AC and Phase Sensing of Nanowires for Biosensing
Abstract
:1. Introduction
2. Materials and Methods
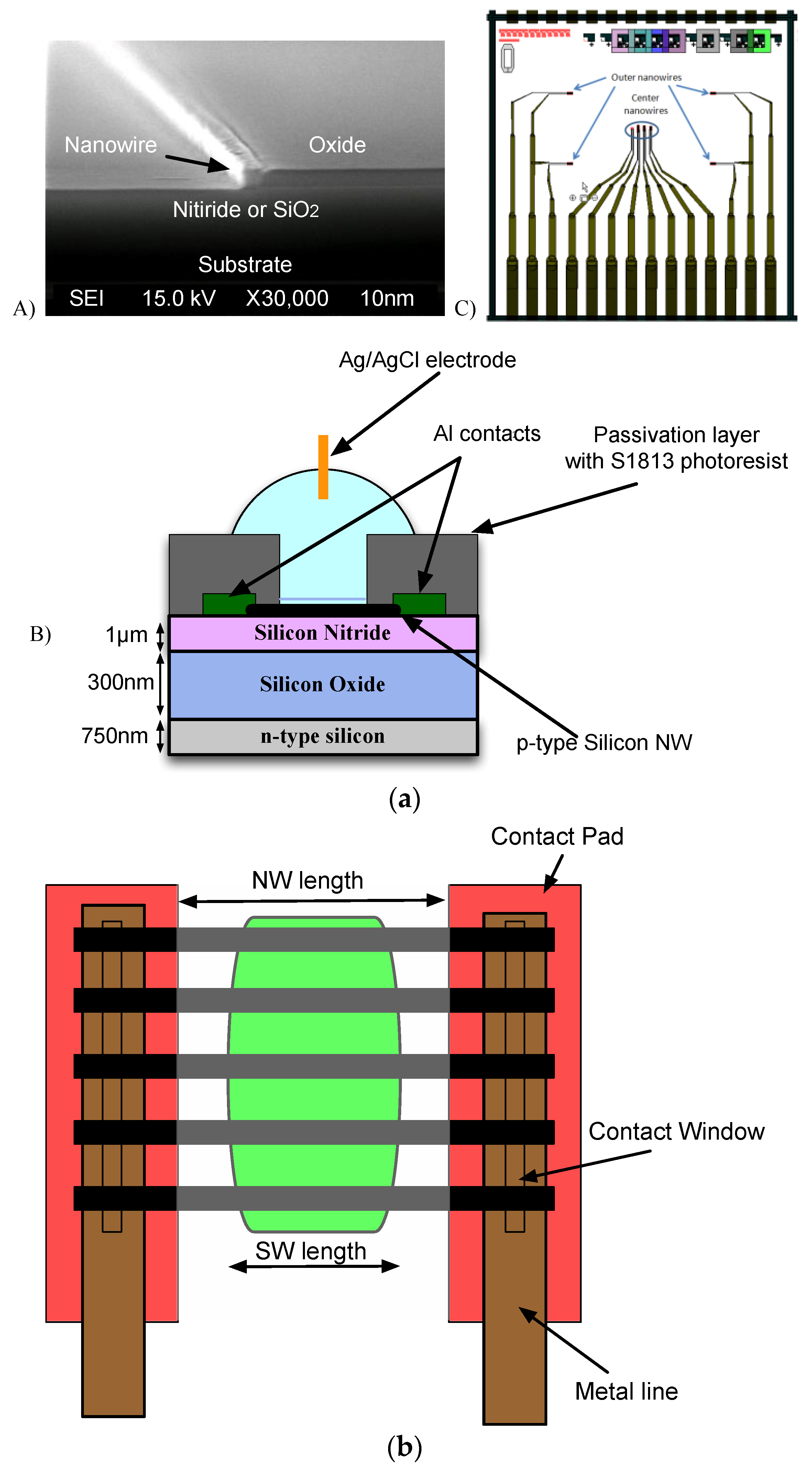
2.1. Device Under Test
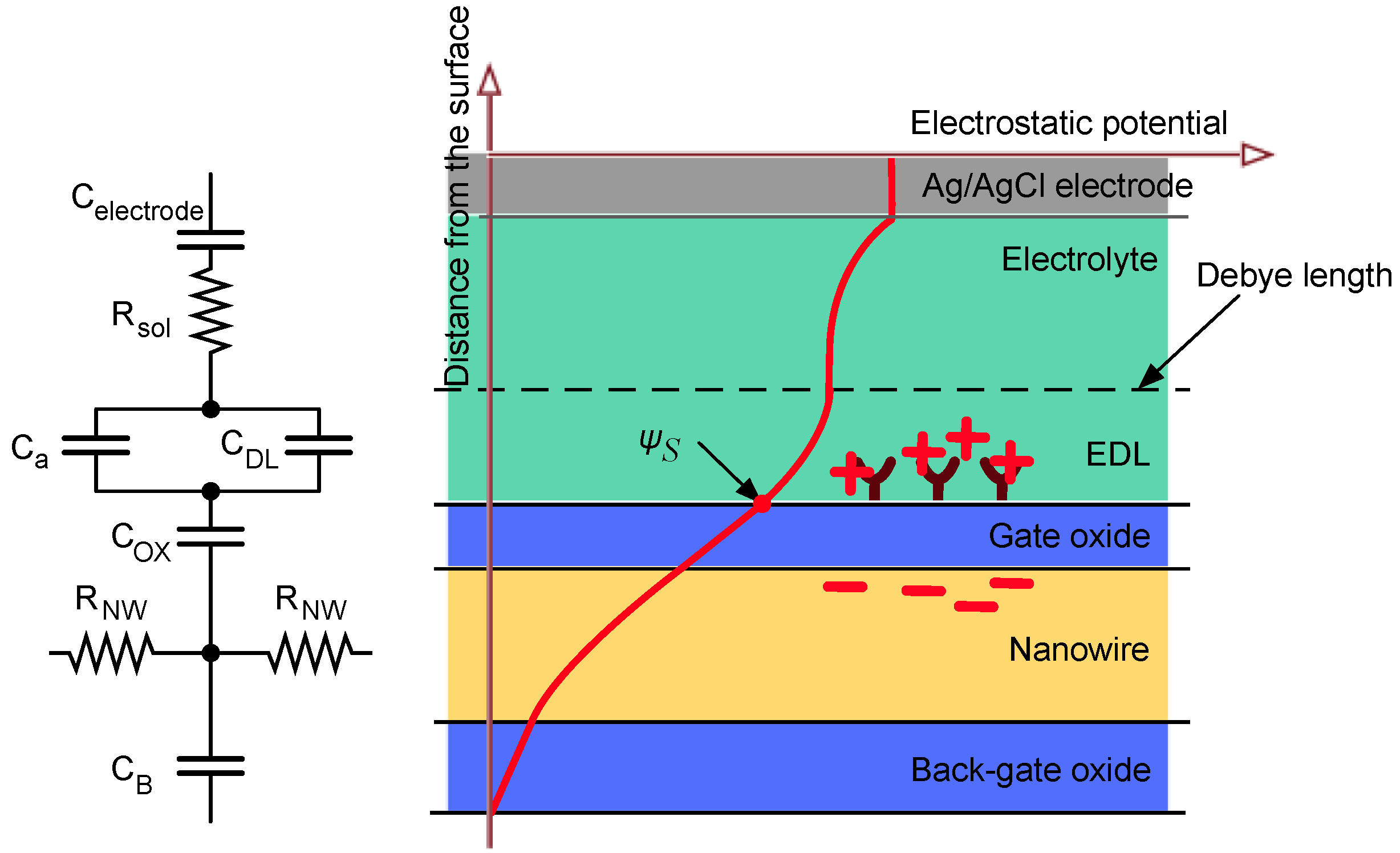
2.2. NW Physical Model
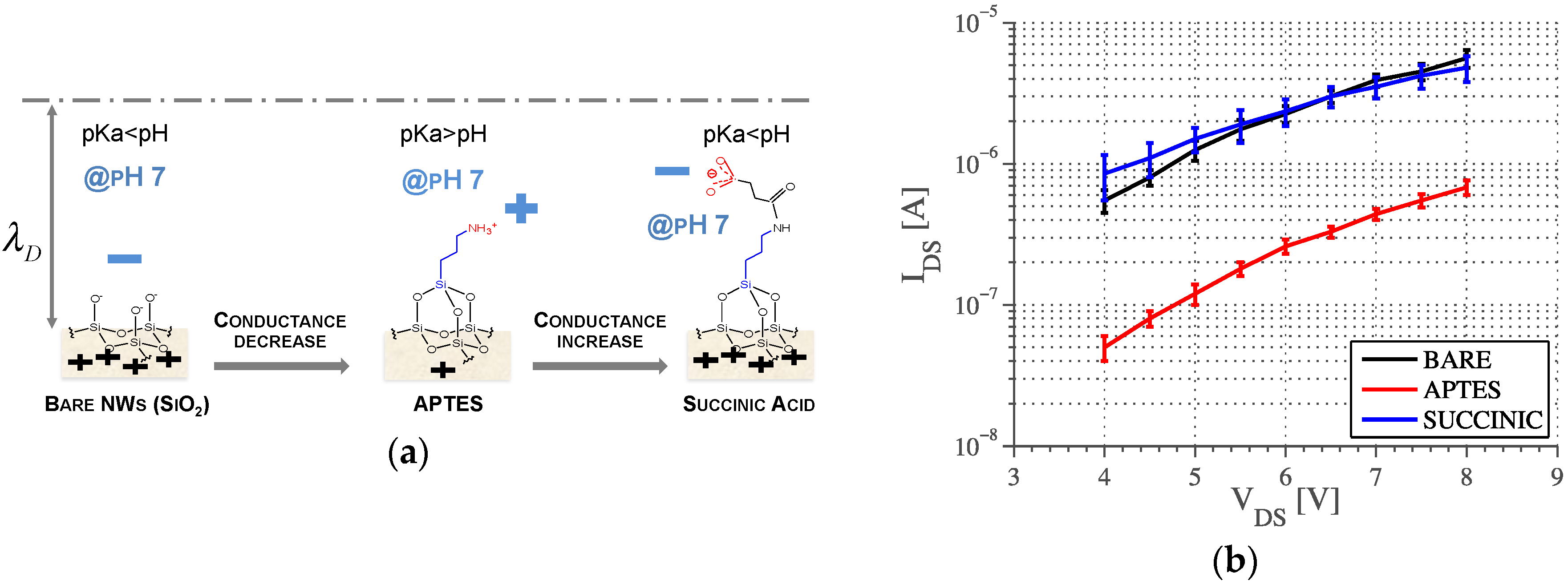
- The concentration of the solution modulates the Debye length according to Equation (2), thus changing the exponential decay of the electrostatic potential (Figure 2, right), modeled as the double layer capacitance CDL.
- Any charge variation due to site-binding (such as pH and molecular interactions at the interface) occurring within the Debye length directly influence the potential profile at the interface according to Poisson’s equation. The concentration of hydrogen ions is usually much smaller than the concentration of salt ions; therefore these play a negligible role in determining the Debye length.
- The functionalization of the NW surface induces, together with local electrostatic potential variation, charge dynamic effects that can be modeled as a differential capacitance CA in parallel with the double layer capacitance [33].
2.3. AC Nanowire Sensing
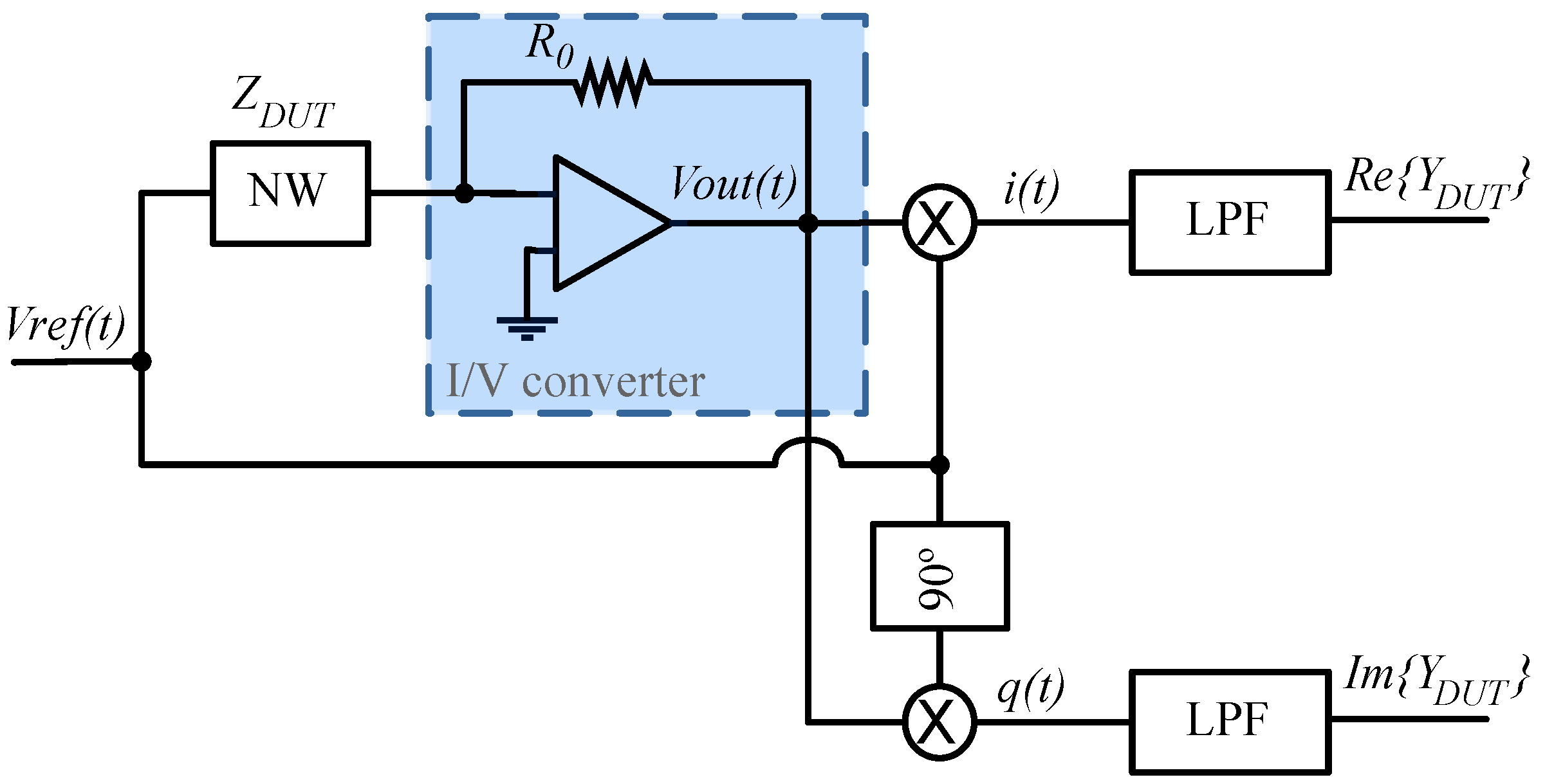
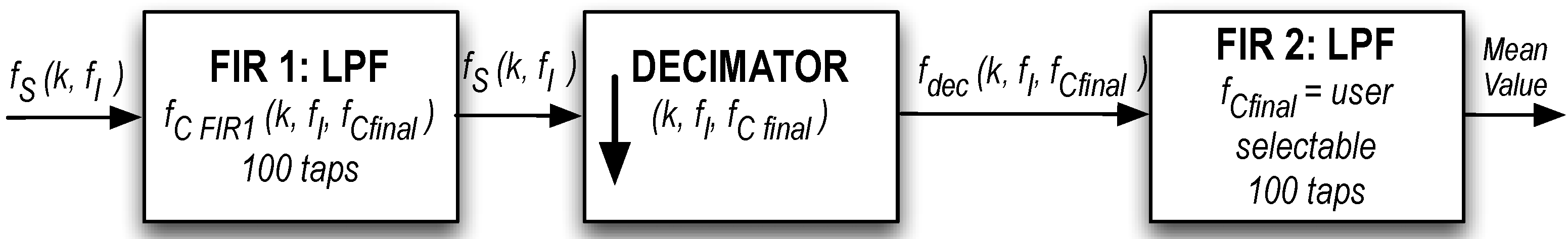
2.4. Impedance Lock-In Principle
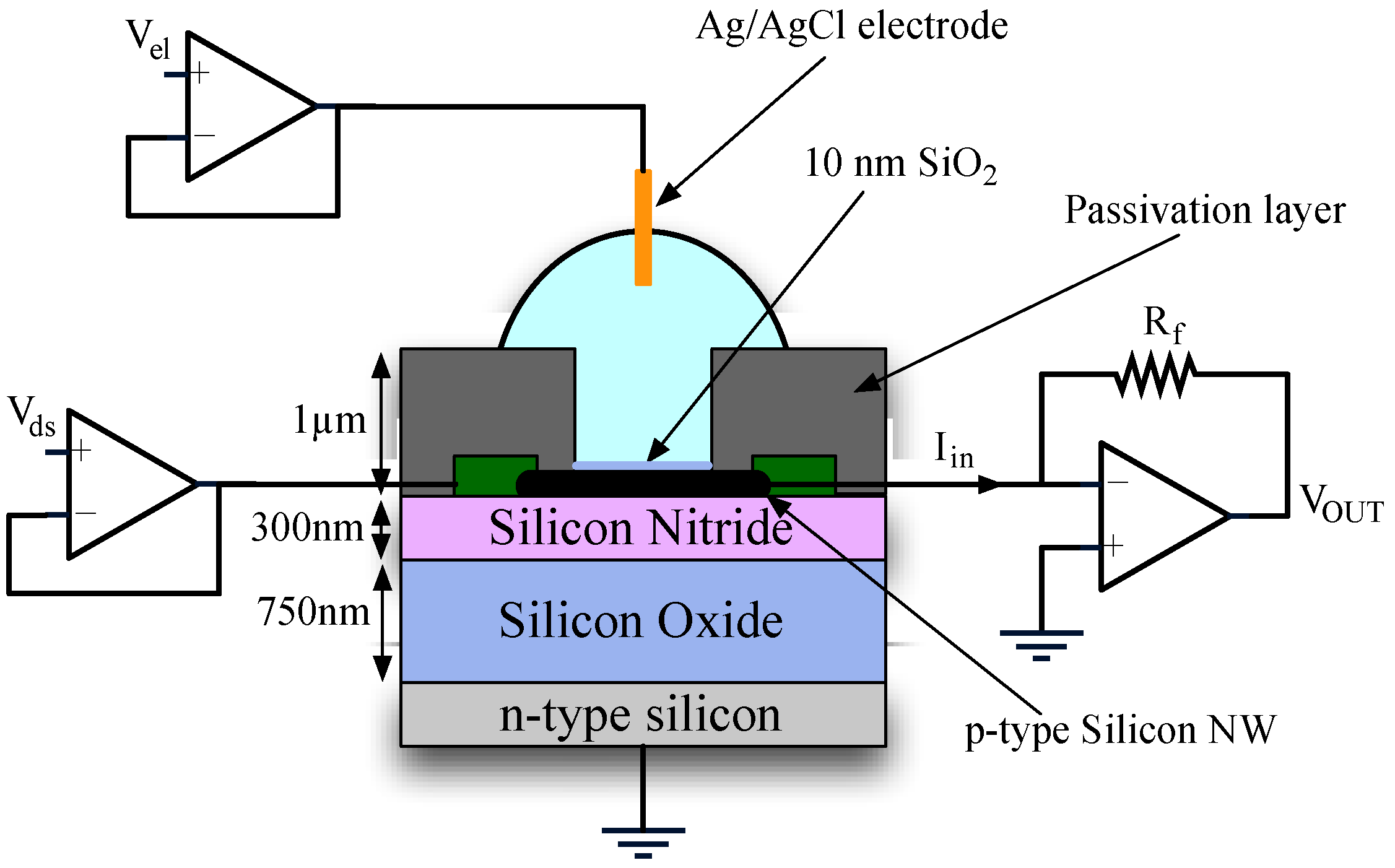
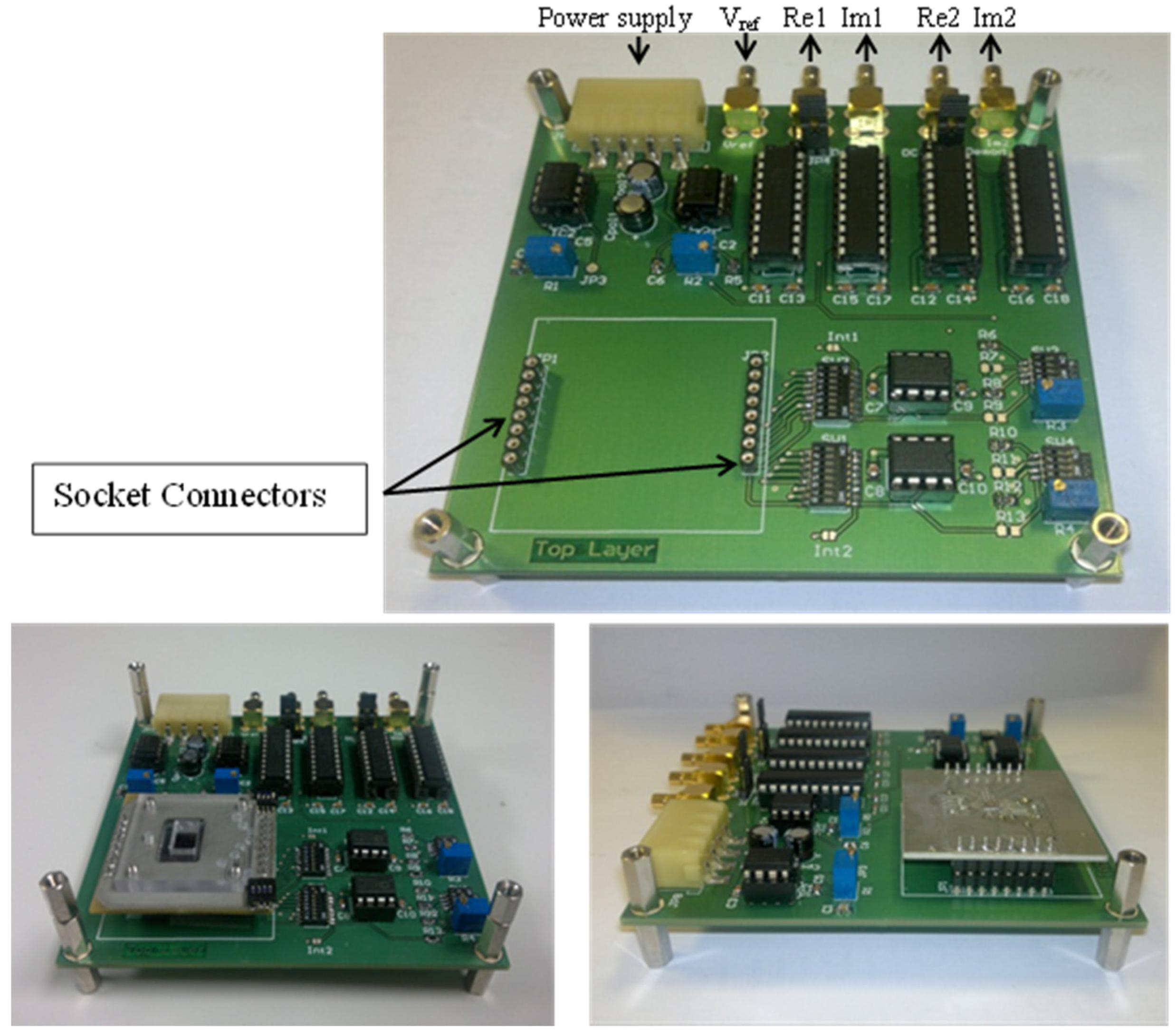
2.5. Circuit Implementation
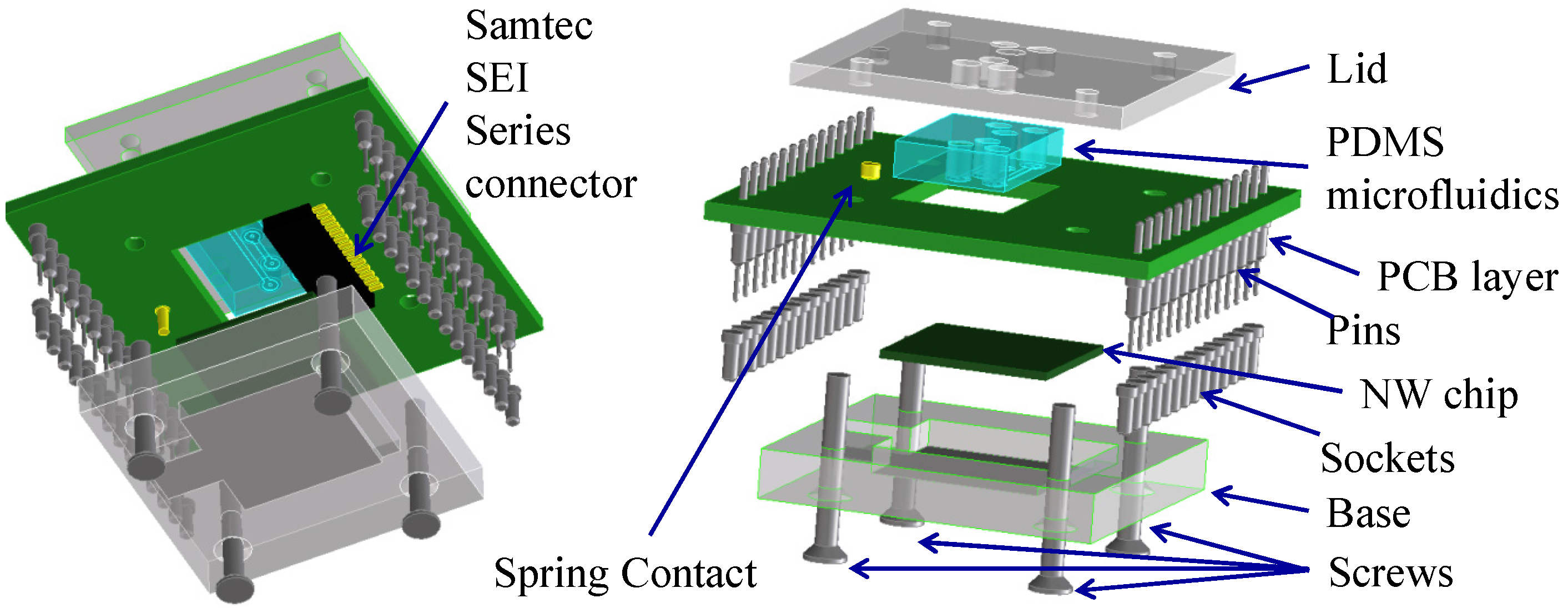
2.6. Microfluidics
- A base, which holds the NW chip and makes contact to the back gate with a small piece of aluminium foil providing. A recess enables automatic alignment of the contact pads of the NW chip with the connector.
- A windowed two-layer PCB, with Samtec® SEI connector, a spring connector and two 4-way surface mount device (SMD) switches. The spring connector contacts the aluminium foil base. The 4-way SMD switches connect the VREF signal to the selected Si-NWs under test.
- A rectangular PDMS microfluidics gasket, designed and moulded as required (e.g., with or without microchannels for solution flow), ensures a tight seal between the sensitive NW chip surface and the bottom layer of the PCB avoiding electrolyte leakage;
- A lid to package the microfluidic gasket as well as the whole device.
3. Results and Discussion
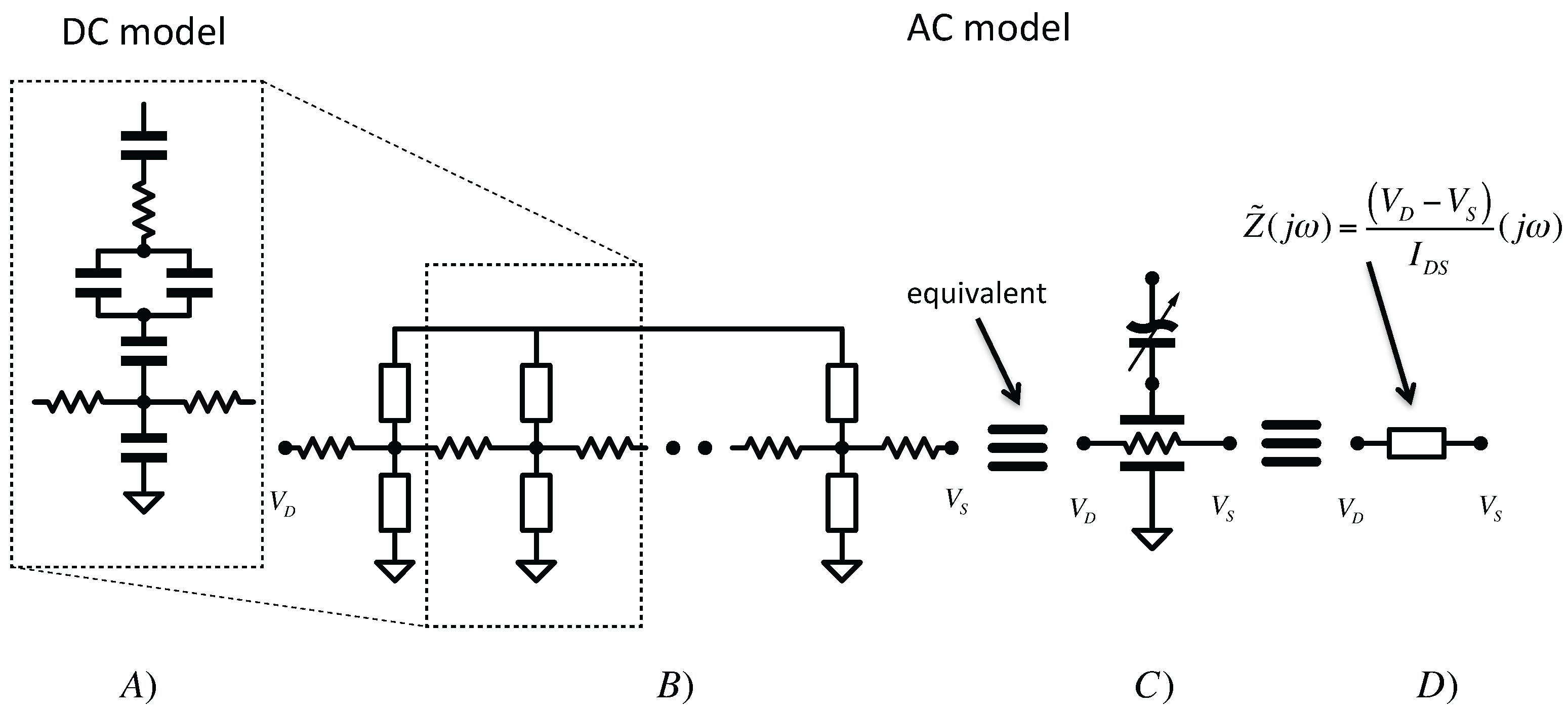
3.1. Equivalent Model
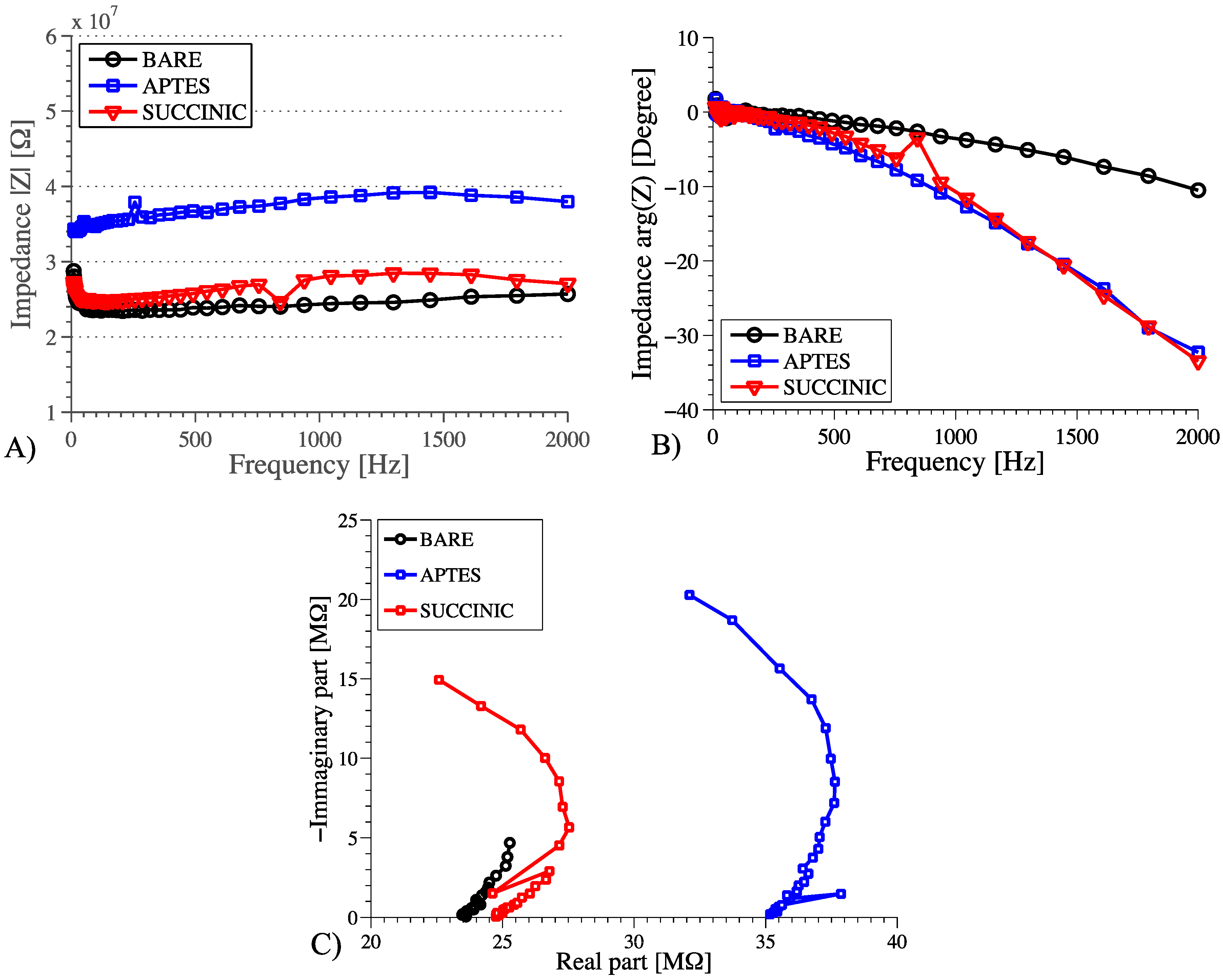
3.2. AC Measurements
- The effect of the charge change following functionalization changes the NW resistance, thus the magnitude of the AC impedance is similar to the DC (bare NW and succinic acid showed the same behavior);
- Surface chemistries with similar effects on NW charge have different properties that can be measured by phase detection and modeled through different values of CA, which should be added in parallel with CDL in the model, as explained in [32].
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| DC | Direct Current |
| NW | Nanowire |
| CMOS | Complementary Metal Oxide Semiconductor |
| Si-NW | Silicon Nanowire |
| PECVD | Plasma Enhanced Chemical Vapor Deposited |
| LPCVD | Low-Pressure Chemical Vapor Deposition |
| RIE | Reactive Ion Etcher |
| PCB | Printed Circuit Board |
| ISFET | Ion-Sensitive Field Effect Transistor |
| EDL | Electrical Double Layer |
| IEP | Isoelectric Point |
| DUT | Device Under Test |
| SMD | Surface Mount Device |
| PDMS | Polydimethylsiloxane |
| APTES | 3-aminopropyltri-ethoxysilane |
References
- Ramgir, N.S.; Yang, Y.; Zacharias, M. Nanowire-based sensors. Small 2010, 6, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Curreli, M.; Zhang, R.; Ishikawa, F.N.; Chang, H.-K.; Cote, R.J.; Zhou, C.; Thompson, M.E. Real-time, label-free detection of biological entities using nanowire-based FETs. IEEE Trans. Nanotechnol. 2008, 7, 651–667. [Google Scholar] [CrossRef]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-free biomarker detection from whole blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chai, K.T.C.; Luo, H.Z.H.; Huang, J.M.; Tay, I.G.K.; Lim, A.E.; Je, M. Multiplexed detection of cardiac biomarkers in serum with nanowire arrays using readout ASIC. Biosens. Bioelectron. 2012, 35, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Krivitsky, V.; Hsiung, L.-C.; Lichtenstein, A.; Brudnik, B.; Kantaev, R.; Elnathan, R.; Pevzner, A.; Khatchtourints, A.; Patolsky, F. Si nanowires forest-based on-chip biomolecular filtering, separation and preconcentration devices: Nanowires do it all. Nano Lett. 2012, 12, 4748–4756. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.M.A.; Lombardini, M.; Sun, K.; Giustiniano, F.; Roach, P.L.; Davies, D.E.; Howarth, P.H.; de Planque, M.R.R.; Morgan, H.; Ashburn, P. Thin film polycrystalline silicon nanowire biosensors. Nano Lett. 2012, 12, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bomer, J.G.; van der Wiel, W.G.; Carlen, E.T.; van den Berg, A. Top-down fabrication of sub-30 nm monocrystalline silicon nanowires using conventional microfabrication. ACS Nano 2009, 3, 3485–3492. [Google Scholar] [CrossRef] [PubMed]
- Zeimpekis, I.; Sun, K.; Hu, C.; Thomas, O.; de Planque, M.R.; Chong, H.M.; Morgan, H.; Ashburn, P. Study of parasitic resistance effects in nanowire and nanoribbon biosensors. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Accastelli, E.; Scarbolo, P.; Ernst, T.; Palestri, P.; Selmi, L.; Guiducci, C. Multi-wire tri-gate silicon nanowires reaching milli-pH unit resolution in one micron square footprint. Biosensors 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Manickam, A.; Johnson, C.A.; Kavusi, S.; Hassibi, A. Interface design for CMOS-integrated Electrochemical Impedance Spectroscopy (EIS) biosensors. Sensors 2012, 12, 14467–14488. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Offermans, P.; Meynants, G.; Tong, H.D.; van Rijn, C.J.M.; Merken, P. A low-power readout circuit for nanowire based hydrogen sensor. Microelectron. J. 2010, 41, 733–739. [Google Scholar] [CrossRef]
- Offermans, P.; Tong, H.D.; van Rijn, C.J.M.; Merken, P.; Brongersma, S.H.; Crego-Calama, M. Ultralow-power hydrogen sensing with single palladium nanowires. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Van den Brink, F.T.G.; Gool, E.; Frimat, J.-P.; Bomer, J.; van den Berg, A.; Le Gac, S. Parallel single-cell analysis microfluidic platform. Electrophoresis 2011, 32, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Zagnoni, M. Miniaturised technologies for the development of artificial lipid bilayer systems. Lab Chip 2012, 12, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Carlen, E.T.; van den Berg, A. Labs-on-a-chip and nanosensors for medical applications and life sciences. In Proceeding of the 2013 IEEE International Electron Devices Meeting, Washington, DC, USA, 9–11 December 2013.
- Zaborowski, M.; Dumania, P.; Tomaszewski, D.; Czupryniak, J.; Kokot, M.; Pałetko, P.; Gotszalk, T.; Grabiec, P. Development of si nanowire chemical sensors. Procedia Eng. 2012, 47, 1053–1056. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, H.T.; Hsueh, T.J.; Chang, S.J.; Hung, F.Y.; Weng, W.Y.; Hsu, C.L.; Dai, B.T. Si nanowire-based humidity sensors prepared on glass substrate. IEEE Sens. J. 2011, 11, 3036–3041. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst limit in dual-gated Si-nanowire FET sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Yoon, H.; Varadan, V.K. Potassium ion sensing with nanowire electrodes on a flexible substrate for early detection of myocardial ischemia. J. Nanotechnol. Eng. Med. 2010, 1. [Google Scholar] [CrossRef]
- Tena-Zaera, R.; Elias, J.; Lévy-Clément, C.; Mora-Seró, I.; Luo, Y.; Bisquert, J. Electrodeposition and impedance spectroscopy characterization of ZnO nanowire arrays. Phys. Status Solidi A 2008, 205, 2345–2350. [Google Scholar] [CrossRef]
- Tirado, M.; Comedi, D.; LaPierre, R.R. Impedance spectroscopy characterization of GaAs nanowire bundles grown by metal-catalyzed molecular beam epitaxy. J. Alloy. Compd 2010, 495, 443–445. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Zhong, Z. Detection beyond the Debye screening length in a high-frequency nanoelectronic biosensor. Nano Lett. 2012, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- García-Romeo, D.; Valero, M.R.; Medrano, N.; Calvo, B.; Celma, S. A high performance LIA-based interface for battery powered sensing devices. Sensors 2015, 15, 25260–25276. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.N.; Chen, S.; Carlen, E.T.; van den Berg, A. All-(111) surface silicon nanowires: Selective functionalization for biosensing applications. ACS Appl. Mater. Interfaces 2010, 2, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Sun, A.; Pang, L.; Venkatesh, A.G.; Hall, D.; Fainman, Y. Integration of Faradaic electrochemical impedance spectroscopy into a scalable surface plasmon biosensor for in tandem detection. Opt. Express 2015, 23, 30237–30249. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ohnuki, H.; Endo, H.; Izumi, M. Impedimetric and amperometric bifunctional glucose biosensor based on hybrid organic-inorganic thin films. Bioelectrochemistry 2015, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.; Reddy, A.K.N. Modern Electrochemistry; Springer: Medford, MA, USA, 2001. [Google Scholar]
- Morgan, H.; Green, N.G. AC Electrokinetic: Colloids and Nanoparticles; Research Studies Press: Baldock, UK, 2002. [Google Scholar]
- Bard, A.J.; Faulkner, L.J. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Shinwari, M.W.; Deen, M.J.; Landheer, D. Study of the electrolyte-insulator-semiconductor field-effect transistor (EISFET) with applications in biosensor design. Microelectron. Reliab. 2007, 47, 2025–2057. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Deen, M.J.; Selvaganapathy, P. Analytic modelling of biotransistors. IET Circuits Devices Syst. 2008, 2, 158–165. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Elfström, N.; Juhasz, R.; Sychugov, I. Surface charge sensitivity of silicon nanowires: Size dependence. Nano Lett. 2007, 7, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, I.; Hao, Z.; Holman, H.-Y.N.; Pisano, A.P. Quantitative studies of long-term stable, top-down fabricated silicon nanowire pH sensors. Appl. Phys. A 2012, 107, 421–428. [Google Scholar] [CrossRef]
- Cui, Y.; Zhong, Z.; Wang, D.; Wang, W.U.; Lieber, C.M. High performance silicon nanowire field effect transistors. Nano Lett. 2003, 3, 149–152. [Google Scholar] [CrossRef]
- Crescentini, M.; Bennati, M.; Tartagni, M. Recent trends for (bio)chemical impedance sensor electronic interfaces. Electroanalysis 2012, 24, 563–572. [Google Scholar] [CrossRef]
- Balanced Modulator/Demodulator. AD630 Datasheet, Rev. F. Available online: https://www.digchip.com/datasheets/parts/datasheet/041/AD630.php (accessed on 12 April 2016).
- Rossi, M.; Bennati, M.; Thei, F.; Tartagni, M. A Low-cost and Portable system for real-time impedimetric measurements and impedance spectroscopy of sensors. In Proceeding of the SENSORDEVICES 2012: Third International Conference on Sensor Device Technologies and Applications, Rome, Italy, 19–24 August 2012; pp. 101–106.

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescentini, M.; Rossi, M.; Ashburn, P.; Lombardini, M.; Sangiorgi, E.; Morgan, H.; Tartagni, M. AC and Phase Sensing of Nanowires for Biosensing. Biosensors 2016, 6, 15. https://doi.org/10.3390/bios6020015
Crescentini M, Rossi M, Ashburn P, Lombardini M, Sangiorgi E, Morgan H, Tartagni M. AC and Phase Sensing of Nanowires for Biosensing. Biosensors. 2016; 6(2):15. https://doi.org/10.3390/bios6020015
Chicago/Turabian StyleCrescentini, Marco, Michele Rossi, Peter Ashburn, Marta Lombardini, Enrico Sangiorgi, Hywel Morgan, and Marco Tartagni. 2016. "AC and Phase Sensing of Nanowires for Biosensing" Biosensors 6, no. 2: 15. https://doi.org/10.3390/bios6020015
APA StyleCrescentini, M., Rossi, M., Ashburn, P., Lombardini, M., Sangiorgi, E., Morgan, H., & Tartagni, M. (2016). AC and Phase Sensing of Nanowires for Biosensing. Biosensors, 6(2), 15. https://doi.org/10.3390/bios6020015






