Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics
Abstract
:1. Introduction
Disease Burdens in the Developing World
2. Lateral Flow Tests
2.1. Impact on Developing Countries
| Company | Product Name | Disease | Analyte/Antigen (Ag) | Required Sample | Detection Time (Min) | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Infectious Diseases | Alere | Binax NOW Filariasis | Lymphatic filariasis | Wuchereria bancrofti | 100 µL WB/S/P | 10 | - | - |
| IMMY | CrAg A | Cryptococcal meningitis | C. neoformans C. gattii | 40 µL of S/CSF | 10 | 100% | 94% | |
| Alere | Binax NOW | Malaria | Plasmodium Ag | 15 µL of WB | 15 | P. falciparum: 99.7%, P. vivax: 93.5% | P. falciparum: 94.2%, P. vivax: 99.8% | |
| Quidel Corp. | Quick Vue RSV Test | Infantile bronchiolitis | Respiratory syncytial virus (RSV) Ag | Nasal swab, aspirate and wash | 15 | 92%-swab; 99%-aspirate; 83%-wash | 92%-swab; 92%-aspirate; 90%-wash | |
| Alere | Alere Determine HIV-1/2 Ag/Ab Combo | AIDS | HIV-1/2 antibodies and free HIV-1 p24 Ag | 50 µL of WB/S/P | 20 | - | 99.75% | |
| Alere | Panbio Dengue Duo Cassette | Dengue fever | IgM and elevated IgG | WB/S/P | 15 | 85.50% | 91.60% | |
| Alere | Alere™ Influenza A & B Test | Influenza | Influenza A and B nucleoprotein Ag | Nasal swab | 10 | Flu A 93.8%; Flu B 77.4% | Flu A 95.8%; Flu B 98% | |
| Alere | Alere Determine TB LAM | Tuberculosis (TB) in HIV positive patients | Lipoarabinomannan (LAM) Ag | 60 µL of Urine | 25 | 51.7% for <100 CD4 cell count | >98% | |
| Trinity Biotech | Uni-Gold™ Legionella Urinary Antigen PLUS | Legionnaire’s Disease | Legionella pneumophila serogroup 1 Ag | 150–200 µL of Urine | 15 | 82.1% | 99.2% | |
| Alere | Binax NOW | Pneumonia | S. pneumoniae Ag | Urine/CSF | 15 | Urine-86%, CSF-97% | Urine-94%, CSF-99% | |
| Cancer | CTK Biotech | On Site PSA Rapid Test | Prostate cancer | Prostate specific antigen (PSA) | 60–90 µL of S/P | 10 | Relative: 100% | Relative: 99% |
| Alere | Alere NMP22 BladderChek | Bladder cancer | Nuclear matrix protein (NMP22) | 4 drops of Urine | 30 | 99% when combined with cystoscopy | 99% NPV along with cystoscopy | |
| Alere | Clearview iFOBT | Colon cancer | Faecal Occult Blood | Faeces | 5 | 93.60% | 99.10% | |
| Arbor Vita Corp. | OncoE6 Cervical Test | Cervical cancer | E6 oncoproteins | Cervical swab | 150 | 84.6% | 98.5% | |
| Quicking Biotech Co., Ltd | CA125 rapid test kit | Ovarian Cancer | CA125 Ag | 100 µL of S | 10 | - | - | |
| Innovation Biotech | AFP Test | Hepatocellular Cancer | Alpha fetoprotein Ag | S/P | 10 | 25 ng/mL | 99% | |
| Cardiac Diseases | LifeSign | StatusFirst CHF NT-proBNP | Congestive Heart Failure | NT-proBNP | 3 drops of P | 15 | 20 pg/mL | - |
| BTNX Inc. | Rapid Response CK-MB Test | Myocardial infarction (MI) | Creatine kinase MB (CKMB) | WB/S/P | 10 | 5 ng/mL | 99.80% | |
| Boditech Med Inc. | ichroma™ CK-MB Test | Myocardial infarction (MI) | Creatine kinase MB (CKMB) | 75 µL of WB/S/P | 12 | 3 ng/mL | - | |
| Response Biomedical Corp. | RAMP MYOGLOBIN TEST | Acute myocardial infarction (AMI) | Myoglobin | WB | 10 | 2.36 ng/mL | - | |
| Trinity Biotech | Meritas® Troponin I | Myocardial infarction (MI) | Troponin I | 200 µL of WB/P | 15 | 0.036 ng/mL | - | |
| BTNX Inc. | RAPID RESPONSE D-DIMER TEST | Venous Thromboembolism (VTE) | D-Dimer | WB/P | 10 | 500 ng/mL | - | |
| American Screening Corp. | Instant-View Troponin I | Acute myocardial infarction (AMI) | Troponin I | 200 µL of WB/S | 10–20 | - | - |
2.2. Principle of a Lateral Flow Immunoassay
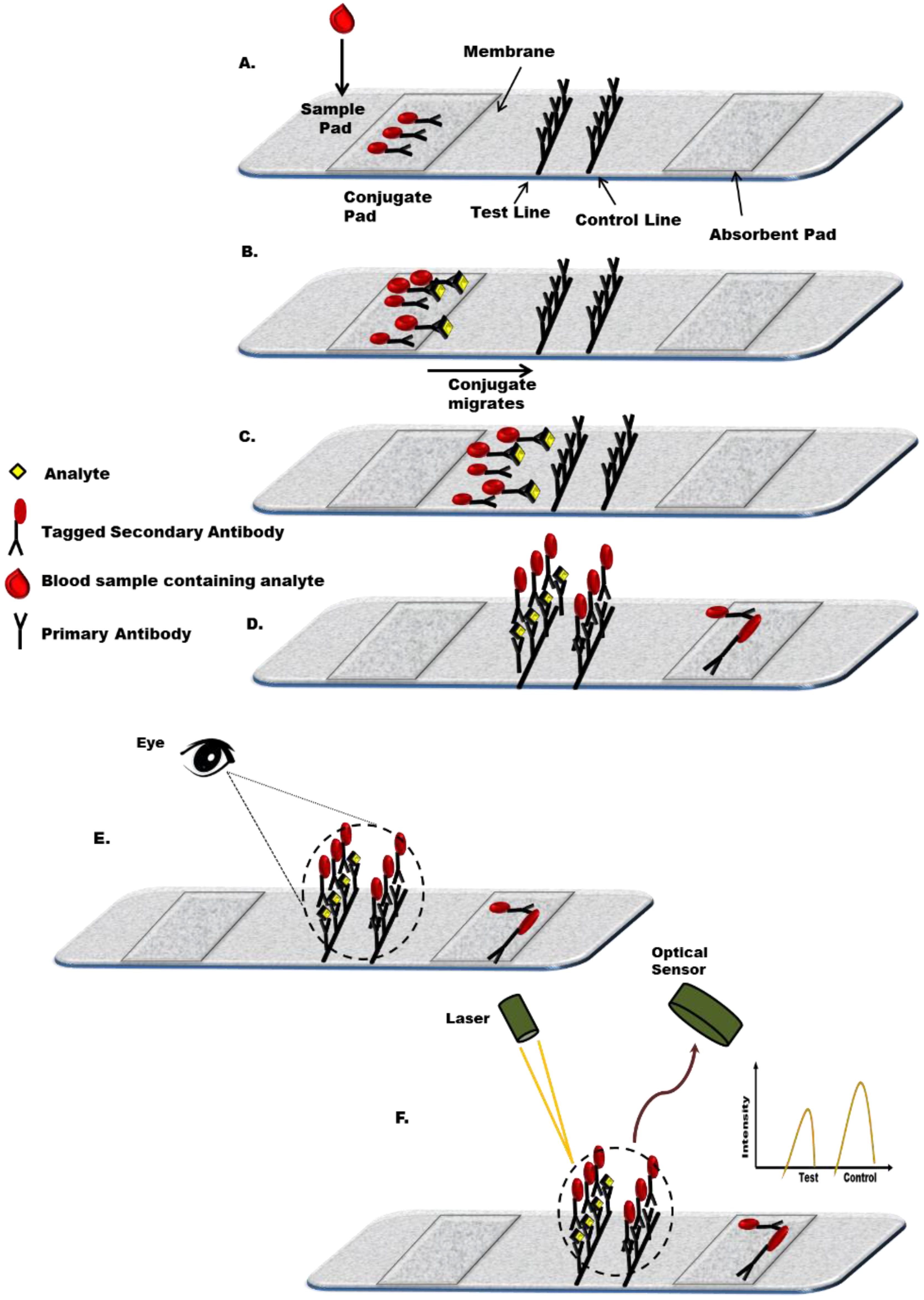
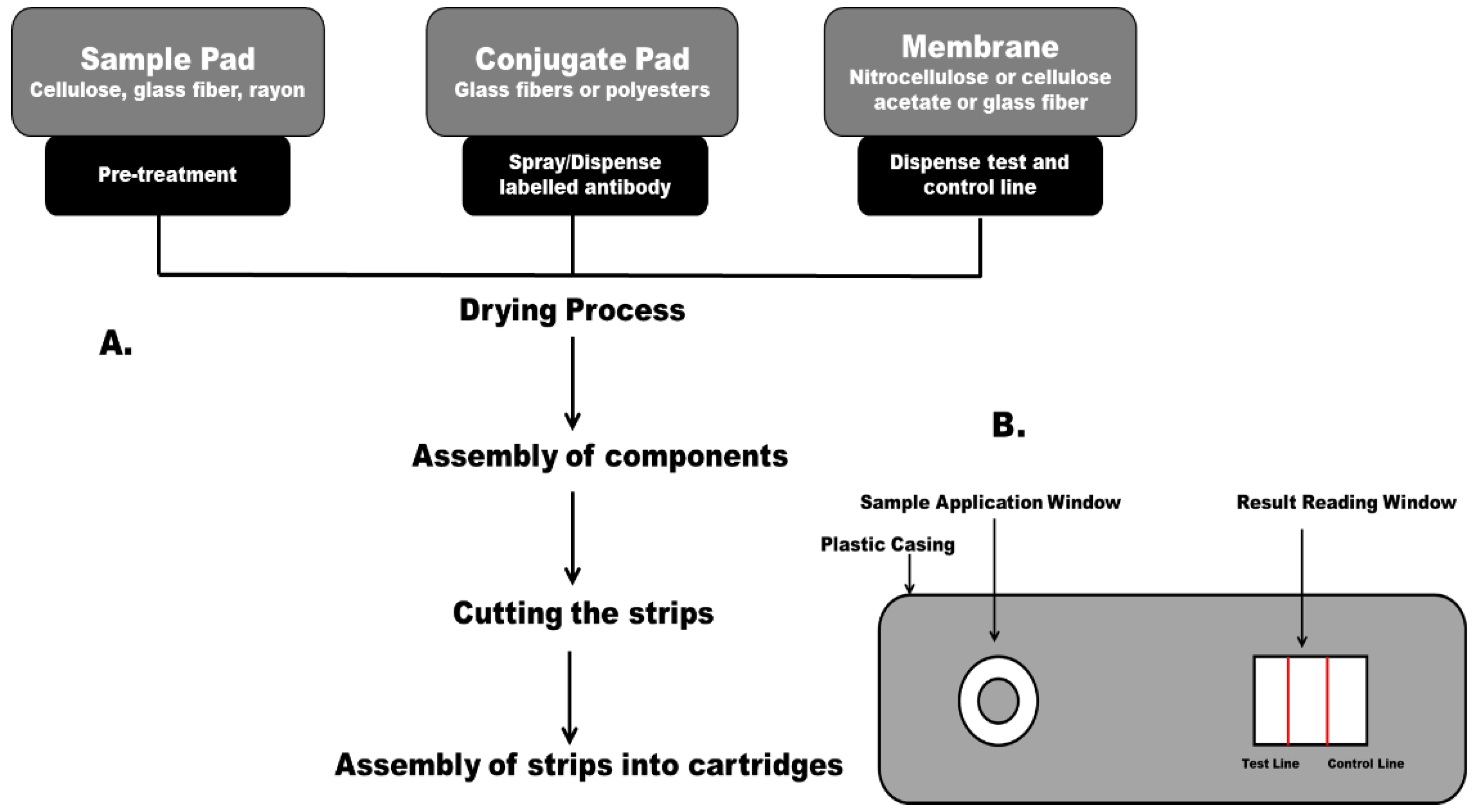
2.2.1. Biorecognition Components
2.2.2. Sample Application Pad
2.2.3. Conjugate Pad
2.2.4. Membrane
2.2.5. Absorbent Pad
2.2.6. Backing Materials for the Membrane
2.2.7. Detection Label
2.2.8. Detection System
2.3. Limitations of “Traditional” POC Approaches
3. The Future: Microfluidics as an Emerging Platform for Point-of-Care Diagnosis
3.1. Microfluidic Devices in Developing Countries
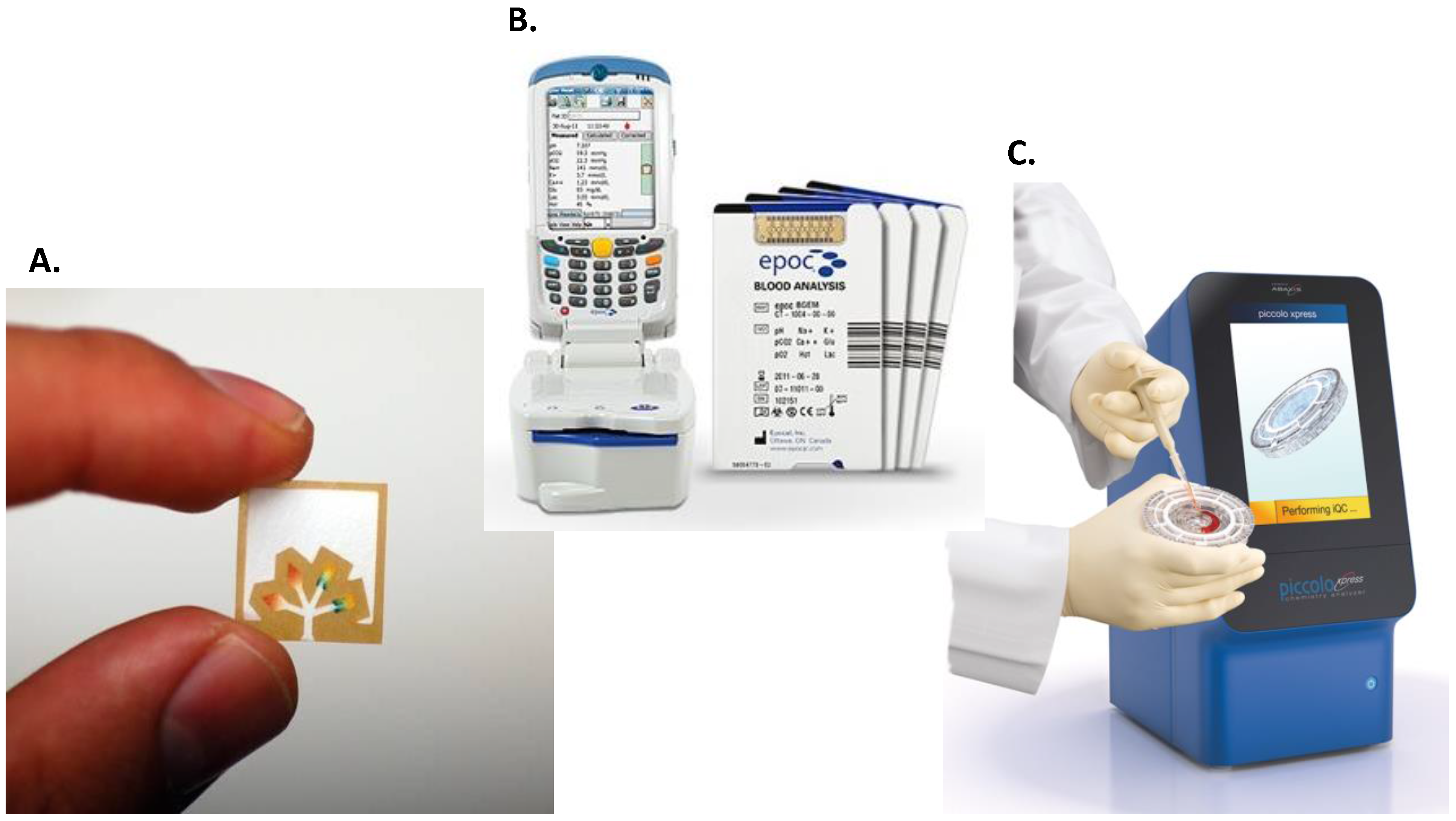
| Company | Test Name | Tested Analyte/Parameter | Sample Required | Components | Training Requirements and Other Characteristics | Results Type and Turnaround Time |
|---|---|---|---|---|---|---|
| Abaxis | The Piccolo® | Blood chemistries | 100 µL of WB, S or P | Disposable test discs and portable analyser. | No operator intervention is required. | Quantitative results in about 12 min. |
| Biosite (Alere) | Alere Triage® MeterPro | Blood/urine chemistries including “waived” Lipid and liver panels | 225 µL of WB, P or urine | Disposable cartridges, and meter. | No training required. Built-in quality controls. | Qualitative results in 15–20 min. |
| Epocal (Alere) | The epoc® Blood Analysis System | Blood chemistries | 92 μL of WB | Test cards and wireless card reader with a host mobile computer. | No training required. RT- test card storage. Automated, built-in QC/QA calibration and quality tests | Quantitative results in about 30 s. |
| Focus Dx (Quest) | Simplexa | Flu A/B & RSV | Nasopharyngeal swabs | PCR platform (3M™ Integrated Cycler) and amplification discs with sample wells. | Used by physician. | Quantitative results in 1 h. |
| HandlyLab (BD) | BD MAXTM GBS Assay IDI-Strep B Assay | Group B Streptococcus (GBS) | Vaginal/rectal swab samples | Cepheid Smart Cycler® RT-PCR system, disposable cartridge and computer system. | Trained personal required. Can be performed in moderate complexity laboratories. | Qualitative results in 1 h. |
| i-STAT Corp (Abbot) | i-STAT Analyser | Blood chemistries; coagulation; cardiac markers | 17–95 μL of WB, depending on cartridge type | Handheld analyser and self-contained cartridges. | Training is required. Portable. Cartridges can be stored at room temperature. | Quantitative results in less than 15 min. |
| Idaho Technologies | FilmArray RP | Panel of respiratory pathogens | Nasopharyngeal swabs | FilmArray instrument and freeze-dried reagents in pouches | No precise measuring or pipetting required. | Qualitative results. 2 min. of hands-on time; about a 1-h turnaround time. |
| IQuum | Liat™ Influenza A/B Assay | H1N1 influenza viral RNA | Nasopharyngeal swab samples | Benchtop analyzer disposable assay tubes | Use under CLIA requirements. Can be operated by minimally trained users. | Qualitative results in less than 30 min. |
| Micronics (Sony) | ABORhCard | ABO and Rh blood typing | 50 μL of fingerstick WB | Disposable cards that with anti-A, B and D antibodies printed into discrete microfluidic channels. | No training required. No refrigeration needed. Long shelf-life. | Qualitative results in approximately 2 min. |
| Sphere Medical | Proxima | Blood chemistry | Arterial WB | Disposable multi-parameter microanalyser, silicon chips. | Use by trained clinician. | Quantitative results in approximately 3 min. |
| TearLab | TearLab Osmolarity System | Osmolarity of human tears (diagnosis of Dry Eye Disease) | 50 nL of tear film | Disposable microchip works in conjunction with a pen that convey data to the osmolarity reader. | Professional in vitro diagnostic use only. | Quantitative results in seconds. |
3.2. Self-Contained and Automated Testing
3.3. No Specialised Training Requirements
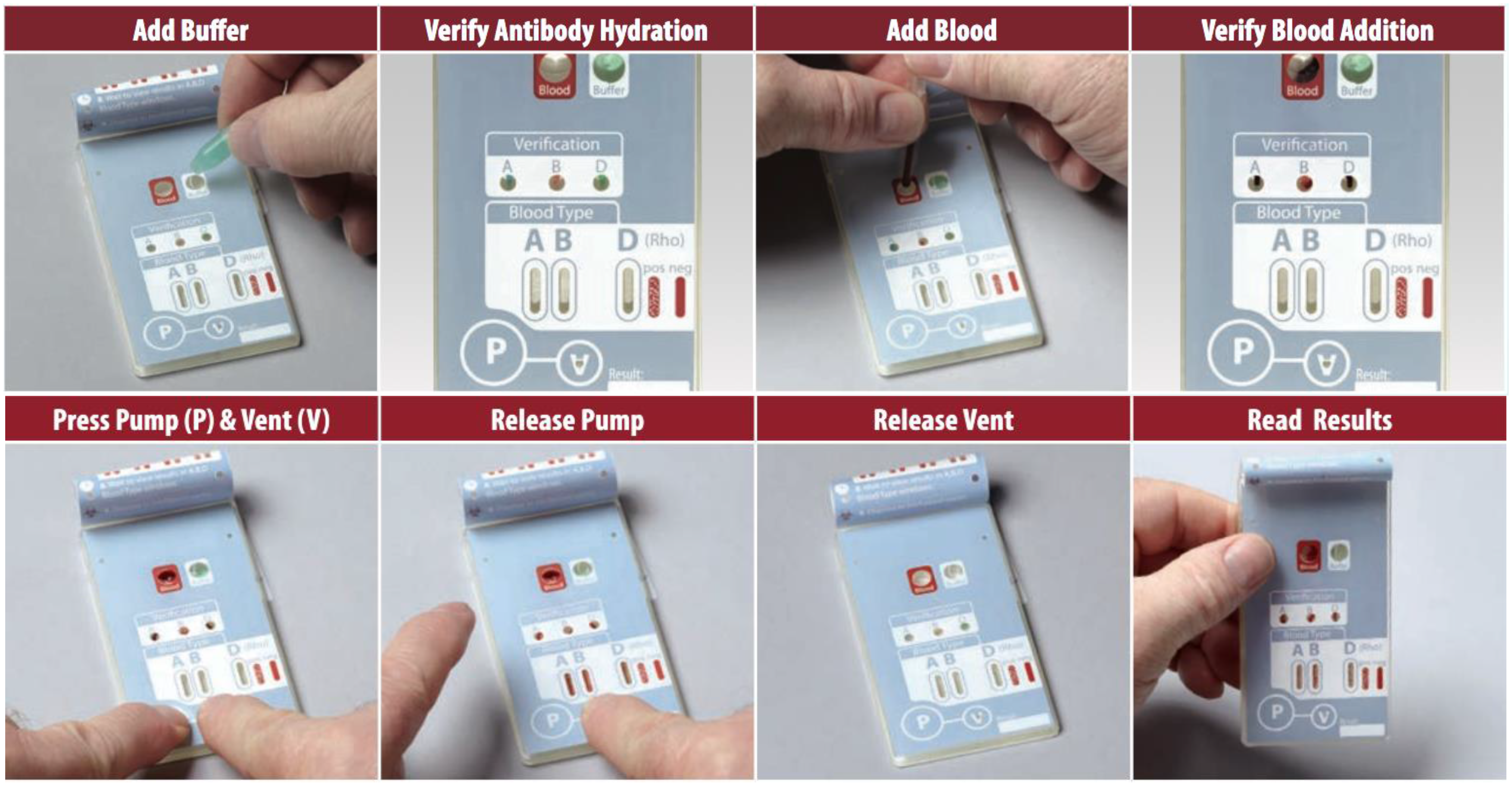
3.4. Easily Interpreted Results
3.5. Necessity of a Robust Test
3.6. General Observations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global Status Report on Noncommunicable Diseases. Available online: http://www.who.int/nmh/publications/ncd_report_full_en.pdf (accessed on 20 May 2015).
- Maher, D.; Ford, N.; Unwin, N.; Frontières, M.S. Priorities for developing countries in the global response to non-communicable diseases. Glob. Health 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- McNerney, R.; Daley, P. Towards a point-of-care test for active tuberculosis: Obstacles and opportunities. Nat. Rev. Microbiol. 2011, 9, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for high impact diagnostics in the developing world. Nature 2006, 444, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Point-Of-Care Diagnostics/Testing Market by Products (Glucose Monitoring & Infectious Diseases Testing Kits, Cardiac & Tumor Markers), by End Users (Self & Professional Monitoring), Over the Counter & Prescription Based-Global Forecast to 2018. Available online: http://www.marketsandmarkets.com/Market-Reports/point-of-care-diagnostic-market-106829185.html (accessed on 20 May 2015).
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Worldometers. Available online: http://www.worldometers.info/world-population/ (accessed on 20 May 2015).
- Jamison, D.T.; Breman, J.G.; Measham, A.R.; Alleyne, G.; Claeson, M.; Evans, D.B.; Jha, P.; Mils, A.; Musgrove, P. Cost-Effective Strategies for the Excess Burden of Disease in Developing Countries. In Priorities in Health; World Bank: Washington, DC, USA, 2006; pp. 59–95. [Google Scholar]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowafi, M.; Pandya, A.; et al. The Global Economic Burden of Noncommunicable Diseases; World Economic Forum: Geneva, Switzerland, 2011; pp. 2–25. [Google Scholar]
- The Emerging Crisis: Noncommunicable Diseases. Available online: http://www.cfr.org/diseases-noncommunicable/NCDs-interactive/p33802?cid=otr-marketing_use-NCDs_interactive/#!/ (accessed on 22 May 2015).
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Singer, J.M.; Plotz, C.M. The latex fixation test: I. Application to the serologic diagnosis of rheumatoid arthritis. Am. J. Med. 1956, 21, 888–892. [Google Scholar] [CrossRef]
- Learmonth, K.M.; McPhee, D.A.; Jardine, D.K.; Walker, S.K.; Aye, T.-T.; Dax, E.M. Assessing Proficiency of Interpretation of Rapid Human Immunodeficiency Virus Assays in Nonlaboratory Settings: Ensuring Quality of Testing. J. Clin. Microbiol. 2008, 46, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, B. Lateral Flow Immunoassay Systems: Evolution from the Current State of the Art to the Next Generation of Highly Sensitive, Quantitative Rapid Assays. In The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques, 4th ed.; Wild, D., Ed.; Newnes: Boston, MA, USA, 2013; pp. 89–107. [Google Scholar]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- McPartlin, D.A.; O’Kennedy, R.J. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Rev. Mol. Diagn. 2014, 14, 979–998. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS World AIDS Day Report 2012. Available online: http://www.unaids.org/sites/default/files/media_asset/JC2434_WorldAIDSday_results_en_1.pdf (accessed on 22 July 2015).
- Kozel, T.R.; Bauman, S.K. CrAg lateral flow assay for cryptococcosis. Expert Opin. Med. Diagn. 2012, 6, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Tangpukdee, N.; Duangdee, C.; Wilairatana, P.; Krudsood, S. Malaria diagnosis: A brief review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Derhaschnig, U.; Laggner, A.N.; Röggla, M.; Hirschl, M.M.; Kapiotis, S.; Marsik, C.; Jilma, B. Evaluation of coagulation markers for early diagnosis of acute coronary syndromes in the emergency room. Clin. Chem. 2002, 48, 1924–1930. [Google Scholar] [PubMed]
- McCord, J.; Nowak, R.M.; McCullough, P.A.; Foreback, C.; Borzak, S.; Tokarski, G.; Tomlanovich, M.C.; Jacobsen, G.; Weaver, W.D. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001, 104, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2014, in press. [Google Scholar]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Nabais, E.; Lopez-Ferber, M. Rapid visual tests: Fast and reliable detection of ochratoxin A. Toxins 2010, 2, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, S.; Hu, X. Signal enhancement in a lateral flow immunoassay based on dual gold nanoparticle conjugates. Clin. Biochem. 2013, 46, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, H.; Gu, H.; Li, J.; Wang, Y.; Wei, M. Development of lateral flow immunoassay system based on superparamagnetic nanobeads as labels for rapid quantitative detection of cardiac troponin I. Mater. Sci. Eng. C 2009, 29, 702–707. [Google Scholar] [CrossRef]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, E.; Myyryläinen, T.; Salminen, T.; Soukka, T.; Pettersson, K. Performance of fluorescent europium (III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal. Biochem. 2012, 428, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Wu, Y.; Xia, X.; Liao, Y.; Li, Q. Fluorescent probe-based lateral flow assay for multiplex nucleic acid detection. Anal. Chem. 2014, 86, 5611–5614. [Google Scholar] [CrossRef] [PubMed]
- Rohrman, B.A.; Leautaud, V.; Molyneux, E.; Richards-Kortum, R.R. A lateral flow assay for quantitative detection of amplified HIV-1 RNA. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, N.; Keiichiro, Y.; Hiromi, U.; Ritsuko, K.; Tadahiro, S.; Kazuyoshi, I.; Masato, S.; Toshiro, M.; Eiichi, T. Detection of influenza virus using a lateral flow immunoassay for amplified DNA by a microfluidic RT-PCR chip. Analyst 2012, 137, 3422–3426. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.T.; Kotanen, C.N.; Carrara, S.; Guiseppi-Elie, A.; Moussy, F.G. Diagnostic tools and technologies for infectious and non-communicable diseases in low-and-middle-income countries. Health Technol. 2013, 3, 271–281. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Perkel, J.M. Microfluidics, macro-impacts. Biotechniques 2012, 52, 131–134. [Google Scholar] [PubMed]
- Pollock, N.R.; Rolland, J.P.; Kumar, S.; Beattie, P.D.; Jain, S.; Noubary, F.; Wong, V.L.; Pohlmann, R.A.; Ryan, U.S.; Whitesides, G.M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152–129. [Google Scholar] [CrossRef] [PubMed]
- Hugo, S.; Land, K.; Madou, M.; Kido, H. A centrifugal microfluidic platform for point-of-care diagnostic applications. South Afr. J. Sci. 2014, 110, 1–7. [Google Scholar] [CrossRef]
- Taylor, B.J.; Howell, A.; Martin, K.A.; Manage, D.P.; Gordy, W.; Campbell, S.D.; Lam, S.; Jin, A.; Polley, S.D.; Samuel, R.A.; et al. A lab-on-chip for malaria diagnosis and surveillance. Malar. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Jiang, X.; Zhao, W.; Liu, S.; Cheng, X.; Sui, G. Microfluidic Platform for Direct Capture and Analysis of Airborne Mycobacterium tuberculosis. Anal. Chem. 2014, 86, 5815–5821. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Morarka, A.; Bodas, D.; Paknikar, K.M. Multiplexed detection of waterborne pathogens in circular microfluidics. Appl. Biochem. Biotechnol. 2012, 167, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.; Schnippel, K.; Ndibongo, B.; Long, L.; Fox, M.P.; Rosen, S. How to estimate the cost of point-of-care CD4 testing in program settings: An example using the Alere Pima Analyzer in South Africa. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Mnyani, C.N.; McIntyre, J.A.; Myer, L. The reliability of point-of-care CD4 testing in identifying HIV-infected pregnant women eligible for antiretroviral therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 60, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.E.; Caron, J.E.; Genzen, J.R. Liver function testing on the Abaxis Piccolo Xpress: Use in Ebola virus disease protocols. Clin. Chim. Acta 2015, 446, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Laksanasopin, T.; Guo, T.W.; Nayak, S.; Sridhara, A.A.; Xie, S.; Olowookere, O.O.; Cadinu, P.; Meng, F.; Chee, N.H.; Kim, J.; et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; Gous, N.; Carmona, S.; Stevens, W. Laboratory Evaluation of the Liat HIV Quant (IQuum) Whole-Blood and Plasma HIV-1 Viral Load Assays for Point-of-Care Testing in South Africa. J. Clin. Microbiol. 2015, 53, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Weigl, B.; Domingo, G.; LaBarre, P.; Gerlach, J. Towards non-and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip 2008, 8, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Dimov, I.K.; Basabe-Desmonts, L.; Garcia-Cordero, J.L.; Ross, B.M.; Ricco, A.J.; Lee, L.P. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 2011, 11, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Nwankire, C.; Gorkin, R.; Siegrist, J.; Gaughran, J.; Chan, D.S.; Ducrée, J. Full integration and automation of immunoassay protocols by rotationally actuated dissolvable film valves. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011.
- Glynn, M.; Kirby, D.; Chung, D.; Kinahan, D.J.; Kijanka, G.; Ducrée, J. Centrifugo-magnetophoretic purification of CD4+ cells from whole blood toward future HIV/AIDS point-of-care applications. J. Lab. Autom. 2013, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L. i-STAT—Combining Chemistry and Haematology in PoCT. Clin. Biochem. Rev. 2010, 31, 81–84. [Google Scholar] [PubMed]
- Mainor, B.H.; Hardwick, W.E.; King, W.D. Evaluation of a portable clinical analyzer in the pediatric emergency department: Analysis of cost and turnaround time. South. Med. J. 2002, 95, 634–638. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O'Kennedy, R. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors 2015, 5, 577-601. https://doi.org/10.3390/bios5030577
Sharma S, Zapatero-Rodríguez J, Estrela P, O'Kennedy R. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors. 2015; 5(3):577-601. https://doi.org/10.3390/bios5030577
Chicago/Turabian StyleSharma, Shikha, Julia Zapatero-Rodríguez, Pedro Estrela, and Richard O'Kennedy. 2015. "Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics" Biosensors 5, no. 3: 577-601. https://doi.org/10.3390/bios5030577
APA StyleSharma, S., Zapatero-Rodríguez, J., Estrela, P., & O'Kennedy, R. (2015). Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors, 5(3), 577-601. https://doi.org/10.3390/bios5030577