ion-sensitive field-effect transistor with elementary charge sensitivity. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Wang, Y.; Dai, P.; Li, T.; Gao, X.; Wang, Y.; Fan, C. Enhanced Sensing of Nucleic Acids with Silicon Nanowire Field Effect Transistor Biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed Electrical Detection of Cancer Markers with Nanowire Sensor Arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-Free Immunodetection with CMOS-Compatible Semiconducting Nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-Free Biomarker Detection from Whole Blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Elfström, N.; Karlström, A.E.; Linnros, J. Silicon Nanoribbons for Electrical Detection of Biomolecules. Nano Lett. 2008, 8, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y.; Rajan, N.K.; Routenberg, D.A.; Modis, Y.; Reed, M.A. Quantification of the affinities and kinetics of protein interactions using silicon nanowire biosensors. Nat. Nanotechnol. 2012, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bedner, K.; Guzenko, V.A.; Tarasov, A.; Wipf, M.; Stoop, R.L.; Just, D.; Rigante, S.; Fu, W.; Minamisawa, R.A.; David, C.; et al. pH Response of Silicon Nanowire Sensors: Impact of Nanowire Width and Gate Oxide. Sens. Mater. 2013, 25, 567–576. [Google Scholar]
- Baumgartner, S.; Vasicek, M.; Bulyha, A.; Heitzinger, C. Optimization of nanowire DNA sensor sensitivity using self-consistent simulation. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Heitzinger, C.; Vacic, A.; Reed, M.A. Predictive simulations and optimization of nanowire field-effect PSA sensors including screening. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Bedner, K.; Guzenko, V.A.; Tarasov, A.; Wipf, M.; Stoop, R.L.; Rigante, S.; Brunner, J.; Fu, W.; David, C.; Calame, M.; et al. Investigation of the dominant 1/f noise source in silicon nanowire sensors. Sens. Actuators B Chem. 2014, 191, 270–275. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst Limit in Dual-Gated Si-Nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Wipf, M.; Bedner, K.; Kurz, J.; Fu, W.; Guzenko, V.A.; Knopfmacher, O.; Stoop, R.L.; Calame, M.; Schönenberger, C. True Reference Nanosensor Realized with Silicon Nanowires. Langmuir 2012, 28, 9899–9905. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Academic Press, Inc.: Cambridge, MA, USA, 1989. [Google Scholar]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef] [PubMed]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoop, R.L.; Wipf, M.; Müller, S.; Bedner, K.; Wright, I.A.; Martin, C.J.; Constable, E.C.; Fanget, A.; Schönenberger, C.; Calame, M. Implementing Silicon Nanoribbon Field-Effect Transistors as Arrays for Multiple Ion Detection. Biosensors 2016, 6, 21. https://doi.org/10.3390/bios6020021
Stoop RL, Wipf M, Müller S, Bedner K, Wright IA, Martin CJ, Constable EC, Fanget A, Schönenberger C, Calame M. Implementing Silicon Nanoribbon Field-Effect Transistors as Arrays for Multiple Ion Detection. Biosensors. 2016; 6(2):21. https://doi.org/10.3390/bios6020021
Chicago/Turabian StyleStoop, Ralph L., Mathias Wipf, Steffen Müller, Kristine Bedner, Iain A. Wright, Colin J. Martin, Edwin C. Constable, Axel Fanget, Christian Schönenberger, and Michel Calame. 2016. "Implementing Silicon Nanoribbon Field-Effect Transistors as Arrays for Multiple Ion Detection" Biosensors 6, no. 2: 21. https://doi.org/10.3390/bios6020021
APA StyleStoop, R. L., Wipf, M., Müller, S., Bedner, K., Wright, I. A., Martin, C. J., Constable, E. C., Fanget, A., Schönenberger, C., & Calame, M. (2016). Implementing Silicon Nanoribbon Field-Effect Transistors as Arrays for Multiple Ion Detection. Biosensors, 6(2), 21. https://doi.org/10.3390/bios6020021






