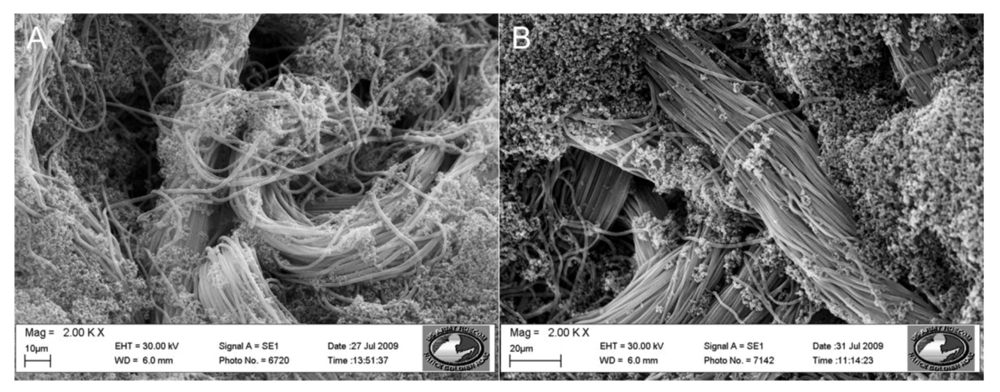
Synthesis of a Functionalized Polypyrrole Coated Electrotextile for Use in Biosensors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.2.1. Functional Group Selection
2.2.2. Dopant Inclusion and Solvent Selection
2.2.3. Post-Polymerization Treatment
2.2.4. Fiber Platform Selection
2.3. Physicochemical Characterization
2.4. Biological Experiments
2.4.1. Optical Analysis
2.4.2. Electrochemical Analysis

3. Results and Discussion
| Platform | Monomer | Dopant | Solvent | Reaction Time | Wash Treatment | Resistance | Figure # | Notes |
|---|---|---|---|---|---|---|---|---|
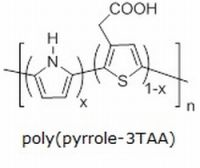
| Nylon 6 | 3-COOH/Pyrrole | none | acetonitrile | 18 h | none | 397 kΩ | 1(A) | Coating even, black, and conformal on fibers. Heavy buildup of polymer clusters along fibers. |
| Nylon 6 | 3-TAA/Pyrrole | none | acetonitrile | 18 h | none | 23.71 kΩ | 1(B) | Coating even, black, and conformal on fibers. Clusters of polymers buds scattered along fibers. |
| Nylon 6 | 3-TAA/Pyrrole | none | acetonitrile | 30 min | none | 189.98 kΩ | none | Coating uneven, gray, and brittle. |
| Nylon 6 | 3-TAA/Pyrrole | 5SSA | acetonitrile | 30 min | none | 291 kΩ | none | Coating uneven, gray, with dark black spots, brittle. |
| Nylon 6 | 3-TAA/Pyrrole | none | methanol | 30 min | none | 35.31 MΩ | none | Coating uneven, gray, slight brittleness. |
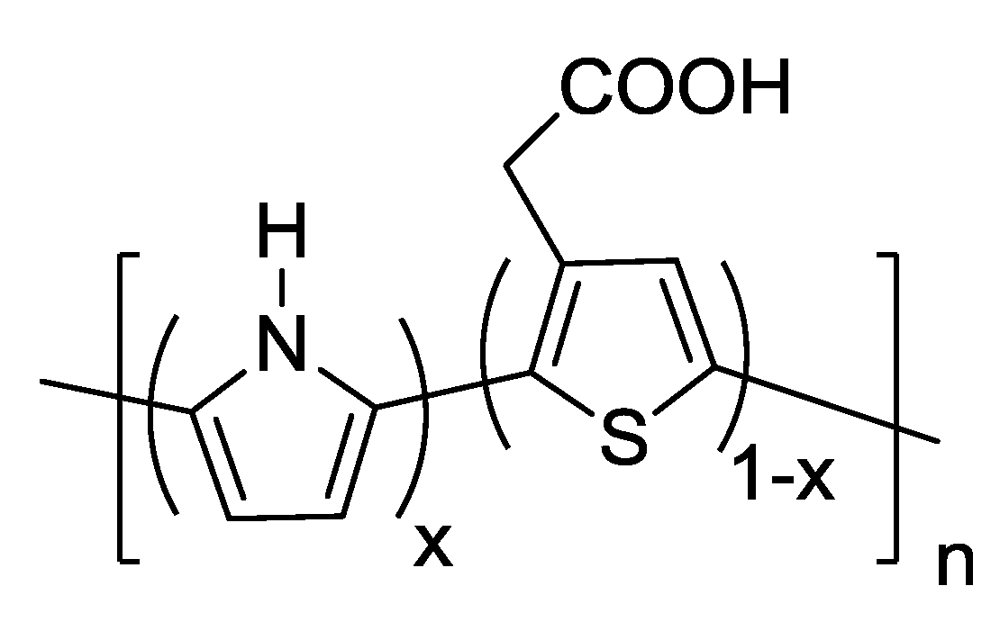
| Nylon 6 | 3-TAA/Pyrrole | 5SSA | methanol | 30 min | none | 710 Ω | 2(A) | Coating uneven and gray with black spots. Polymer forms a solid sheet across fibers. |
| Nylon 6 | 3-TAA/Pyrrole | none | water | 30 min | none | 557 Ω | none | Coating smooth and even, black, slightly brittle |
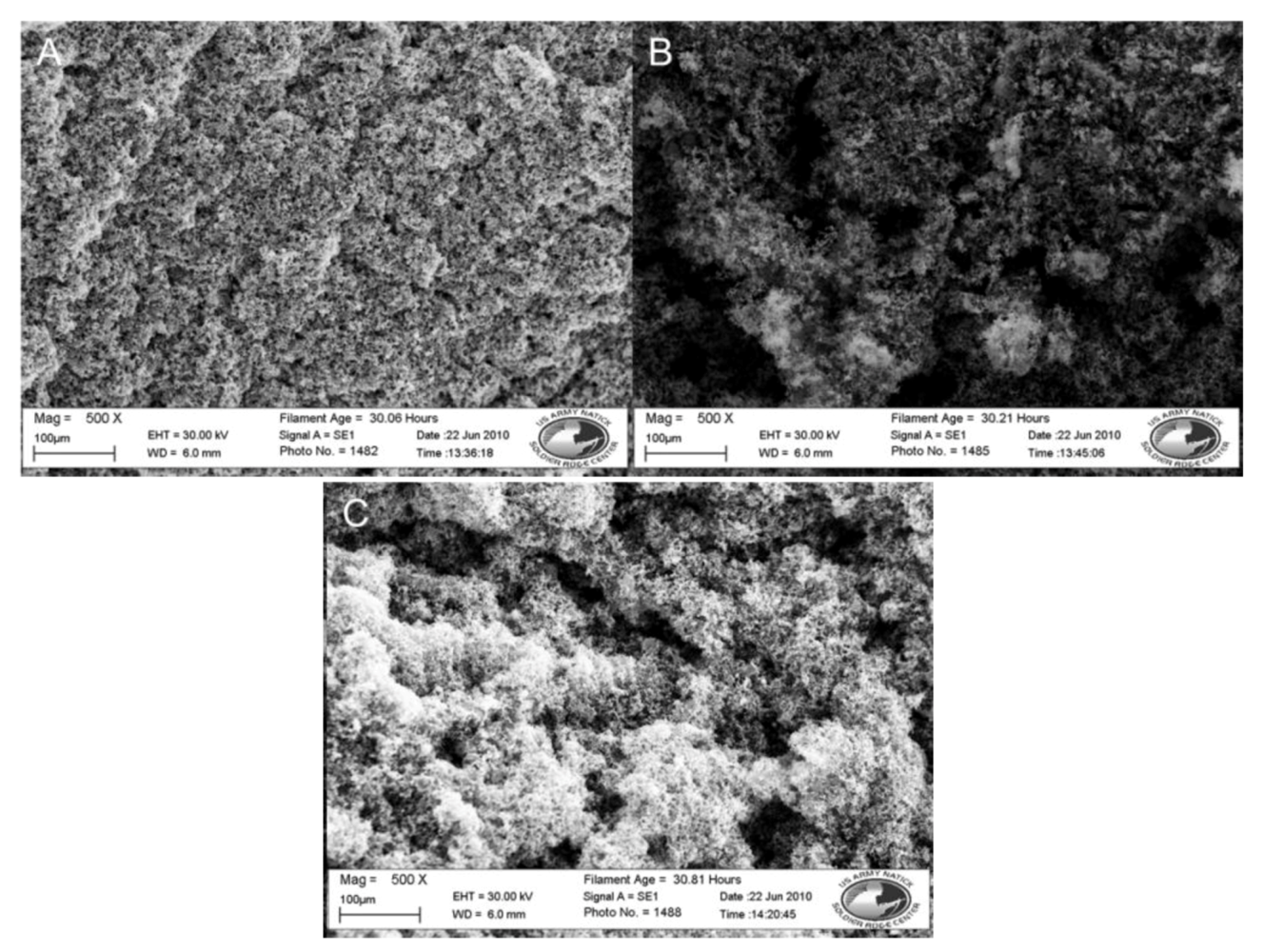
| Nylon 6 | 3-TAA/Pyrrole | 5SSA | water | 30 min | none | 91.5 Ω, 51.2 Ω | 2(B)3(A) | Coating smooth, even, and black. Polymer clusters are small and build along fiber surfaces |
| Nylon 6 | 3-TAA/Pyrrole | 5SSA | water | 30 min | DI water wash and sonication | 60.5 Ω | 3(C) | Coating smooth, even, and black. Coating is slightly lighter than other samples with better porosity |
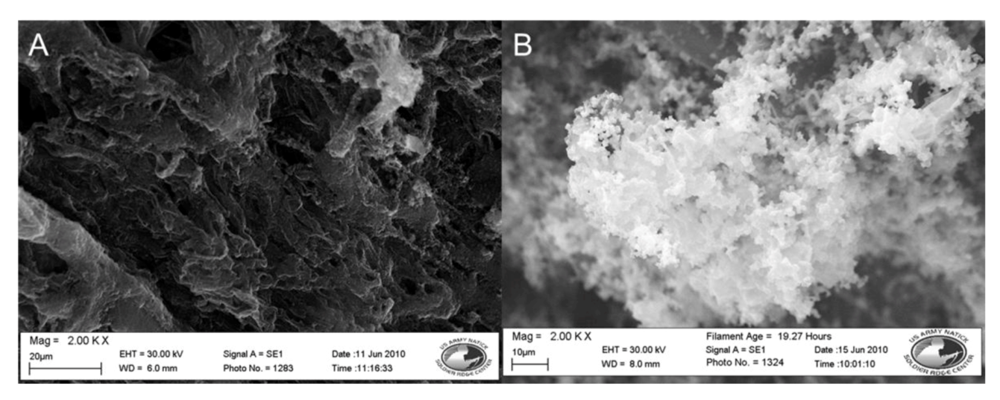
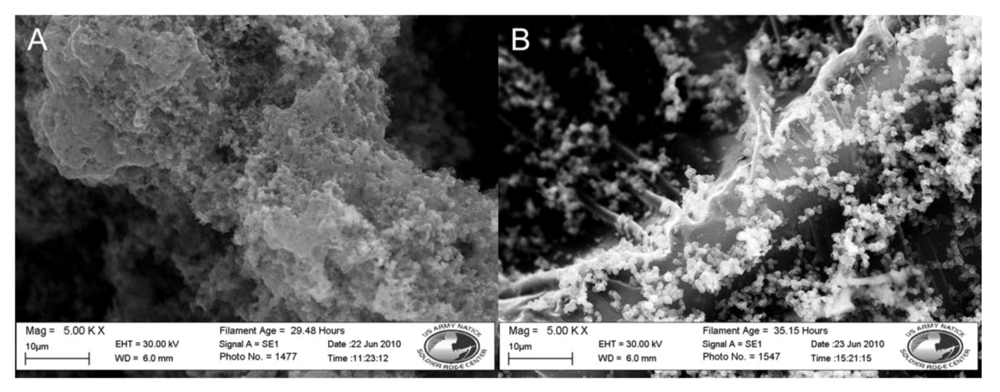
| Nylon 6 | 3-TAA/Pyrrole | 5SSA | water | 30 min | DI water wash | 42.6 Ω | 3(B)4(A) | Coating smooth, even, black, and slightly brittle. Coating is slightly lighter than unwashed sample |
| Polypropylene | 3-TAA/Pyrrole | 5SSA | water | 30 min | DI water wash | 55.1 Ω | 4(B) | Coating smooth, even, and black. Not brittle. Coating is conformal along fibers. |
3.1. Functional Group Selection
3.2. Dopant Inclusion and Solvent Selection
3.3. Post-Polymerization Treatment
3.4. Fiber Platform Selection
3.5. Biorecognition Element Attachment
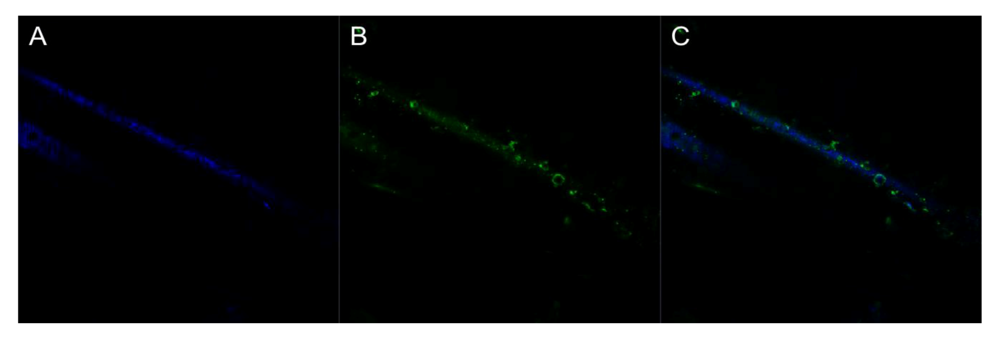
3.5.1. Optical Analysis
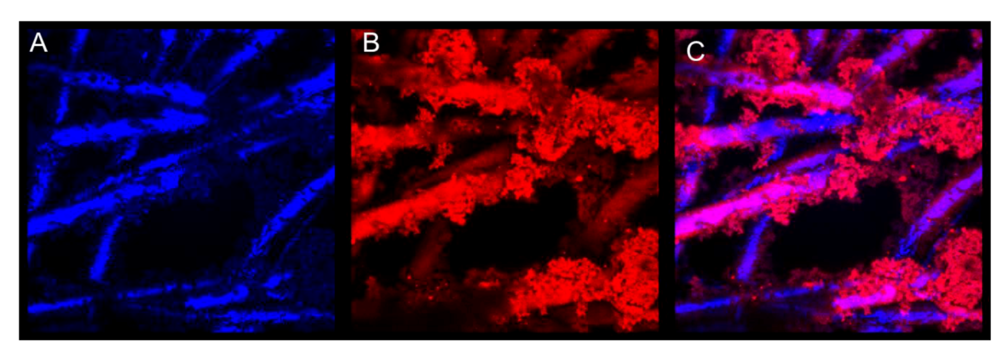
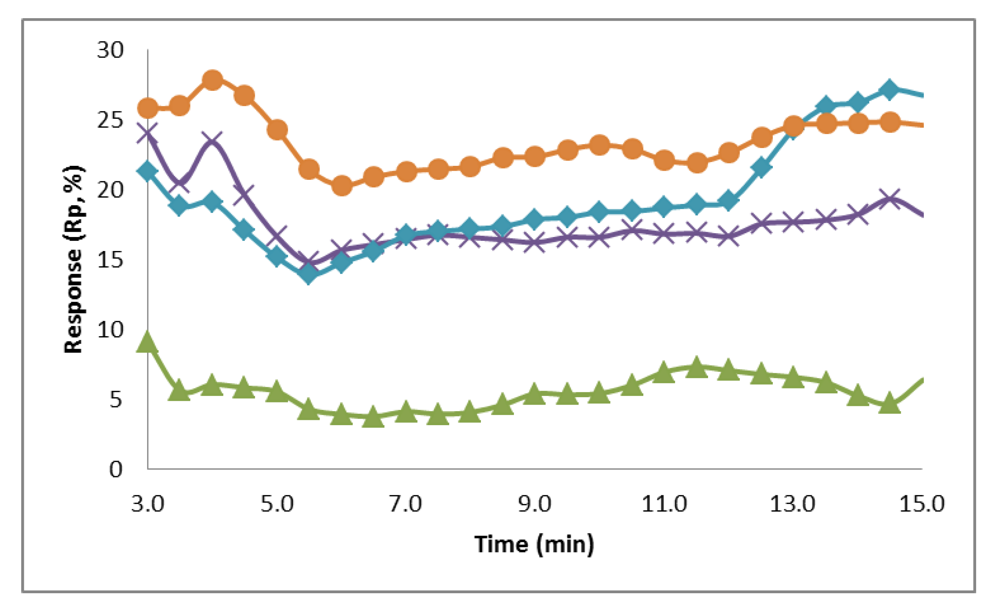
3.5.2. Electrochemical Analysis
4. Conclusions
Acknowledgments
References
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor recognition elements. Curr. Issues Mol. Biol. 2008, 10, 1–12. [Google Scholar]
- Setterington, E.B.; Cloutier, B.C.; Ochoa, J.M.; Cloutier, A.K.; Patel, P.J.; Alocilja, E.C. Rapid, sensitive, and specific immunomagnetic separation of foodborne pathogens. Int. J. Food Saf. Nutr. Publ. Health 2011, 4, 83–100. [Google Scholar] [CrossRef]
- Torres-Chavolla, E.; Alocilja, E.C. Aptasensors for detection of microbial and viral pathogens. Biosens. Bioelectron. 2009, 24, 3175–3182. [Google Scholar]
- Zhang, D.; Huarng, M.C.; Alocilja, E.C. A multiplex nanoparticle-based bio-barcoded DNA sensor for the simultaneous detection of multiple pathogens. Biosens. Bioelectron. 2010, 26, 1736–1742. [Google Scholar] [CrossRef]
- Cahn, T.M. Biosensors. In Sensor Physics and Technology Series; Grattan, K.T.V., Augousti, A., Eds.; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Scott, A.O. Biosensors for Food Analysis; Woodhead Publishing: Cambridge, UK, 1998; pp. 13–27. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000; Volume XVI. [Google Scholar]
- Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M.; Elwary, S.; Turner, A. (Eds.) Springer Science+Business Media LLC: New York, NY, USA, 2008; pp. 341–370.
- Bhattacharyya, D.; Senecal, K.; Marek, P.; Senecal, A.; Gleason, K.K. High surface area flexible chemiresistive biosensor by oxidative chemical vapor deposition. Adv. Funct. Mater. 2011, 21, 4328–4337. [Google Scholar]
- Gregory, V.R.; Kimbrell, W.C.; Kuhn, H.H. Electrically conductive non-metallic textile coatings. J. Coated Fabr. 1991, 20, 167–175. [Google Scholar]
- Heisey, C.L.; Wightman, J.P.; Pittman, E.H.; Kuhn, H.H. Surface and adhesion properties of polypyrrole-coated textiles. Text. Res. J. 1993, 63, 247–256. [Google Scholar]
- Kuhn, H.H.; Kimbrell, W.C. Method for Making Electrically Conductive Textile Materials; Milliken Research Corporation: Spartanburg, SC, USA, 1989. [Google Scholar]
- Kuhn, H.H.; Kimbrell, W.C.; Fowler, J.E.; Barry, C.N. Properties and applications of conductive textiles. Synthetic Met. 1993, 57, 3707–3712. [Google Scholar] [CrossRef]
- McGraw, S.K.; Anderson, M.J.; Alocilja, E.C.; Marek, P.J.; Senecal, K.J.; Senecal, A.G. Antibody immobilization on conductive polymer coated nonwoven fibers for biosensors. Sensor Transducer J. 2011, 13, 142–149. [Google Scholar]
- Granato, F.; Scampicchio, M.; Bianco, A.; Mannino, S.; Bertarelli, C.; Zerbi, G. Disposable electrospun electrodes based on conducting nanofibers. Electroanalysis 2008, 20, 1374–1377. [Google Scholar] [CrossRef]
- Marks, R.S.; Novoa, A.; Thomassey, D.; Cosnier, S. An innovative strategy for immobilization of receptor proteins on to an optical fiber by use of poly(pyrrole-biotin). Anal. Bioanal. Chem. 2002, 374, 1056–1063. [Google Scholar]
- Konry, T.; Novoa, A.; Shemer-Avni, Y.; Hanuka, N.; Cosnier, S.; Lepellec, A.; Marks, R.S. Optical fiber immunosensor based on a poly(pyrrole-benzophenone) film for the detection of antibodies to viral antigen. Anal. Chem. 2005, 77, 1771–1779. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar]
- Vaddiraju, S.; Senecal, K.; Gleason, K.K. Novel strategies for the deposition of -COOH functionalized conducting copolymer films and the assembly of inorganic nanoparticles on conducting polymer platforms. Adv. Funct. Mater. 2008, 18, 1929–1938. [Google Scholar] [CrossRef]
- Alocilja, E.C.; Radke, S.M. Market analysis of biosensors for food safety. Biosens. Bioelectron. 2003, 18, 841–846. [Google Scholar]
- Frontiers in Biosensorics II: Practical Applications, 1st ed.; Scheller, F.W.; Schubert, F.; Fedrowitz, J. (Eds.) Birkhäuser Basel: Basel, Switzerland, 2000; Volume 2, pp. 121–140.
- Swain, A. Biosensors: A new realism. Ann. Biol. Clin. Paris 1992, 50, 175–179. [Google Scholar]
- Myers, R.E. Chemical oxidative polymerization as a synthetic route to electrically conducting polypyrroles. J. Electron. Mater. 1986, 15, 61–69. [Google Scholar]
- Armes, S.P. Optimum reaction conditions for the polymerization of pyrrole by iron(III) chloride in aqueous solution. Synthetic Met. 1987, 20, 365–371. [Google Scholar]
- Novak, P. Limitations of polypyrrole synthesis in water and their causes. Electrochim. Acta 1992, 37, 1227–1230. [Google Scholar]
- Stanke, D.; Hallensleben, M.L.; Toppare, L. Oxidative polymerization of some N-alkylpyrroles with ferric chloride. Synthetic Met. 1995, 73, 267–272. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Elsevier Science: London, UK, 2008. [Google Scholar]
- Avlyanov, J.K.; Kuhn, H.H.; Josefowicz, J.Y.; MacDiarmid, A.G. In situ deposited thin films of polypyrrole: Conformational changes induced by variation of dopant and substrate surface. Synthetic Met. 1997, 84, 153–154. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McGraw, S.K.; Alocilja, E.; Senecal, A.; Senecal, K. Synthesis of a Functionalized Polypyrrole Coated Electrotextile for Use in Biosensors. Biosensors 2012, 2, 465-478. https://doi.org/10.3390/bios2040465
McGraw SK, Alocilja E, Senecal A, Senecal K. Synthesis of a Functionalized Polypyrrole Coated Electrotextile for Use in Biosensors. Biosensors. 2012; 2(4):465-478. https://doi.org/10.3390/bios2040465
Chicago/Turabian StyleMcGraw, Shannon K., Evangelyn Alocilja, Andre Senecal, and Kris Senecal. 2012. "Synthesis of a Functionalized Polypyrrole Coated Electrotextile for Use in Biosensors" Biosensors 2, no. 4: 465-478. https://doi.org/10.3390/bios2040465
APA StyleMcGraw, S. K., Alocilja, E., Senecal, A., & Senecal, K. (2012). Synthesis of a Functionalized Polypyrrole Coated Electrotextile for Use in Biosensors. Biosensors, 2(4), 465-478. https://doi.org/10.3390/bios2040465