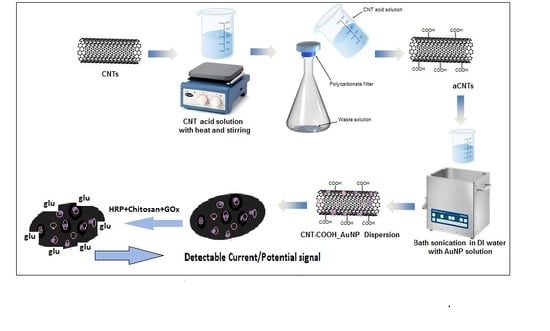
Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Fabrication of Functionalized Buckypaper
| BP Sample | Composition and processing | GOx Immobilization |
|---|---|---|
| BP-1 |
|
|
| BP-2 |
|
|
| BP-3 |
|
|
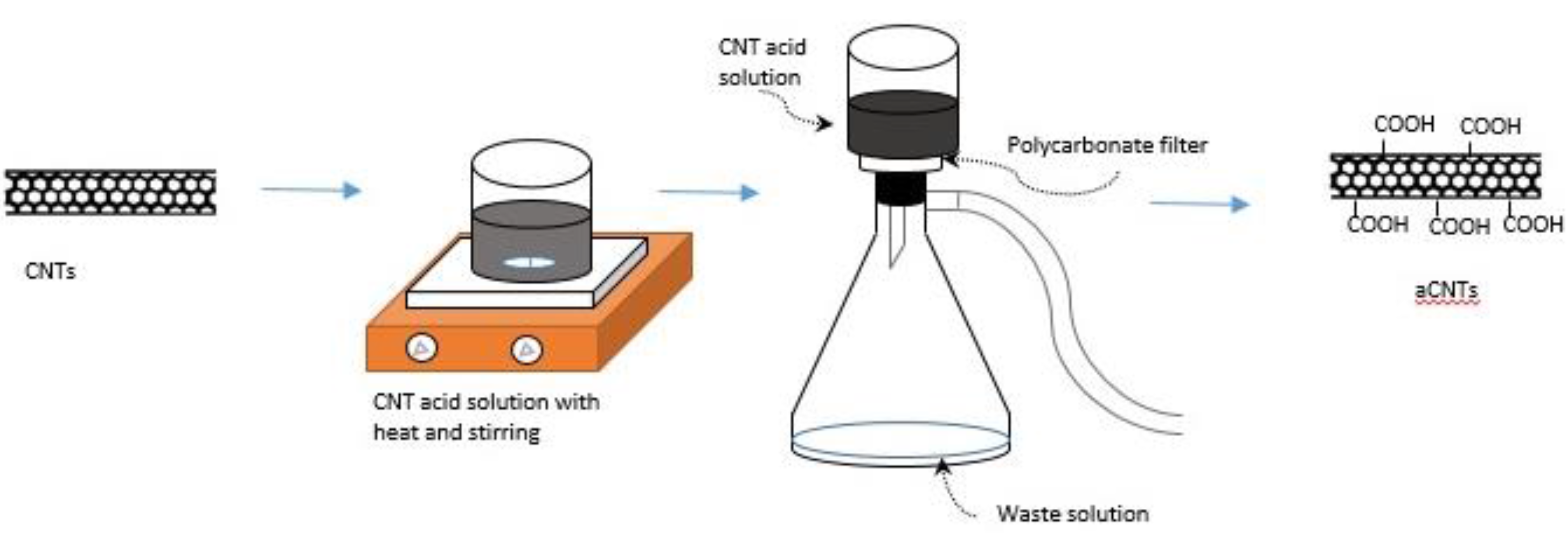
2.2.2. Characterization of Buckypaper
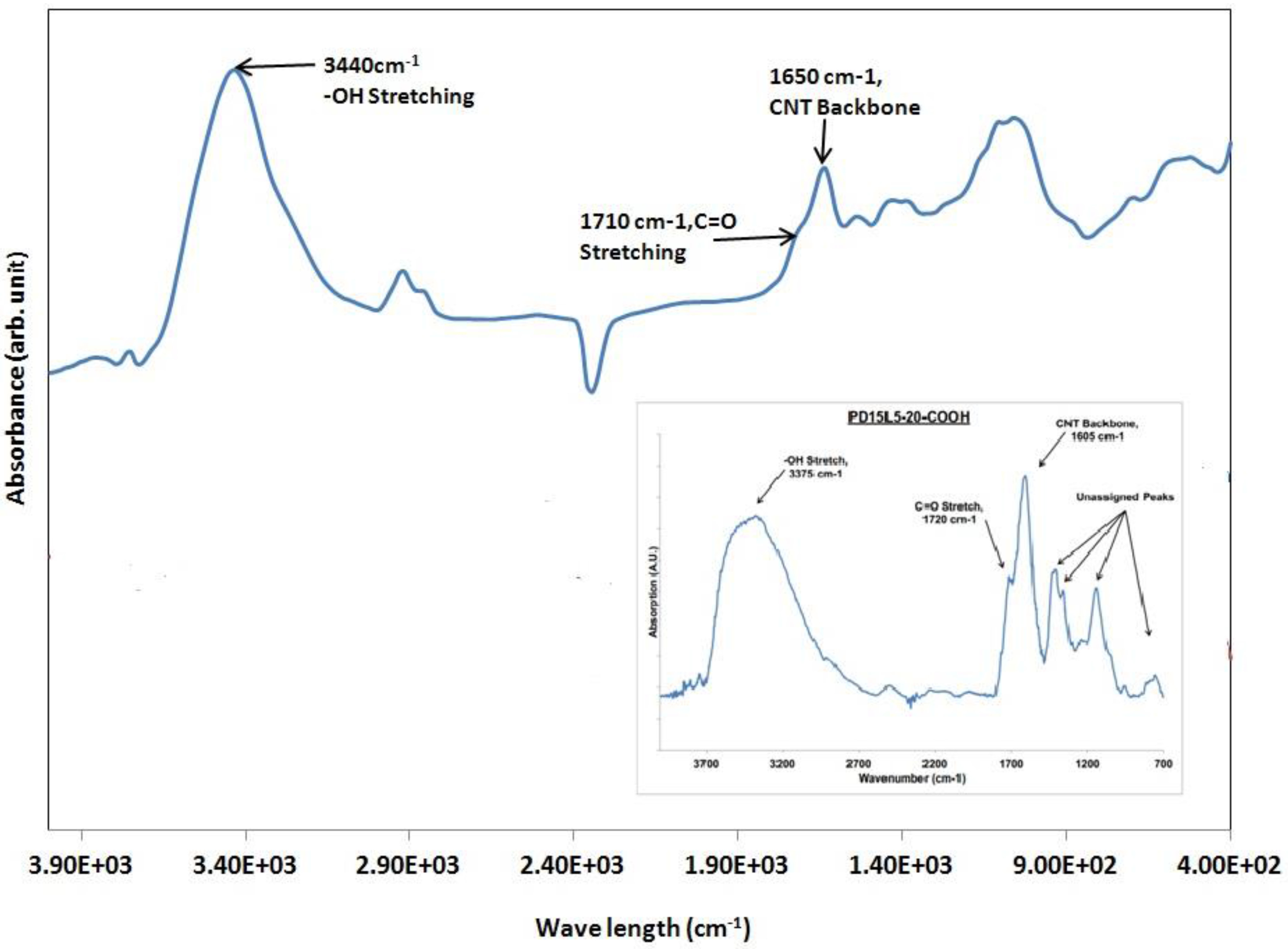
Fourier Transform Infrared Spectroscopy (FTIR) for Acid Modified Carbon Nanotubes
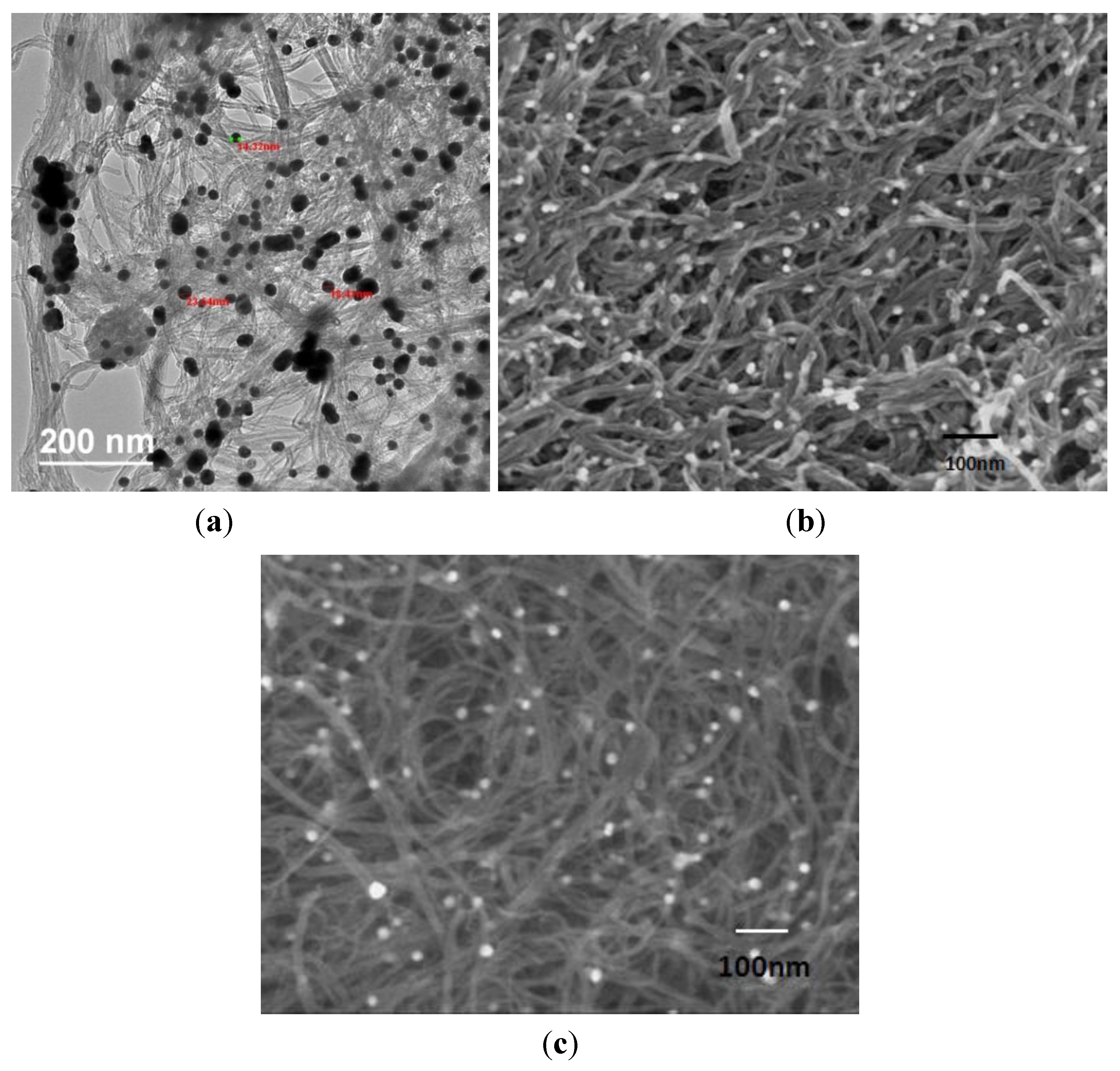
Electron Microscopy and Electrical Conductivity
Electrochemical Characterization and Sensor Evaluation
3. Results and Discussion
3.1. Structure and Morphology for Buckypaper Electrode
3.2. Electrochemical Properties for Buckypaper Biosensors
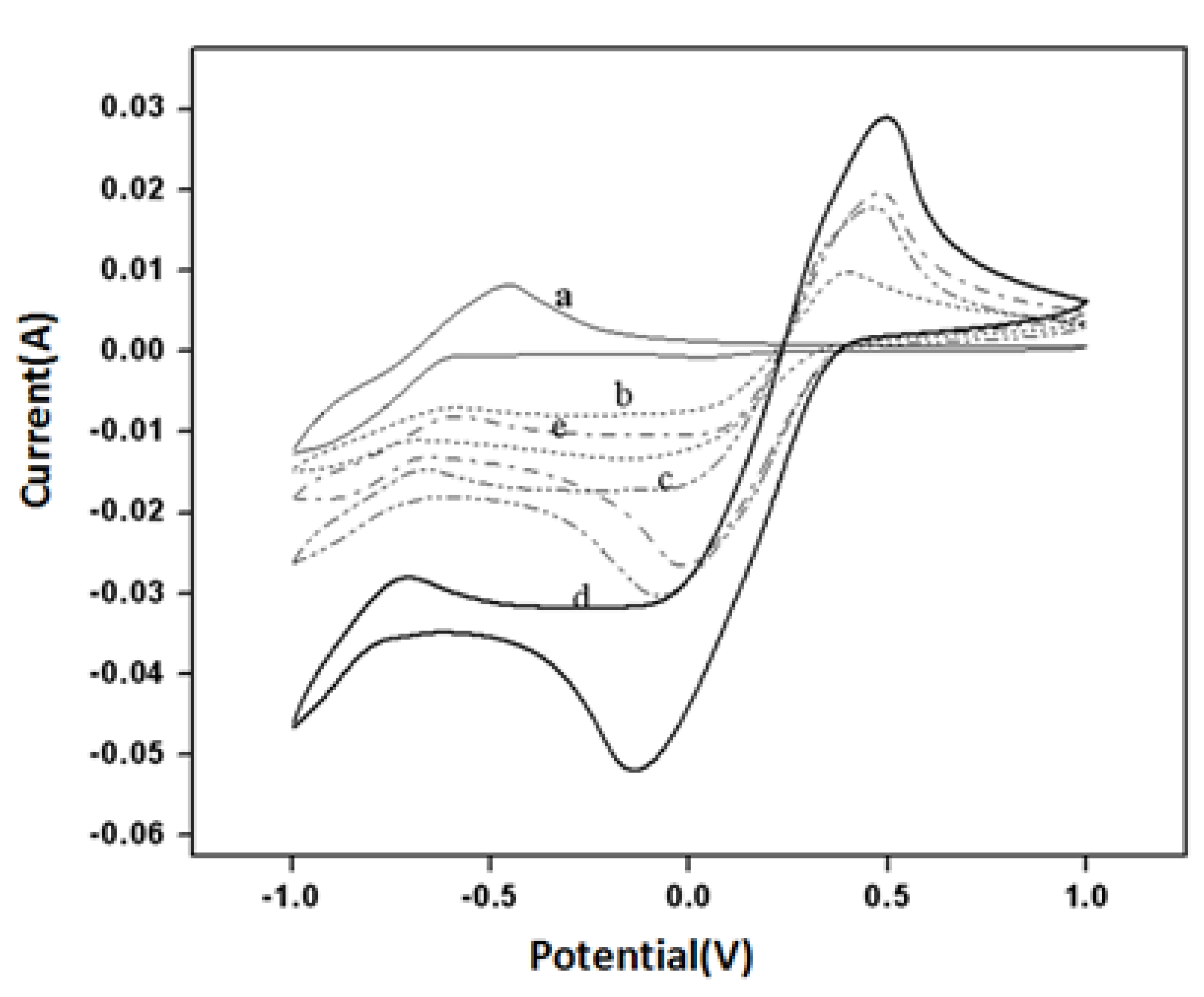
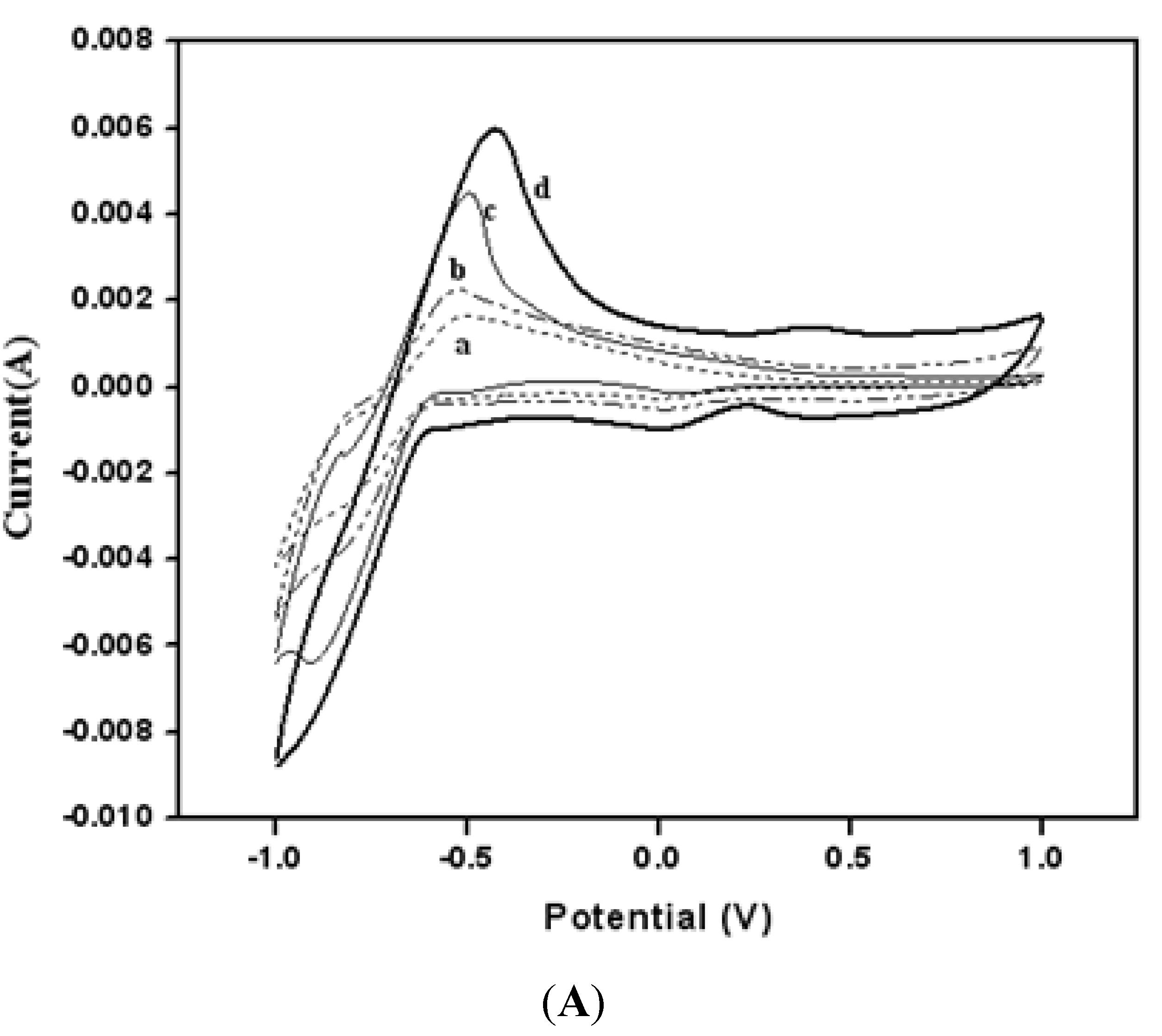
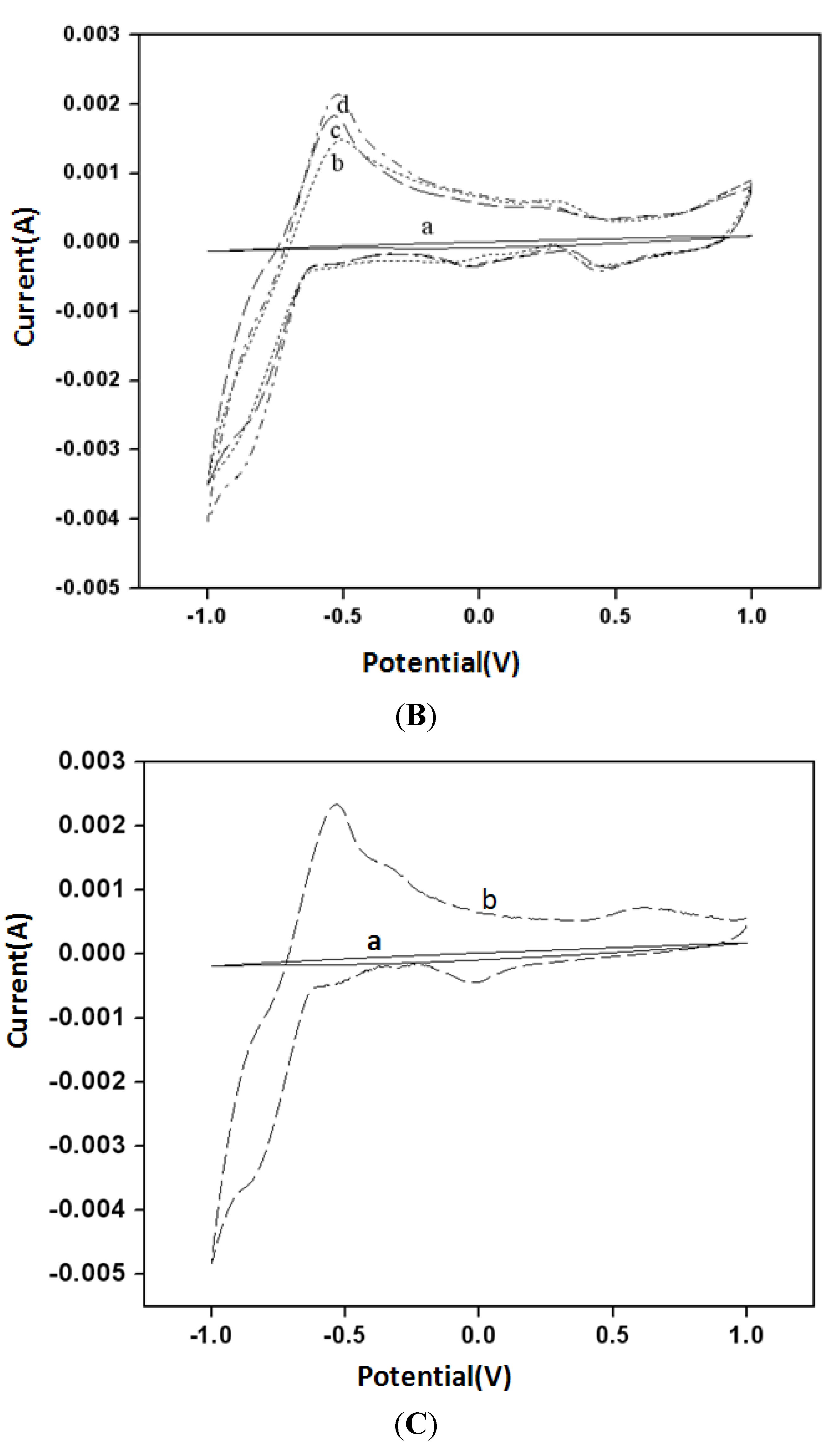
3.3. Amperometry and Glucose Biosensor Sensitivity
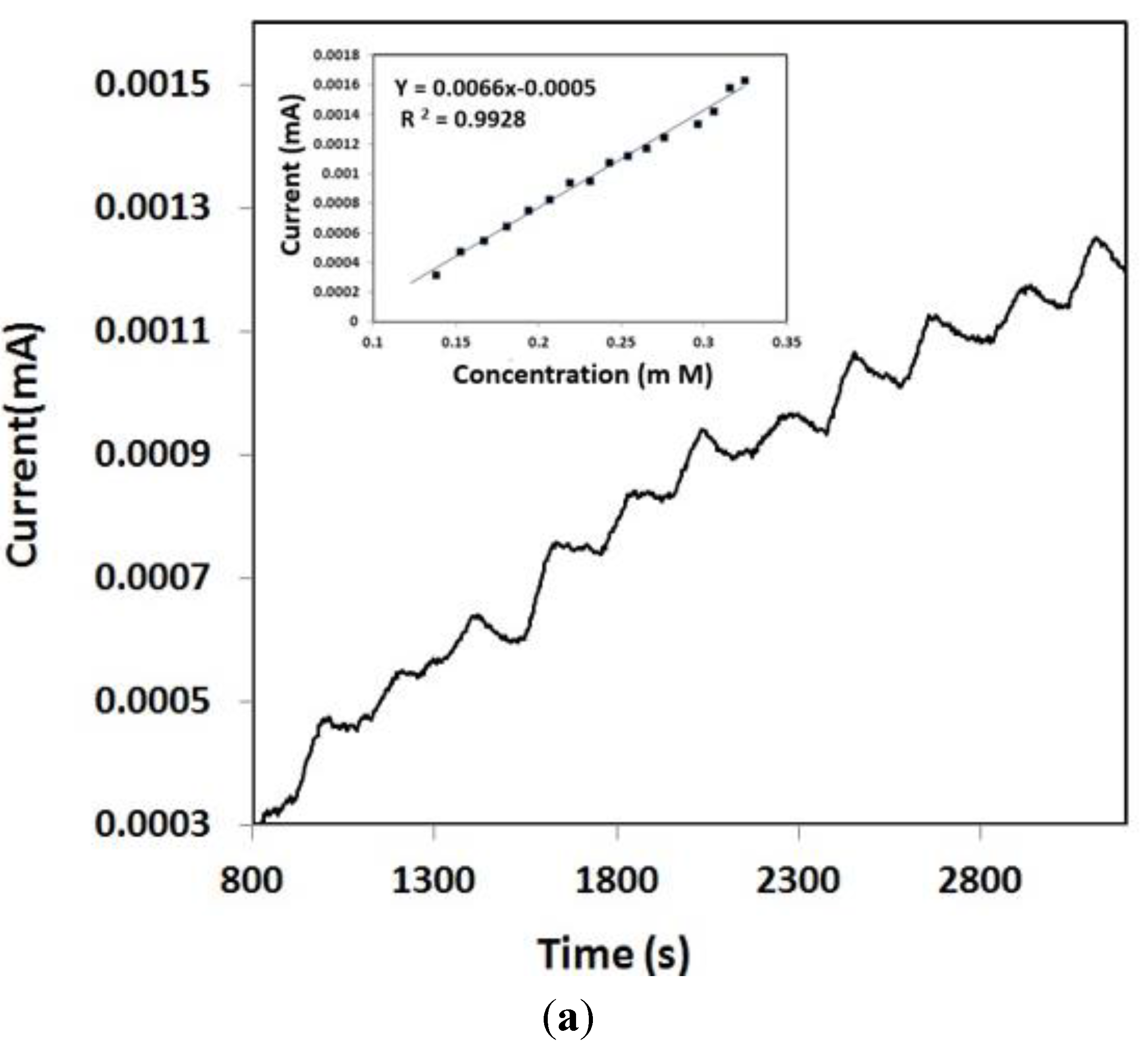
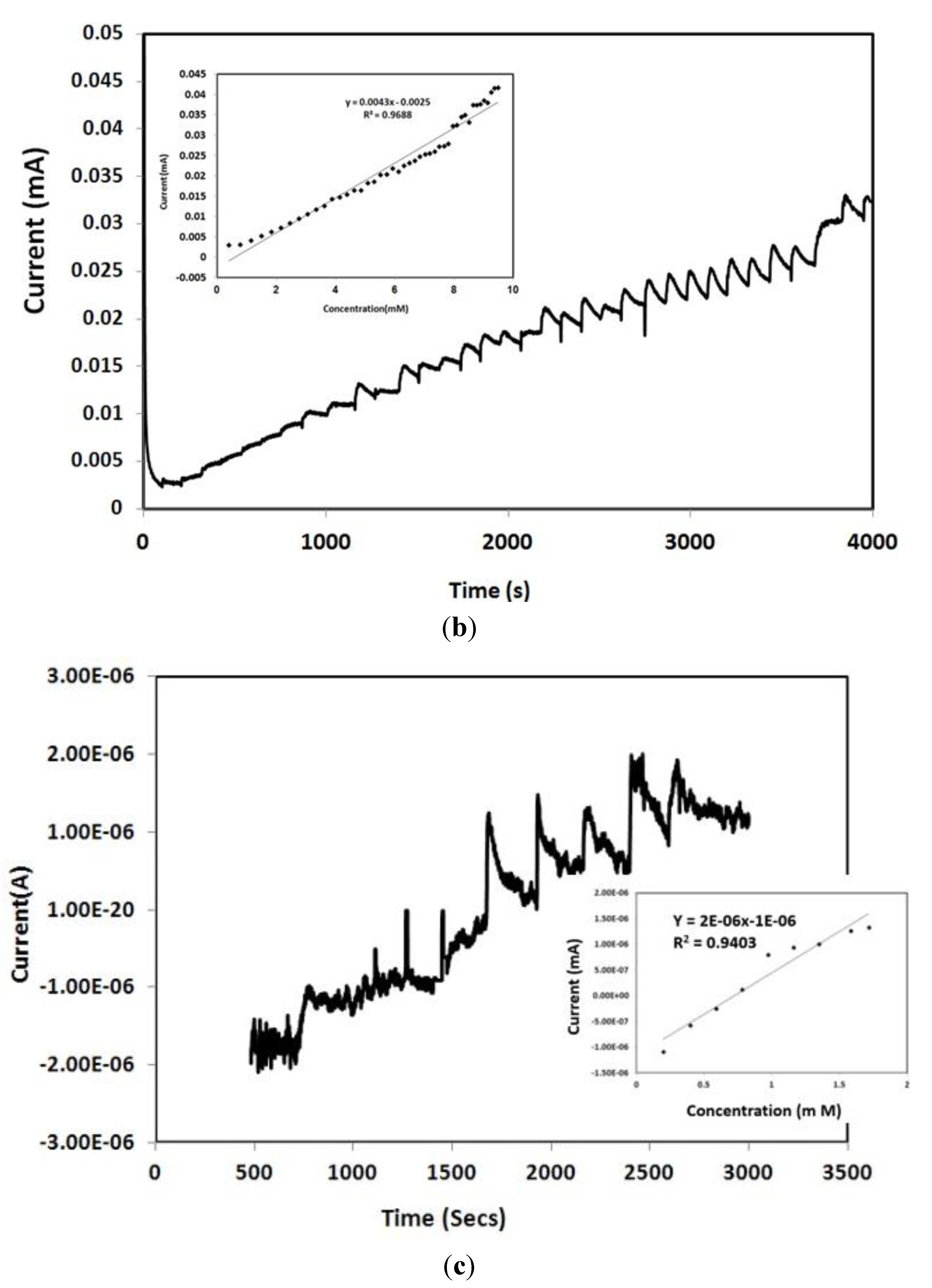
3.4. Selectivity of the Biosensor
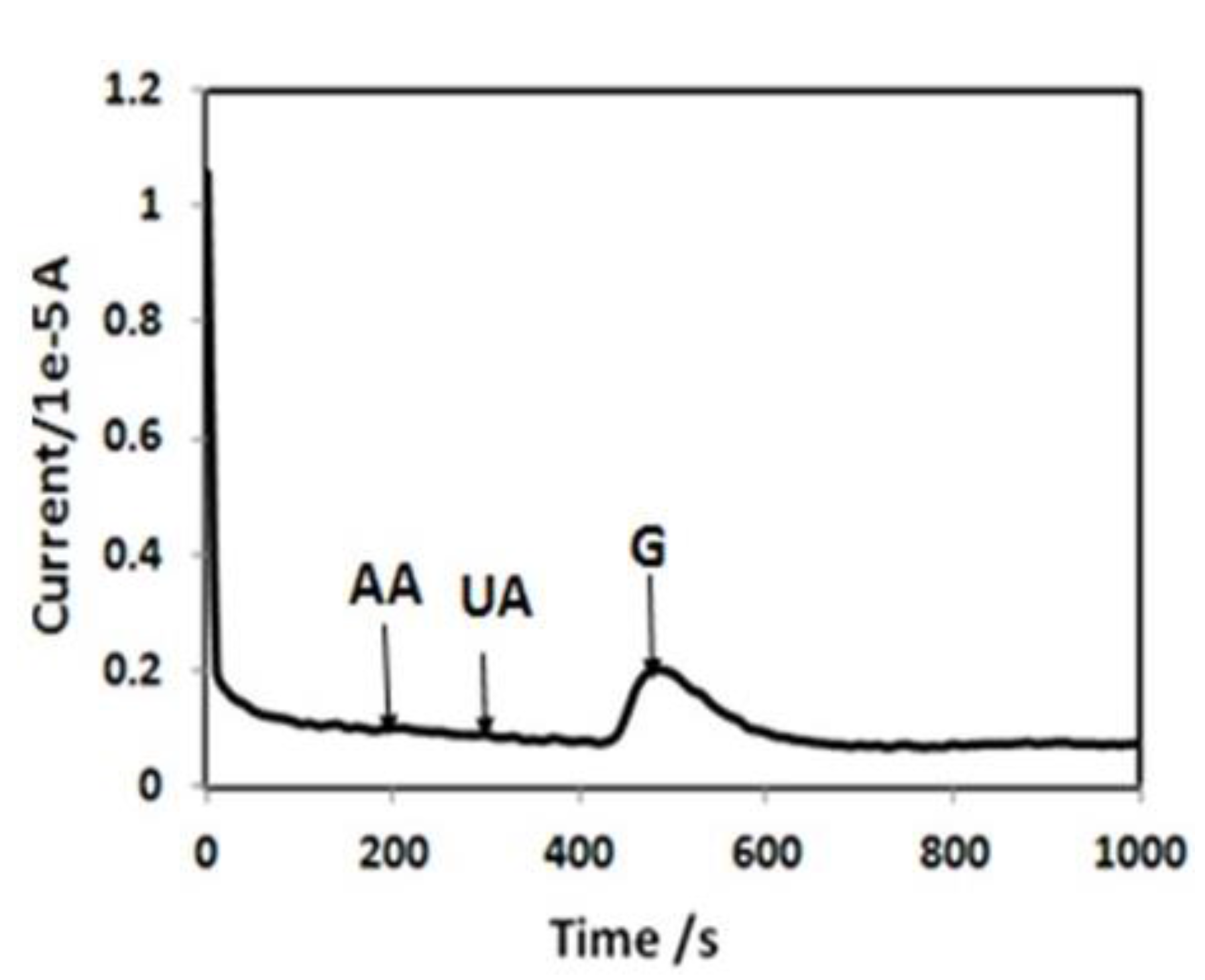
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siskin, E. Insulin hyperglycemia in Epilepsy. Arch. Neurol. Psychiatry 1936, 36, 331–341. [Google Scholar]
- Fryer, B.M.; Schernthaner, G.; Heller, S.R. Hypoglycemia and cardiovascular risks. Diabetes Care 2011, 34, S132–S137. [Google Scholar]
- Lin, C.; Park, Y.; Liang, Z.; Zhang, C.; Wang, B. Science and applications of buckypaper. Encyclopaedia Nanosci. Nanotech. 2011, 22, 461–482. [Google Scholar]
- Lin, Y.; Fang, L.; Yi, T.; Ren, Z. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar]
- Ahmadalinezhad, A.; Guosheng, W.; Chen, A. Mediator-free electrochemical biosensor based on buckypaper with enhanced stability and sensitivity for glucose detection. Biosens. Bioelectron. 2011, 30, 287–293. [Google Scholar]
- Kumar, N.A.; Kim, S.H.; Kim, J.S.; Kim, J.T.; Jeong, Y.T. Functionalization of multi-walled carbon nanotubes with cysteamine for the construction of CNT/Gold nanoparticle hybrid nanostructures. Surf. Rev. Lett. 2009, 16, 487–492. [Google Scholar]
- Kumar, N.A.; Bund, A.; Cho, B.G.; Lim, K.T.; Jeong, Y.T. Novel amino-acid-based polymer/multi-walled carbon nanotube bio-nanocomposites: Highly water dispersible carbon nanotubes decorated with gold nanoparticles. Nanotechnology 2009, 20, 225608–225616. [Google Scholar]
- Parviz, N.; Farnoush, F.; Bagher, L.; Ganjali, M.R. Glucose biosensor based on MWCNTs-gold nanoparticles in a nafion film on the glassy carbon electrode using flow injection FFT continuous cyclic voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1213–1224. [Google Scholar]
- Cai, D.; Yu, Y.; Lan, Y.; Dufort, F.J.; Xiong, G.; Paudel, T.; Ren, Z.; Wagner, D.J.; Chiles, T.C. Glucose sensors made of novel carbon nanotube-gold nanoparticle composites. Biofactors 2007, 30, 271–277. [Google Scholar]
- Zhang, F.; Cho, S.S.; Yang, S.H.; Seo, S.S.; Cha, G.S.; Nam, H. Gold nanoparticle-based mediatorless biosensor prepared on microporous electrode. Electroanalysis 2006, 18, 217–222. [Google Scholar]
- German, N.; Ramanaviciene, A.; Voronovic, J.; Ramanavicius, A. Glucose biosensor based on graphite electrodes modified with glucose oxidase and colloidal gold nanoparticles. Microchim. Acta 2010, 168, 221–229. [Google Scholar]
- Chang, A.; Orth, A.; Le, B.; Menchavez, P.; Miller, L. Performance analysis of the OneTouch® UltraVue™ blood glucose monitoring system. J. Diabetes Sci. Technol. 2009, 3, 1158–1165. [Google Scholar]
- Pasta, M.; Mantia, F.L.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochim. Acta 2010, 55, 5561–5568. [Google Scholar]
- Dash, S.; Munichandraaiah, N. Non-enzymatic electroanalysis of glucose on electrodeposited Au-PEDOT dendrites. ECS Eletrochem. Lett. 2013, 2, B17–B20. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, H.; Gaillard, M.; Gonzalez, L.; Chatterjee, J. Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors. Biosensors 2014, 4, 449-460. https://doi.org/10.3390/bios4040449
Papa H, Gaillard M, Gonzalez L, Chatterjee J. Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors. Biosensors. 2014; 4(4):449-460. https://doi.org/10.3390/bios4040449
Chicago/Turabian StylePapa, Henry, Melissa Gaillard, Leon Gonzalez, and Jhunu Chatterjee. 2014. "Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors" Biosensors 4, no. 4: 449-460. https://doi.org/10.3390/bios4040449
APA StylePapa, H., Gaillard, M., Gonzalez, L., & Chatterjee, J. (2014). Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors. Biosensors, 4(4), 449-460. https://doi.org/10.3390/bios4040449