Fiber-Optic Fluoroimmunoassay System with a Flow-Through Cell for Rapid On-Site Determination of Escherichia coli O157:H7 by Monitoring Fluorescence Dynamics
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Construction of Flow-Through System for E. coli O157:H7 Detection
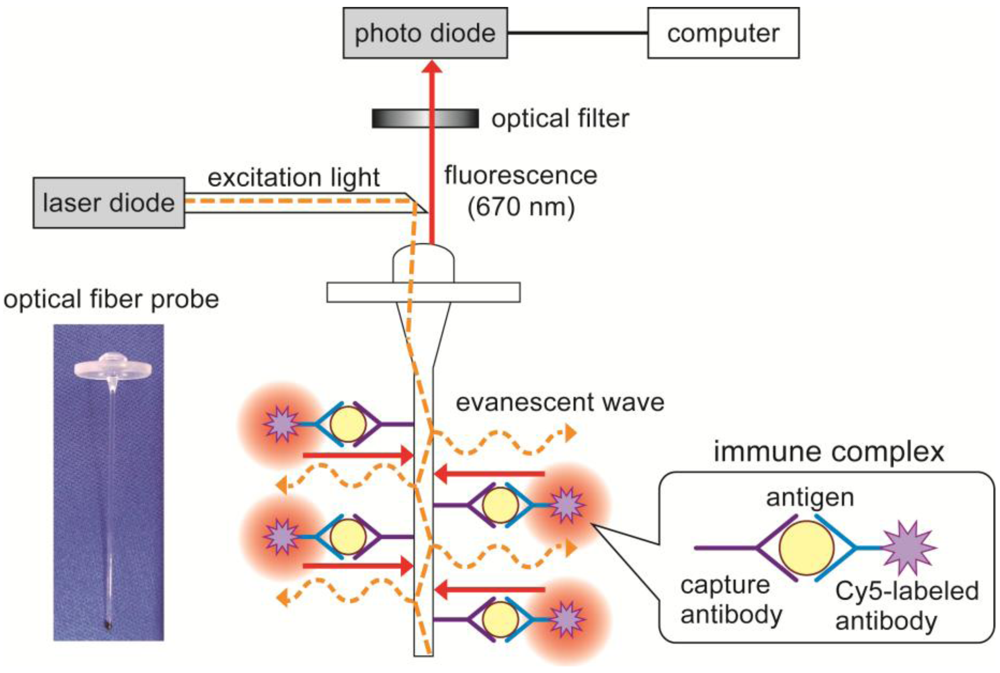
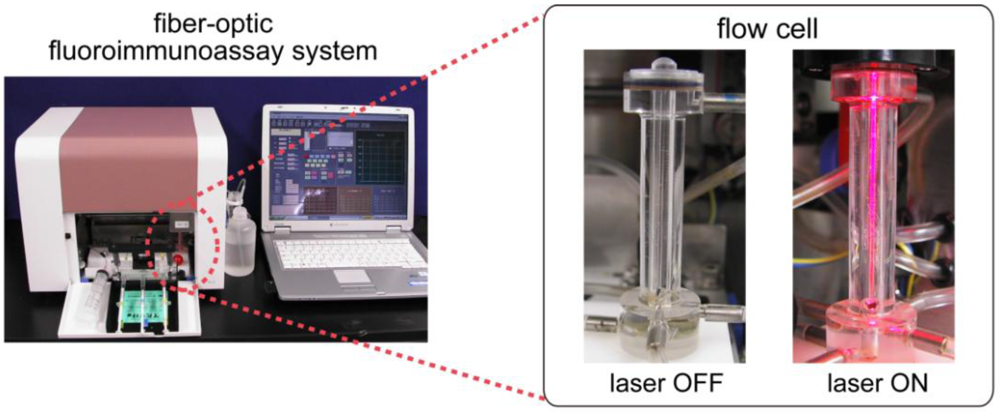
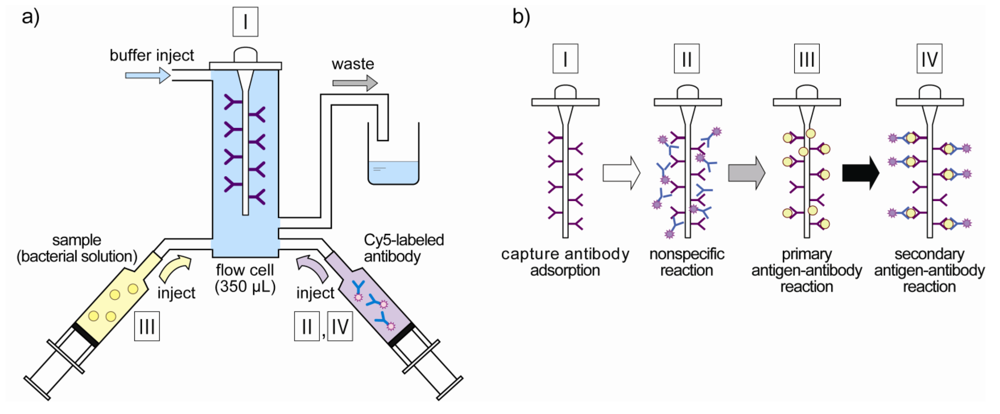
2.3. Characterization of the Flow-Through System
3. Results and Discussion
3.1. Measurement of E. coli O157:H7

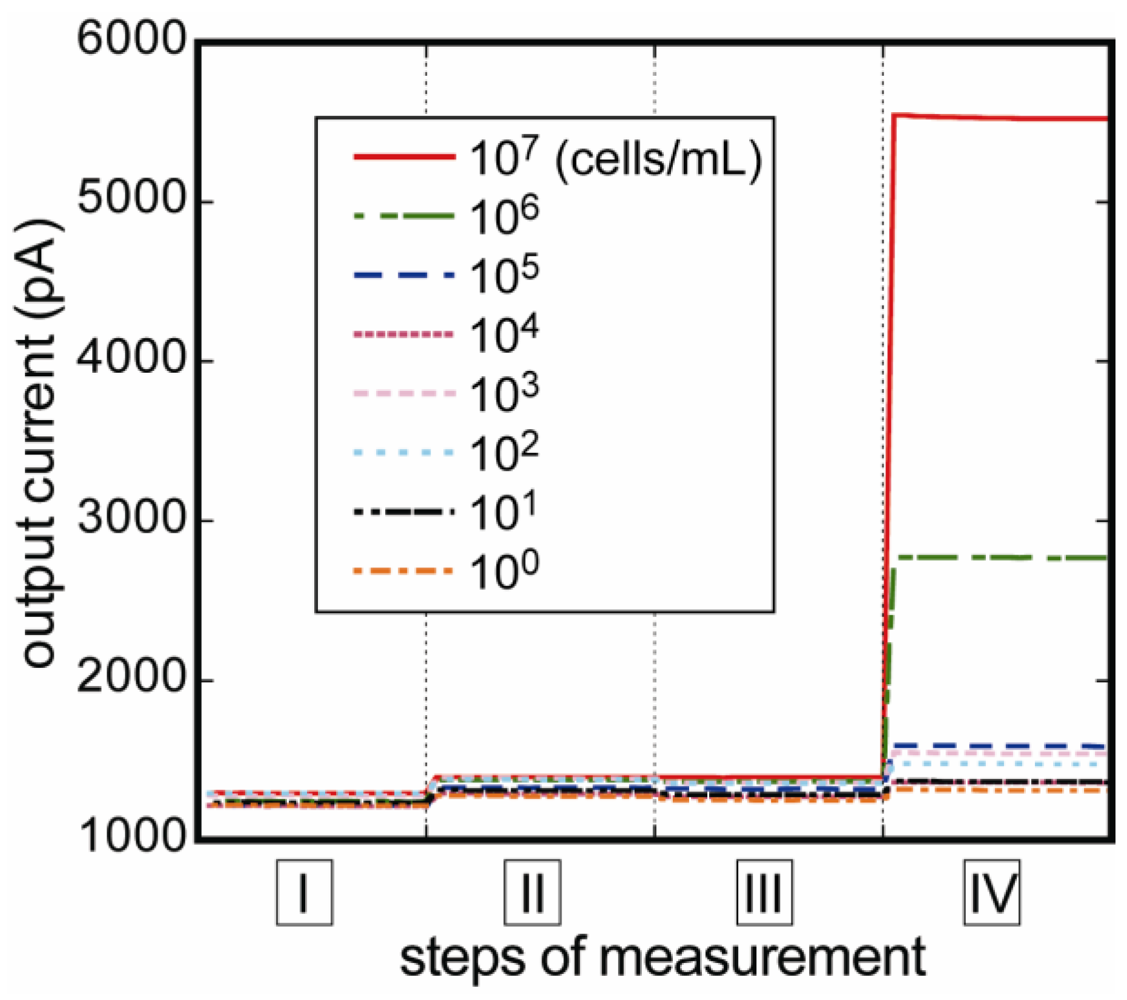
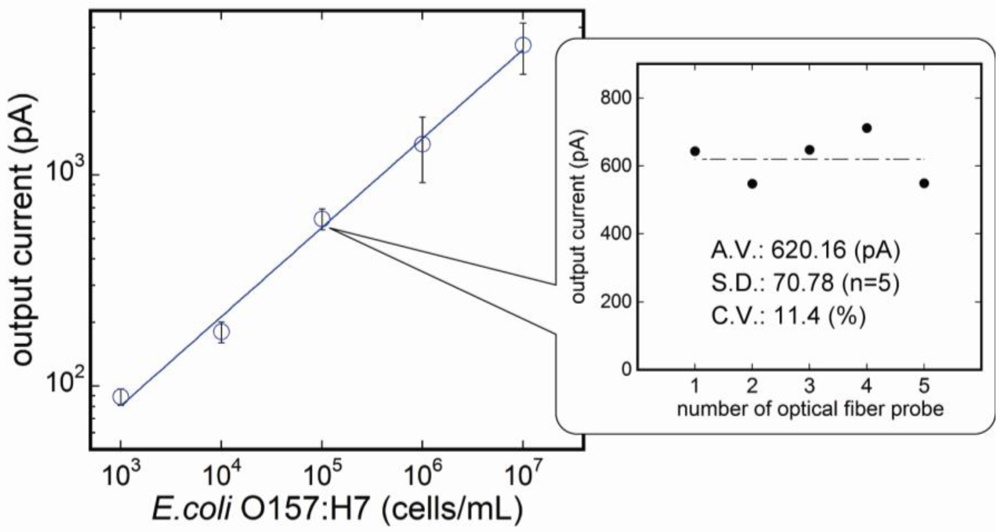
3.2. Reproducibility of the Fluoroimmunosensor
3.3. Fluorescence Monitoring and Measuring of E. coli O157:H7 by the Flow-Through System

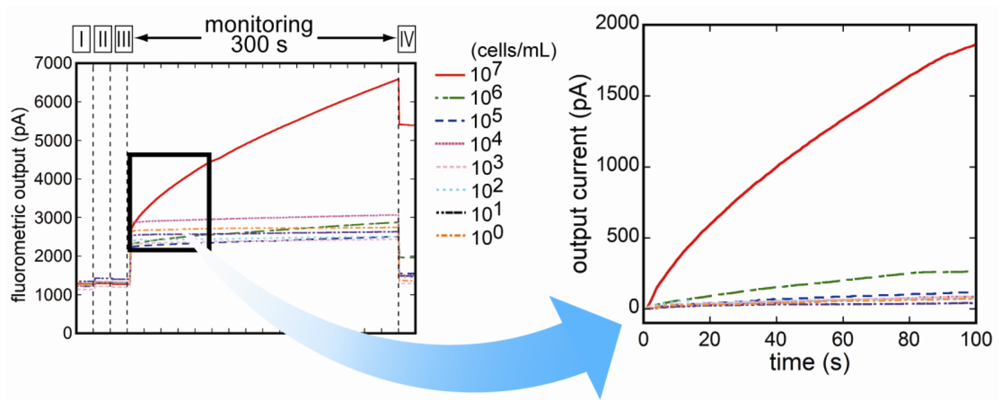
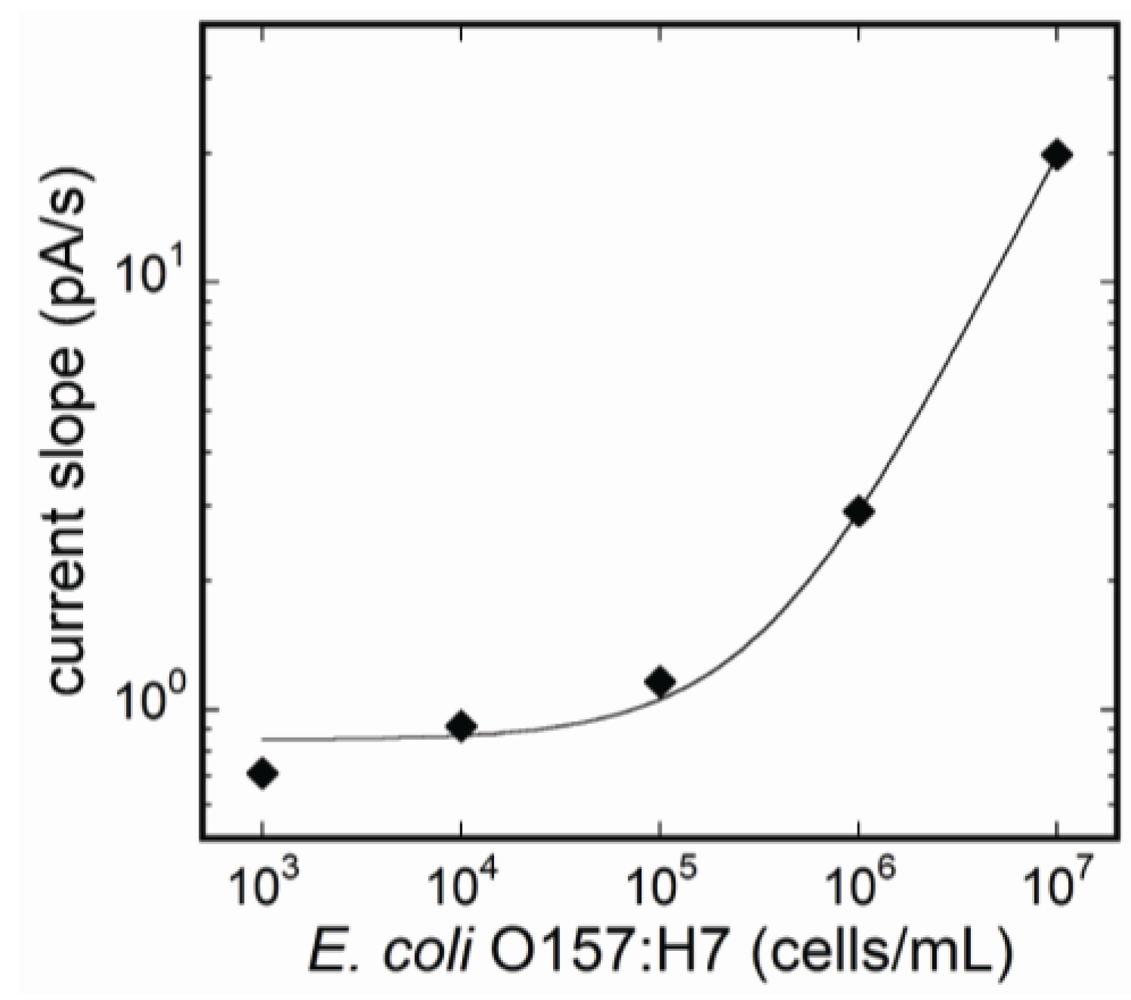
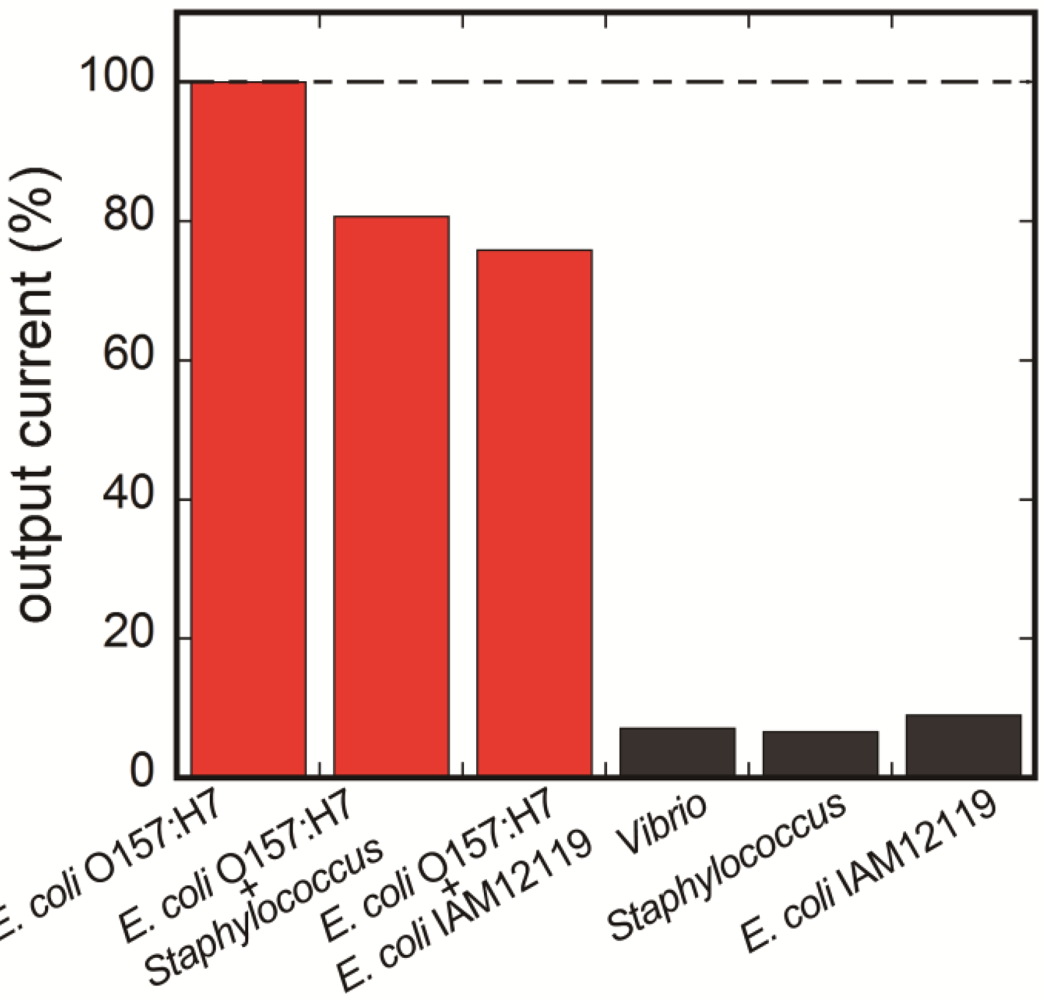
3.4. Selectivity of the Fluoroimmunosensor for E. coli O157:H7
4. Conclusions
Acknowledgments
References
- Tilden, J.J.; Young, W.; McNamara, A.M.; Custer, C.; Boesel, B.; Lambert-Fair, M.A.; Majkowski, J.; Vugia, D.; Werner, S.B.; Hollingsworth, J.; Morris, J.G.J. A new route of transmission for Escherichia coli: Infection from dry fermented salami. Am. J. Publ. Health 1996, 86, 1142–1145. [Google Scholar] [CrossRef]
- O’Brien, A.O.; Lively, T.A.; Chen, M.E.; Rothman, S.W.; Formal, S.B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet 1983, 321. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; Blake, P.A.; Cohen, M.L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Mead, P.S.; Griffin, P.M. Escherichia coli O157:H7. Lancet 1998, 352, 1207–1212. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar]
- Michino, H.; Araki, K.; Minami, S.; Takaya, S.; Sakai, N.; Miyazaki, M.; Ono, A.; Yanagawa, H. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 1999, 150, 787–796. [Google Scholar] [CrossRef]
- Chapman, P.A.; Cerdan, Malo, A.T.; Siddons, C.A.; Harkin, M. Use of commercial enzyme immunoassays and immunomagnetic separation systems for detecting Escherichia coli O157 in bovine fecal samples. Appl. Environ. Microbiol. 1997, 63, 2549–2553. [Google Scholar]
- Hammack, T.S.; Feng, P.; Amaguaña, R.M.; June, G.A.; Sherrod, P.S.; Andrews, W.H. Comparison of sorbitol MacConkey and hemorrhagic coli agars for recovery of Escherichia coli O157:H7 from brie, ice cream, and whole milk. J. AOAC Int. 1997, 80, 335–340. [Google Scholar]
- Edberg, S.C.; Kontnick, C.M. Comparison of β-glucuronidase-based substrate systems for identification of Escherichia coli. J. Clin. Microbiol. 1986, 24, 368–371. [Google Scholar]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Wang, H.; Sharpe, A.N. An immuno-capturing and concentrating procedure for Escherichia coli O157:H7 and its detection by epifluorescence microscopy. Food Microbiol. 1998, 15, 559–565. [Google Scholar] [CrossRef]
- West, J.M.; Tsuruta, H.; Kantrowitz, E.R. A fluorescent probe-labeled Escherichia coli aspartate transcarbamoylase that monitors the allosteric conformational state. J. Biol. Chem. 2004, 279, 945–951. [Google Scholar]
- Pérez, F.G.; Mascini, M.; Tothill, I.E.; Turner, A.P. Immunomagnetic separation with mediated flow injection analysis amperometric detection of viable Escherichia coli O157. Anal. Chem. 1998, 70, 2380–2386. [Google Scholar] [CrossRef]
- Wright, D.J.; Chapman, P.A.; Siddons, C.A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol. Infect. 1994, 113, 31–39. [Google Scholar] [CrossRef]
- Su, X.L.; Li, Y. Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157:H7. Anal. Chem. 2004, 76, 4806–4810. [Google Scholar] [CrossRef]
- Mo, X.T.; Zhou, Y.P.; Lei, H.; Deng, L. Microbalance-DNA probe method for the detection of specific bacteria in water. Enzyme Microb. Technol. 2002, 30, 583–589. [Google Scholar] [CrossRef]
- Wang, R.F.; Cao, W.W.; Johnson, M.G. 16S rRNA-based probes and polymerase chain reaction method to detect Listeria monocytogenes cells added to foods. Appl. Environ. Microbiol. 1992, 58, 2827–2831. [Google Scholar]
- Su, X.; Low, S.; Kwang, J.; Chew, V.H.T.; Li, S.F.Y. Piezoelectric quartz crystal based veterinary diagnosis for Salmonella enteritidis infection in chicken and egg. Sens. Actuator. B Chem. 2001, 75, 29–35. [Google Scholar] [CrossRef]
- Uchida, H.; Fujitani, K.; Kawai, Y.; Kitazawa, H.; Horii, A.; Shiiba, K.; Saito, K.; Saito, T. A new assay using surface plasmon resonance (SPR) to determine binding of the Lactobacillus acidophilus group to human colonic mucin. Biosci. Biotechnol. Biochem. 2004, 68, 1004–1010. [Google Scholar] [CrossRef]
- Jindou, S.; Soda, A.; Karita, S.; Kajino, T.; Béguin, P.; Wu, J.H.D.; Inagaki, M.; Kimura, T.; Sakka, K.; Ohmiya, K. Cohesin-dockerin interactions within and between Clostridium josui and Clostridium thermocellum: Binding selectivity between cognate dockerin and cohesin domains and species specificity. J. Biol. Chem. 2004, 279, 9867–9874. [Google Scholar]
- Hardegger, D.; Nadal, D.; Bossart, W.; Altwegg, M.; Dutly, F. Rapid detection of Mycoplasma pneumoniae in clinical samples by real-time PCR. J. Microbiol. Methods 2000, 41, 45–51. [Google Scholar] [CrossRef]
- Ahmed, N.; Mohanty, A.K.; Mukhopadhyay, U.; Batish, V.K.; Grover, S. PCR-based rapid detection of Mycobacterium tuberculosis in blood from immunocompetent patients with pulmonary tuberculosis. J. Clin. Microbiol. 1998, 36, 3094–3095. [Google Scholar]
- Gunasekera, T.S.; Attfield, P.V.; Veal, D.A. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl. Environ. Microbiol. 2000, 66, 1228–1232. [Google Scholar] [CrossRef]
- Marks, R.S.; Bassis, E.; Bychenko, A.; Levine, M.M. Chemiluminescent optical fiber immunosensor for detecting cholera antitoxin. Opt. Eng. 1997, 36, 3258–3264. [Google Scholar] [CrossRef]
- Petrosova, A.; Konry, T.; Cosnier, S.; Trakht, I.; Lutwama, J.; Rwaguma, E.; Chepurnov, A.; Mühlberger, E.; Lobel, L.; Marks, R.S. Development of a highly sensitive, field operable biosensor for serological studies of Ebola virus in central Africa. Sens. Actuator. B Chem. 2007, 122, 578–586. [Google Scholar] [CrossRef]
- Sobarzo, A.; Paweska, J.T.; Herrmann, S.; Amir, T.; Marks, R.S.; Lobel, L. Optical fiber immunosensor for the detection of IgG antibody to Rift Valley fever virus in humans. J. Virol. Methods 2007, 146, 327–334. [Google Scholar] [CrossRef]
- Petrovich, M.N.; van Brakel, A.; Poletti, F.; Mukasa, K.; Austin, E.; Finazzi, V.; Petropoulos, P.; O'Driscoll, E.; Watson, M.; DelMonte, T.; Monro, T.M.; Dakin, J.P.; Richardson, D.J. Microstructured Fibers for Sensing Applications. In Proceedings of the SPIE Photonic Crystals and Photonic Crystal Fibers for Sensing Applications, Boston, MA, USA, October 2005; Du, H.H., Ed.; SPIE: Washington, DC, USA, 2005; pp. 78–92. [Google Scholar]
- Stewart, G.; Jin, W.; Culshaw, B. Prospects for fibre-optic evanescent-field gas sensors using absorption in the near-infrared. Sens. Actuator. B Chem. 1997, 38, 42–47. [Google Scholar] [CrossRef]
- Monro, T.M.; Belardi, W.; Furusawa, K.; Baggett, J.C.; Broderick, N.G.R.; Richardson, D.J. Sensing with microstructured optical fibres. Meas. Sci. Technol. 2001, 12, 854–859. [Google Scholar] [CrossRef]
- Lim, D.V. Detection of microorganisms and toxins with evanescent wave, fiber optic biosensors. Proc. IEEE 2003, 91, 902–907. [Google Scholar] [CrossRef]
- Golden, J.P.; Saaski, E.W.; Shriver-Lake, L.C.; Anderson, G.P.; Ligler, F.S. Portable multichannel fiber optic biosensor for field detection. Opt. Eng. 1997, 36, 1008–1013. [Google Scholar]
- Leskinen, S.D.; Schlemmer, S.M.; Kearns, E.A.; Lim, D.V. Detection of E. coli O157:H7 in Complex Matrices under Varying Flow Parameters with a Robotic Fluorometric Assay System. In Proceedings of the SPIE Frontiers in Pathogen Detection: From Nanosensors to Systems, San Francisco, CA, USA, February 2009; Fauchet, P.M., Ed.; SPIE: Washington, DC, USA, 2010; pp. 71670J1–71670J10. [Google Scholar]
- Taniguchi, M.; Akai, E.; Koshida, T.; Hibi, K.; Kudo, H.; Otsuka, K.; Saito, H.; Yano, K.; Endo, H.; Mitsubayashi, K. A fiber optic immunosensor for rapid bacteria determination. IFMBE Proc. 2007, 15, 308–311. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, W.; Si, C.; Ying, Y. Subtractive inhibition assay for the detection of E. coli O157:H7 using surface plasmon resonance. Sensors 2011, 11, 2728–2739. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miyajima, K.; Koshida, T.; Arakawa, T.; Kudo, H.; Saito, H.; Yano, K.; Mitsubayashi, K. Fiber-Optic Fluoroimmunoassay System with a Flow-Through Cell for Rapid On-Site Determination of Escherichia coli O157:H7 by Monitoring Fluorescence Dynamics. Biosensors 2013, 3, 120-131. https://doi.org/10.3390/bios3010120
Miyajima K, Koshida T, Arakawa T, Kudo H, Saito H, Yano K, Mitsubayashi K. Fiber-Optic Fluoroimmunoassay System with a Flow-Through Cell for Rapid On-Site Determination of Escherichia coli O157:H7 by Monitoring Fluorescence Dynamics. Biosensors. 2013; 3(1):120-131. https://doi.org/10.3390/bios3010120
Chicago/Turabian StyleMiyajima, Kumiko, Tomoyuki Koshida, Takahiro Arakawa, Hiroyuki Kudo, Hirokazu Saito, Kazuyoshi Yano, and Kohji Mitsubayashi. 2013. "Fiber-Optic Fluoroimmunoassay System with a Flow-Through Cell for Rapid On-Site Determination of Escherichia coli O157:H7 by Monitoring Fluorescence Dynamics" Biosensors 3, no. 1: 120-131. https://doi.org/10.3390/bios3010120
APA StyleMiyajima, K., Koshida, T., Arakawa, T., Kudo, H., Saito, H., Yano, K., & Mitsubayashi, K. (2013). Fiber-Optic Fluoroimmunoassay System with a Flow-Through Cell for Rapid On-Site Determination of Escherichia coli O157:H7 by Monitoring Fluorescence Dynamics. Biosensors, 3(1), 120-131. https://doi.org/10.3390/bios3010120



