Melanin-Related Materials in Electrochemical Sensors for Monitoring the Environment and Food
Abstract
1. Introduction
2. Role of Melanin for Electrochemical Sensors
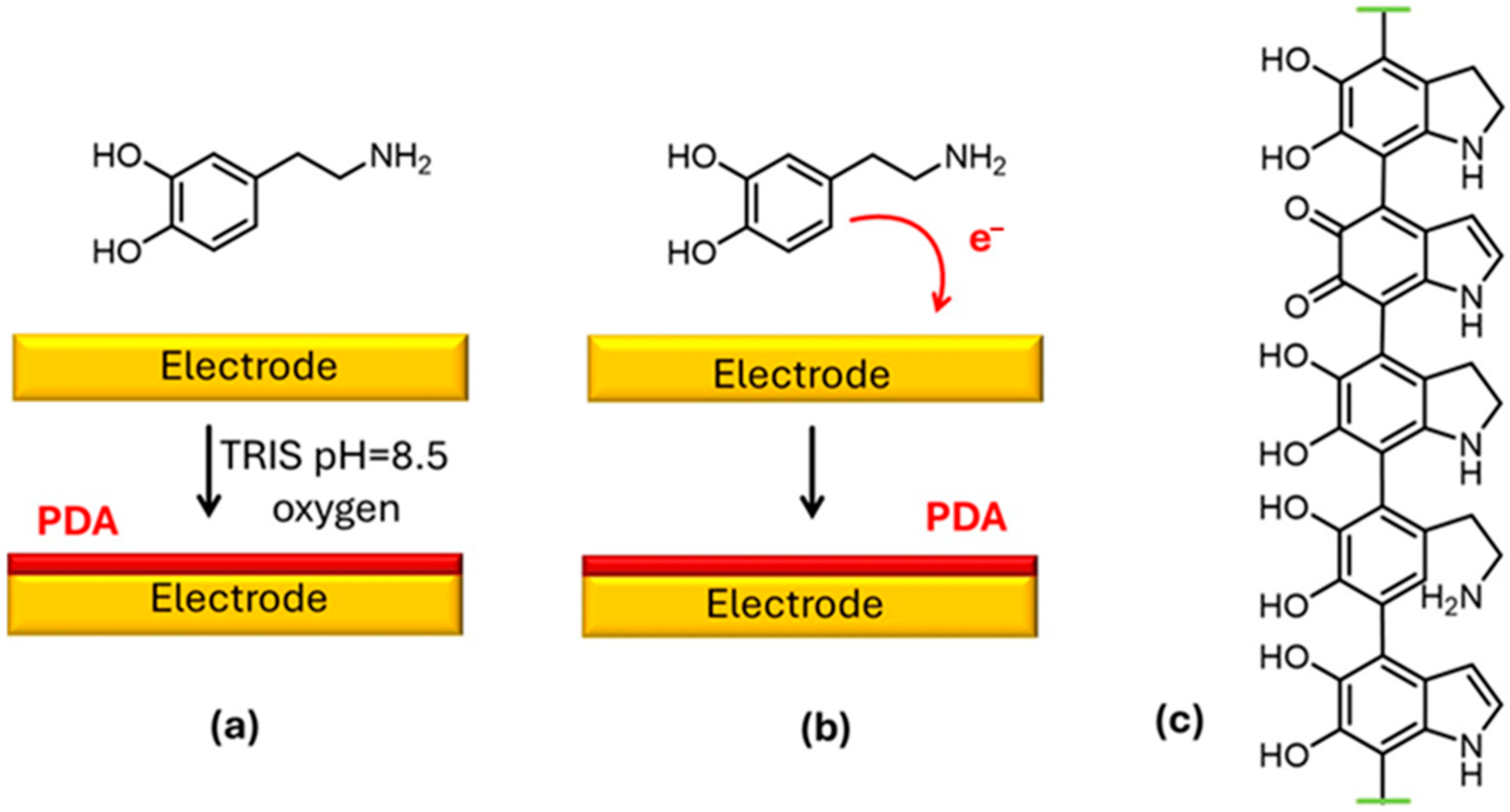
2.1. Melanin Formation and Functional Groups
2.2. Kinds of Electrochemical Sensors
3. Environmental Applications
3.1. Heavy Metal Ion Detection
Ref. | Analyte | Analyte Concentration in Real Samples | LOD | Stability | Linear Response Range | Regulatory Threshold in Potable Water | Regulatory Threshold in Superficial Water |
|---|---|---|---|---|---|---|---|
| [51] | Cd2+, Pb2+, Cu2+, and Fe2+ ions in water and soil | 50–200 ppb Spiked addition | 1.46, 2.86, 17.95, 50.23 ppb, respectively | After 1 year, the electrode still detected all four HMIs. | Cd2+: 3–10 ppb, Pb2+: 9–21 ppb, Cu2+: 20–50 ppb, and Fe2+: 50–100 ppb | WHO: Cd2+: 0.003 mg/L, Pb2+: 0.01 mg/L, Cu2+: 2 mg/L Bureau of Indian Standards: Fe2+: 0.3 mg/L | WHO: Cd2+: 0.005 ppm, Pb2+: 0.05 ppm ppb, Cu2+: 1.5 ppm WFD: Fe2+: 0.73 mg/L |
| [50] | Cd+2, Pb+2, and Cu+2 in tap water | 50–300 ppb Spiked addition | 1.43, 2.41, and 2.48 ppb, respectively, in individual detection; 3.43 for Pb2+ and 3.47 for Cu2+ for simultaneous detection | Nd | Cd+2: 2–16 ppb, Pb+2: 3–15 ppb, and Cu+2: 8–18 ppb | WHO: Cd2+: 3 ppb, Pb2+: 10 ppb, Cu2+: 2000 ppb | WHO: Cd2+: 0.005 ppm, Pb2+: 0.05 ppm, Cu2+: 1.5 ppm |
| [52] | Hexavalent chromium in tap water and river water | Tap water: 0.0490; 0.506; 1.01; 4.96 μg/mL | 3.0 μg L−1 | After 30 days, the signals remained at 97.73% of their initial value. | 0.01 μg mL−1 to 112 μg mL−1 | EPA: 100 ppb | EPA: 18 µg/L as a 24 h average for saltwater |
| River water: 0.0510; 0.515; 1.02; 4.88 μg/mL | |||||||
| Spiked additions: 0.050; 0.50; 1.0; 5.0 μg/mL, respectively | |||||||
| [53] | Pb(II) in lake water | Yangzong lake water: BDL; 0.98; 10.06; 20.05 μg/L | 0.03 μg L−1 | A 3.4% decrease in the peak current after four weeks | 0.1–150 μg L−1 | WHO: Pb2+: 10 ppb | WHO: Pb2+: 0.05 ppm |
| Datun lake water: 5.81; 6.78; 11.90; 15.84 | |||||||
| Spiked additions: 0.00; 1.00; 10.00; 20.00 μg/L for Yangzong lake water and 0.00; 1.00; 6.00; 10.00 μg/L for Datun lake water, respectively | |||||||
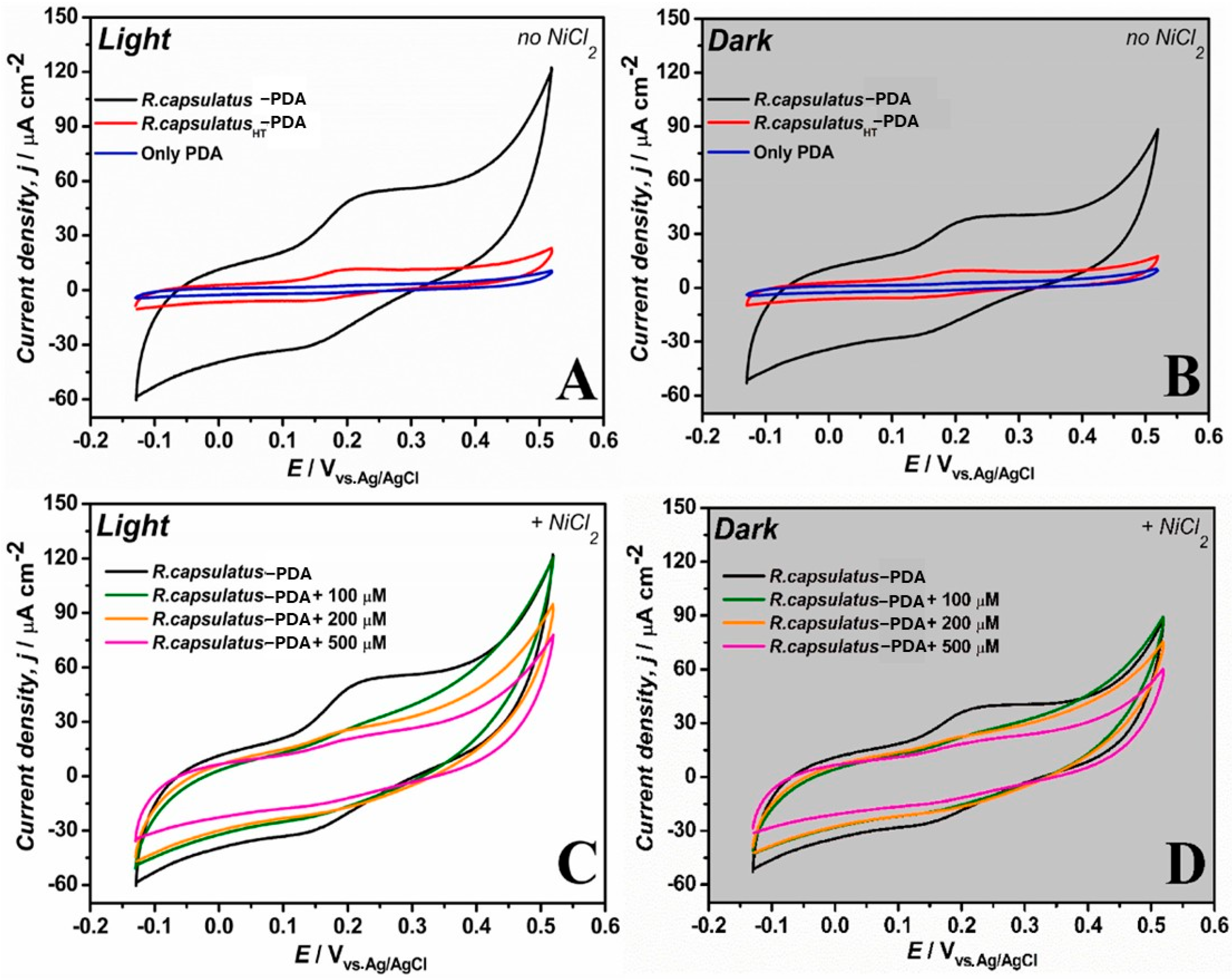
| [54]. | NiCl2 and CuSO4 in beer | 100 μM, 200 μM, and 500 μM (spiked addition) | ND | ND | ND | WHO: 70 µg/L for Ni EPA: 1.3 mg/L for Cu | ND |
| Reference | Base Electrode | Electrode Modifier | Precursors | Mechanism of Modification |
|---|---|---|---|---|
| [51] | GCE | DA, GO, L-Alanine | ALA/pDA/rGO | Drop casting |
| [50] | GCE | DA, MAX phase (Ti3AlC2) | Ti3AlC2 MAX phase, PDA | Drop casting |
| [52] | Screen-printed carbon electrodes | DA, ZIF-8 cubic nanocrystals, HAuCl4 | ZIF-8, AuNPs, PDA | Drop casting |
| [53] | mGCE | Fe3O4 magnetic NPs, DA, KMNO4 | Fe3O4@PDA@MnO2 | ND |
| [54] | poly-3-hydroxypolybutyrate (PHB)–carbon nanofibers (CFs) | DA, R. capsulatus cells | Purple non-sulfur bacteria, redox-adhesive PDA matrix | Drop casting |
3.2. Detecting Drugs and Pharmaceutical Products
| Ref | Analyte | Analyte Concentration in Real Samples | LOD | Stability | Linear Response Range | Regulatory Threshold in Potable Water | Regulatory Threshold in Superficial Water |
|---|---|---|---|---|---|---|---|
| [56] | PA | ND | 0.1 μM | After 2 days, 91.17% of the original redox peak current | from 0.3 to 10 μM | ND | ND |
| [57] | PA in seawater, hospital effluent, and wastewater | 10−10 mg/mL to 3.3 mg/mL (spiked addition) | 0.55 × 10−10 mg/mL | ND | ND | ND | ND |
| [58] | TMP in soil and lake water samples | Soil: 18.33; 29.75; 37.19; 48.69 μmol/L | 0.017 μmol/L | The signal response decreased to 95.3% of the original current response after 7 days. | 0.05–50 μmol/L | ND | ND |
| Lake water: 19.75; 29.60; 39.44; 47.35 μmol/L | |||||||
| Spiked additions: 20.00; 30.00; 40.00; 50.00 μmol/L, respectively | |||||||
| [59] | OTC in tap water samples | With DPV: ND; 0.97; 9.82 μM | 6.88 nM with DPV and 5.56 nM with CV | The oxidation current signal of OTC retained 87% of its initial value after 30 days. | 10 nM–104 nM | ND | ND |
| With CV: ND; 1.02; 9.94 μM | |||||||
| Spiked additions: 0; 1; 10 μM, respectively | |||||||
| [60] | CPZ in human serum, urine, and lake water samples | Human serum: 56.0; 92.0; 472 nM | 0.42 nM | After 14 days, the oxidation peak currents of PDA and CPZ retained 95.2% and 96.5% of their initial values, respectively. | 1 nM–10 μM | ND | ND |
| Urine: 53.0; 105.0; 483 nM | |||||||
| Lake water: 53.0; 91.0; 465 nM | |||||||
| Spiked additions: 50.0; 100; 500 nM, respectively | |||||||
| [61] | CPZ in milk, eggs, and lake water | Milk: 0.00493; 0.0995; 0.505 μM | 0.18 nM | After 4 weeks, the peak current response maintained 95.9% of its initial value. | 0.0005–85 μM | ND | ND |
| Eggs: 0.00518; 0.103; 0.497 μM | |||||||
| Lake water: 0.00517; 0.0988; 0.505 μM | |||||||
| [62] | β-NADH in food and environmental and biological samples | Avocado juice, apple juice, human serum, human urine, lake water, and river water samples Spiked addition using amperometric technique: 0–30 µM DPV: 0–25 µM | amperometric technique: 0.0062 μM DPV: 3.17 μM | After 15 days, the current intensity retained 73.6% of its initial value. | Amperometric technique: 0.018–674 μM DPV: 5–450 μM | ND | ND |
| Reference | Base Electrode | Electrode Modifier | Precursors | Mechanism of Modification |
|---|---|---|---|---|
| [56] | GCE | Neodymium nitrate hexahydrate (Ni), DA, graphite powder | Nd/PDA-rGO | Drop coating |
| [57] | Gold electrode | DA | MIP (PDA) | Electropolymerization |
| [58] | ANME | 3D-CAuNRs, PDA, pPY, TMP | MIP/pDA/3D-CAuNRs/ANME | Chronoamperometry scanning, electropolymerization |
| [59] | GCE | DA, AuNPs, FeCl3⋅6H2O, EDC/NHS | Apt/Au@PDA@NH2-MIL-101(Fe) | Coating (not specified) |
| [60] | GCE sensor | Cu-MIP/ZMO/MWCNTs | Zn (AC)2⋅2H2O; Mn(AC)2⋅4H2O; urea; MWCNTs; tetrabutylammonium tetrafluoroborate (TABTFB); dopamine; CuCl2⋅2H2O | Drop coating, electropolymerization |
| [61] | GCE sensor | N-HCS@MIP | Tetraethoxysilane; (3-aminopropyl)triethoxysilane; dopamine hydrochloride; silica nanoparticles; N2; EDOT; | Drop coating |
| [62] | GCE | DA, HAuCl4, bulk TiC powder | Au@PDA/TiC | Drop casting |
3.3. Detecting Pesticides and Other Related Contaminants
| Reference | Analyte | Analyte Concentration Range in Real Samples | LOD | Stability | Linear Response Range | Regulatory Threshold |
|---|---|---|---|---|---|---|
| [64] | Dichlorvos in water | From 0 to 6 ± 0.5 × 10−3 mg/L | 1.318 × 10−3 mg/L | After 3 days, current decreased by 6.84%. | 0.007–0.025 mg/L | WHO: 0.005 mg/L (HBV); 0.001 mg/L (drinking water) EPA: 0.0058 μg/L (chronic AWQC); 0.001 mg/L (drinking water) |
| [65] | ATZ in tomatoes, apples, and Lijiang River water | Tomato: 3.91 × 10−11 mol/L | 3.47 × 10−13 mol/L | After 15 days, photocurrent decreased by <6.42%. | 1.00 × 10–12–1.00 × 10–5 mol/L | WHO: 0.002 mg/L (drinking water) EPA: 9.7 μg/L (CE-LOC); 0.003 mg/L (drinking water) |
| Apple: 1.66 × 10−11 mol/L | ||||||
| Lijiang River water: ND | ||||||
| [66] | DDT in food samples (radish) | 0; 102.32; 0.893; 0.093 µM (spiked additions: 0; 100; 1; 0.01 µM, respectively) | 6 × 10−12 mol/L | After 4 weeks the sensor retained 97.26% of the Rct value. | 1× 10−11–1× 10−3 mol/L | WHO: 0.001 mg/L (drinking water) EPA: 0.0010 µg/L (chronic AWQC, average over 24 h) |
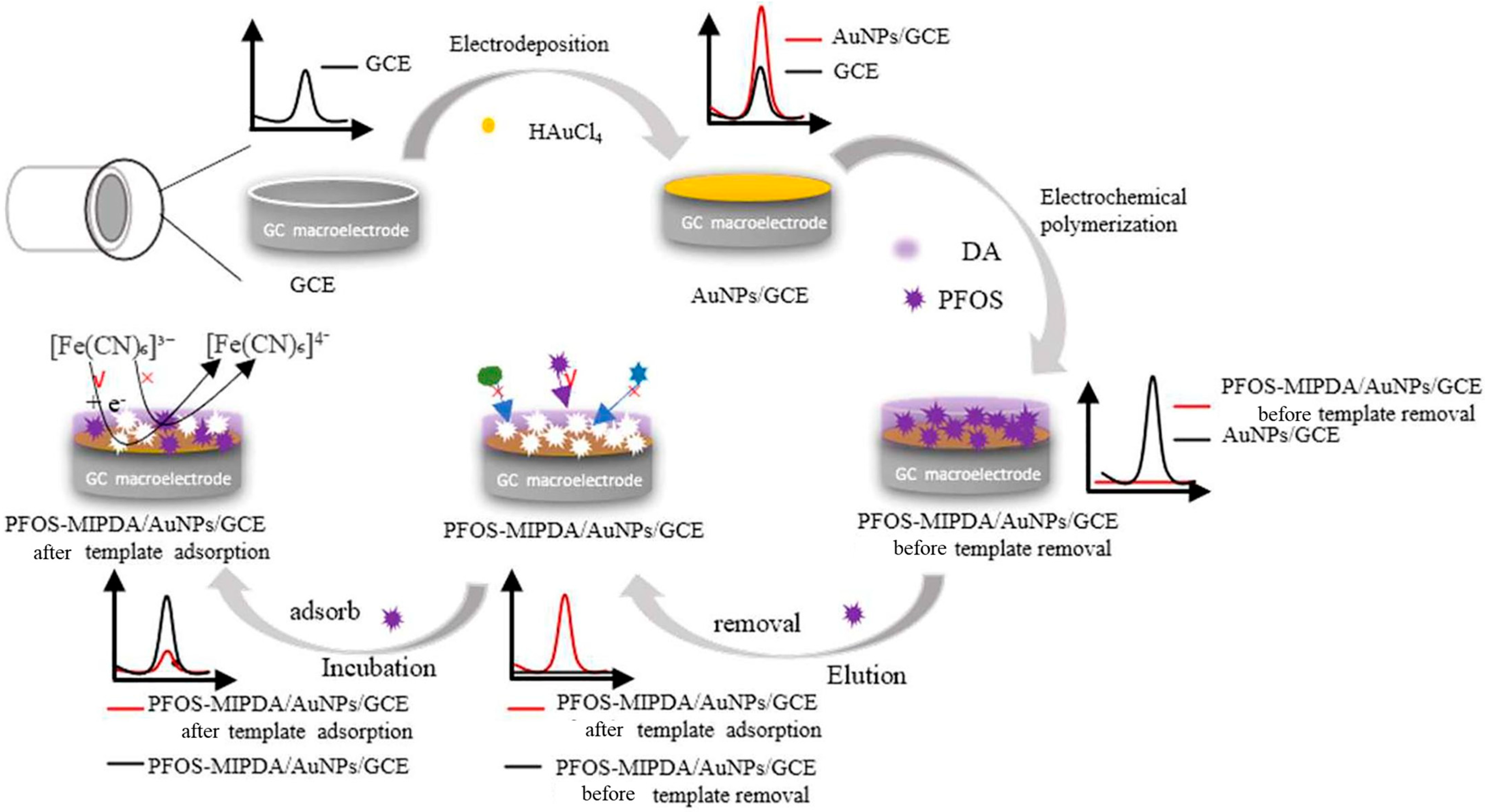
| [68] | PFOS in lake, canal, and tap water | Tap water: ND; 0.0102; 0.0208; 0.0393 µmol/L | 0.0042 µmol/L | After 1 day, peak current decreased to 41.23%. | 0.01–8.00 µmol/L | EPA: 4 ppt (MCL of drinking water) |
| Lake water: ND; 0.0100; 0.0208; 0.0416 µmol/L | ||||||
| Canal water: ND; 0.0105; 0.0195; 0.0393 µmol/L | ||||||
| Spiked additions: 0; 0.01; 0.02; 0.04 µmol/L, respectively | ||||||
| [71] | TCP in water, soil, and food samples | River water: ND; 0.098; 0.99; 4.90; 9.94; 14.81 µM | 0.0042 µM | After 12 days, current decreased by ≈7%. | 0.019−190.7 and 212.7−1649 µM | WHO: 0.1 mg/L (drinking water) EPA: MCLG of 0.002 mg/L |
| Tap water: ND; 0.094; 0.98; 4.91; 9.97; 14.76 µM | ||||||
| Soil: ND; 0.097; 0.98; 4.87; 9.78; 14.80 µM | ||||||
| Red wine: ND; 0.098; 0.98; 4.97; 9.86; 14.63 µM | ||||||
| Apple juice: ND; 0.098; 0.98; 4.98; 9.87; 14.80 µM | ||||||
| Spiked additions: 0; 0.1; 1.0; 5.0; 10.0; 15.0 µM, respectively | ||||||
| [70] | RC in seawater and lake water | ND | 0.06 mM | After 36 days, current decreased by 2.01%. | 1–100 μM 1.2–4 mM | ND |
| [69] | HQ in river water | 49.81± 2.24 μM and 100.28± 5.13 μM (spiked additions: 50 and 100 μM, respectively) | 0.047 μM | After 1 month, the response current was 93.01% of the original response current. | 5.0 × 10−6 to 1.0 × 10−2 M | ND |
| CAT in river water | 47.21 ± 5.13 and 99.61 ± 3.78 (spiked additions: 50 and 100 μM, respectively) | 0.018 μM | After 1 month, the response current was 96.35% of the original response current. | 5.0 × 10−8 to 2.0 × 10−3 M | ND |
| Reference | Base Electrode | Electrode Modifier | Precursors | Mechanism of Modification |
|---|---|---|---|---|
| [64] | GCE sensor | ZrO2@PDA | Zirconium chloride octahydrate; dopamine hydrochloride | ND |
| [65] | FTO electrode | GLD/Cs/ Zn0.5Cd0.5S/Ti3C2/G4/hemin DNAzyme | Glutaraldehyde; chitosan; zinc acetate; cadmium acetate dihydrate; dopamine; thioacetamide; hemin; LbCas12a; ssDNA-FQ; crRNA; G-quadruplex, ATZ aptamer, and activator strand DNA | Drop coating method |
| [66] | GCE sensor | PDA@Fe3O4-MIP MNPs | Fe3O4 nanoparticles; dopamine hydrochloride; BPA | Drop casting |
| [68] | GCE sensor | PFOS-MIPPDA/AuNPs | HAuCl4 × 4H2O; KCl; dopamine hydrochloride; PFOS | Solution immersion and electropolymerization |
| [71] | SPCE sensor | α-Bi2O3 MPs/PDA-RGO | Bismuth nitrate; hydroxylamine hydrochloride; GO; dopamine | Drop casting |
| [70] | HOPG sensor | NPC-Au@Ag | Gold (III) chloride trihydrate; silver nitrate; cuttlefish bought from a local supermarket | Topological transformation technology and drop casting |
| [69] | GCE sensor | Mo, N, S-IPCS | Sodium molybdate; thioacetamide; dopamine hydrochloride | Drop casting |
4. Food Applications
| Ref | Analyte | Analyte Concentration in Real Samples | LOD | Stability | Linear Response Range | Regulatory Threshold in Potable Water | Regulatory Threshold in Superficial Water |
|---|---|---|---|---|---|---|---|
| [72] | Salmonella typhimurium (S. Typhimurium) in food | Tap water, milk, and pork ≈ 104–106 CFU/mL (spiked additions) | 10 CFU/mL | ND | 102–108 CFU/mL | ND | ND |
| [48] | Chloramphenicol in dairy products | Pure milk: 0.47; 0.98; 5.04 × 10−5 mol L−1 | 2.0 × 10−8 mol L−1 | After 30 days, the current response was 90.5% of the initial value. | 5.0 × 10−7–5.0 × 10−4 mol L−1 | ND | ND |
| Milk powder: 0.46; 0.97; 5.08 × 10−5 mol L− 1 | |||||||
| Yoghurt: 0.45; 0.99; 5.03 × 10−5 mol L−1 | |||||||
| Spiked additions: 0.50; 1.00; 5.00 × 10−5 mol L−1, respectively |
| Reference | Base Electrode | Electrode Modifier | Precursors | Mechanism of Modification |
|---|---|---|---|---|
| [72] | Carbon working electrode | LPS, DA | LPS-imprinted MIP | Drop casting |
| [48] | GCE | DA, straw biogas residue, β-cyclodextrin | Biogas residue biochar @polydopamine (BRB@PDA), β-cyclodextrin | Drop casting and electrodeposition |
5. Others
| Reference | Analyte | LOD | Stability | Linear Response Range | Regulatory Threshold |
|---|---|---|---|---|---|
| [73] | Hg2+ in solution | 0.3 ppb | ND | 1 to 10 nM | WHO: 0.006 mg/L (drinking water) EPA: 0.002 mg/L (drinking water) |
| Thiophenol | 3 ppb | 1 to 20 μM | NA | ||
| [22] | Ascorbic acid; HQ | NA | After 32 h, photocurrent decreased by 20%. | NA | NA |
| [74] | K+ | 10−5.4 M | ND | 10−5 to 10−2 M | NA |
| Reference | Electrode Substrate Type | Electrode Modifier | Precursors | Mechanism of Modification |
|---|---|---|---|---|
| [73] | Paper-based electrode | AuNP PDA | H[AuCl4]; dopamine hydrochloride | Electroless deposition |
| [22] | n-type Si electrode | cPDA | Dopamine | Solution immersion and annealing |
| [74] | GCE (K+-ISE) | PDA-AgNPs | Dopamine; silver nitrate | Solution immersion |
6. Outlook and Perspectives
| Reviews | Application | Topic of Interest | Year of Publication |
|---|---|---|---|
| This review | Environmental and food | Assesses the problem of melanin’s low conductivity and proposes innovative methodologies to increase it. | 2025 |
| Recent Advances in Polydopamine-based Electrochemical Biosensors | Diagnostic and therapeutic | PDA is only described as able to increase the electrocatalytic properties of an electrode, without focusing on its limitations. | 2022 |
| Polydopamine films: Electrochemical growth and sensing applications | General/environmental | Focuses on the influence of other parameters, such as thickness, on PDA’s conductivity. | 2022 |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Jeon, D.-J.; Paik, S.; Ji, S.; Yeo, J.-S. Melanin-based structural coloration of birds and its biomimetic applications. Appl. Microsc. 2021, 51, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Y.; Allen, M.C.; Deheyn, D.D.; Yue, X.; Zhao, J.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Bio-Inspired Structural Colors Produced via Self-Assembly of Synthetic Melanin Nanoparticles. ACS Nano 2015, 9, 5454–5460. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T. Photodynamics of Melanin Radicals: Contribution to Photoprotection by Melanin. Photochem. Photobiol. 2023, 99, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. New trends in photobiology: Photoprotection by melanin. J. Photochem. Photobiol. B, Biol. 1991, 9, 135–160. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Vicenzi, S.; Pane, A.; Montalti, M. Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin. Antioxidants 2025, 14, 376. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Melanin and Light. Chem. Eur. J. 2024, 30, e202400461. [Google Scholar] [CrossRef]
- Caldas, M.; Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Melanin nanoparticles as a promising tool for biomedical applications–a review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Polydopamine Nanosystems in Drug Delivery: Effect of Size, Morphology, and Surface Charge. Nanomaterials 2024, 14, 303. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Liu, M.; Huang, H.; Zhang, X.; Wei, Y. Polydopamine-based functional materials and their applications in energy, environmental, and catalytic fields: State-of-the-art review. Chem. Eng. J. 2020, 387, 124019. [Google Scholar] [CrossRef]
- Menichetti, A.; Ramacciotti, F.; Sciutto, G.; Focarete, M.L.; Montalti, M.; Prati, S.; Gualandi, C. Nanofibrous Photothermal Materials from Natural Resources: A Green Approach for Artwork Restoration. ACS Appl. Mater. Interfaces 2024, 16, 69829–69838. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Song, W.; Yang, H.; Liu, S.; Yu, H.; Li, D.; Li, P.; Xing, R. Melanin: Insights into structure, analysis, and biological activities for future development. J. Mater. Chem. B 2023, 11, 7528–7543. [Google Scholar] [CrossRef] [PubMed]
- Hemmatpour, H.; De Luca, O.; Crestani, D.; Stuart, M.C.A.; Lasorsa, A.; van der Wel, P.C.A.; Loos, K.; Giousis, T.; Haddadi-Asl, V.; Rudolf, P. New insights in polydopamine formation via surface adsorption. Nat. Commun. 2023, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. The Photophysics and Photochemistry of Melanin- Like Nanomaterials Depend on Morphology and Structure. Chem.–A Eur. J. 2021, 27, 16309–16319. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Ferrazzano, L.; Montalti, M. Reversible Supramolecular Noncovalent Self-Assembly Determines the Optical Properties and the Formation of Melanin-like Nanoparticles. J. Phys. Chem. Lett. 2022, 13, 9829–9833. [Google Scholar] [CrossRef]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter 2009, 5, 3754–3760. [Google Scholar] [CrossRef]
- Delparastan, P.; Malollari, K.G.; Lee, H.; Messersmith, P.B. Direct Evidence for the Polymeric Nature of Polydopamine. Angew. Chem. Int. Ed. 2019, 58, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, F.; Meng, Y.; Yang, Q.; Wang, J.; Zhang, D.W.; Wang, Y. Carbonized polydopamine layer-protected silicon substrates for light-addressable electrochemical sensing and imaging. Talanta 2023, 254, 124124. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Menichetti, A.; Degli Esposti, L.; Montesi, M.; Panseri, S.; Bassi, G.; Montalti, M.; Lazzarini, L.; Adamiano, A.; Iafisco, M. Fluorescent Carbon Dots from Food Industry By-Products for Cell Imaging. J. Funct. Biomater. 2023, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine–Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Szewczyk, J.; Aguilar-Ferrer, D.; Coy, E. Polydopamine films: Electrochemical growth and sensing applications. Eur. Polym. J. 2022, 174, 111346. [Google Scholar] [CrossRef]
- McGinness, J.; Corry, P.; Proctor, P. Amorphous Semiconductor Switching in Melanins. Science 1974, 183, 853–855. [Google Scholar] [CrossRef]
- Eom, T.; Lee, J.; Lee, S.; Ozlu, B.; Kim, S.; Martin, D.C.; Shim, B.S. Highly Conductive Polydopamine Coatings by Direct Electrochemical Synthesis on Au. ACS Appl. Polym. Mater. 2022, 4, 5319–5329. [Google Scholar] [CrossRef]
- Reali, M.; Gouda, A.; Bellemare, J.; Ménard, D.; Nunzi, J.-M.; Soavi, F.; Santato, C. Electronic Transport in the Biopigment Sepia Melanin. ACS Appl. Bio Mater. 2020, 3, 5244–5252. [Google Scholar] [CrossRef]
- Camus, A.; Reali, M.; Rozel, M.; Zhuldybina, M.; Soavi, F.; Santato, C. High conductivity Sepia melanin ink films for environmentally benign printed electronics. Proc. Natl. Acad. Sci. USA 2022, 119, e2200058119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, M.; Malollari, K.G.; Shin, J.; Winkler, S.M.; Zheng, Y.; Park, J.H.; Grigoropoulos, C.P.; Messersmith, P.B. Laser-induced graphitization of polydopamine leads to enhanced mechanical performance while preserving multifunctionality. Nat. Commun. 2020, 11, 4848. [Google Scholar] [CrossRef]
- Li, H.; Marshall, T.; Aulin, Y.V.; Thenuwara, A.C.; Zhao, Y.; Borguet, E.; Strongin, D.R.; Ren, F. Structural evolution and electrical properties of metal ion-containing polydopamine. J. Mater. Sci. 2019, 54, 6393–6400. [Google Scholar] [CrossRef]
- Ozlu, B.; Moraes Silva, S.; Moulton, S.E.; Shim, B.S. Synergistic Polydopamine/Graphene Oxide Hybrids: Elevating Electrochemical Performance. ACS Electrochem. 2025, 1, 453–464. [Google Scholar] [CrossRef]
- Aguilar-Ferrer, D.; Szewczyk, J.; Coy, E. Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: Physico-chemical properties and perspectives. Catal. Today 2022, 397–399, 316–349. [Google Scholar] [CrossRef]
- Li, R.; Parvez, K.; Hinkel, F.; Feng, X.; Müllen, K. Bioinspired Wafer-Scale Production of Highly Stretchable Carbon Films for Transparent Conductive Electrodes. Angew. Chem. Int. Ed. 2013, 52, 5535–5538. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Polydopamine-Based Nanoprobes Application in Optical Biosensing. Biosensors 2023, 13, 956. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment. Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Petropoulos, V.; Mordini, D.; Montorsi, F.; Akturk, M.; Menichetti, A.; Olivati, A.; Petrozza, A.; Morandi, V.; Maiuri, M.; Gianneschi, N.C.; et al. Photochemical Pathways and Light-Enhanced Radical Scavenging Activity of 1,8-Dihydroxynaphthalene Allomelanin. J. Am. Chem. Soc. 2025, 147, 10031–10043. [Google Scholar] [CrossRef]
- Hong, L.; Simon, J.D. Current Understanding of the Binding Sites, Capacity, Affinity, and Biological Significance of Metals in Melanin. J. Phys. Chem. B 2007, 111, 7938–7947. [Google Scholar] [CrossRef]
- Larsson, B.S. Interaction Between Chemicals and Melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Montalti, M. Functionalization of and through Melanin: Strategies and Bio-Applications. Int. J. Mol. Sci. 2023, 24, 9689. [Google Scholar] [CrossRef] [PubMed]
- Madadelahi, M.; Romero-Soto, F.O.; Kumar, R.; Tlaxcala, U.B.; Madou, M.J. Electrochemical sensors: Types, applications, and the novel impacts of vibration and fluid flow for microfluidic integration. Biosens. Bioelectron. 2025, 272, 117099. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging contaminants: A One Health perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Nema, S.; Patel, M.; Jaiswal, S.; Dhand, C.; Dwivedi, N. Polydopamine modified Ti3AlC2 MAX phase promotes electrochemical heavy metal detection. Surf. Interfaces 2024, 51, 104752. [Google Scholar] [CrossRef]
- Patel, M.; Bisht, N.; Prabhakar, P.; Sen, R.K.; Kumar, P.; Dwivedi, N.; Ashiq, M.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Ternary nanocomposite-based smart sensor: Reduced graphene oxide/polydopamine/alanine nanocomposite for simultaneous electrochemical detection of Cd2+, Pb2+, Fe2+, and Cu2+ ions. Environ. Res. 2023, 221, 115317. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, T.; Feng, Y.; Liu, X.; Lan, M.; Zuo, S. Ordered hollow mesoporous carbon cubes loaded with Au nanoparticles for high-performance electrochemical detection of hexavalent chromium. Sens. Actuators B: Chem. 2024, 399, 134757. [Google Scholar] [CrossRef]
- Wang, L.; Lei, T.; Ren, Z.; Jiang, X.; Yang, X.; Bai, H.; Wang, S. Fe3O4@PDA@MnO2 core-shell nanocomposites for sensitive electrochemical detection of trace Pb(II) in water. J. Electroanal. Chem. 2020, 864, 114065. [Google Scholar] [CrossRef]
- Franco, J.H.; Stufano, P.; Labarile, R.; Lacalamita, D.; Lasala, P.; Fanizza, E.; Trotta, M.; Farinola, G.M.; Grattieri, M. Intact photosynthetic bacteria-based electrodes for self-powered metal ions monitoring. Biosens. Bioelectron.: X 2024, 21, 100552. [Google Scholar] [CrossRef]
- Farré, M.I.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Venkatesh, P.; Sudhakar, K.; Gayathri, G.S. Neodymium-polydopamine-reduced graphene oxide nanocomposite: A high-performance electrochemical sensor for paracetamol detection. Inorg. Chem. Commun. 2025, 173, 113840. [Google Scholar] [CrossRef]
- Tlili, A.; Attia, G.; Fourati, N.; Zerrouki, C.; Yaakoubi, N.; Othmane, A. Molecularly imprinted polydopamine-based sensor for accurate paracetamol monitoring in environmental samples. Emergent Mater. 2025, 8, 2805–2814. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Z.; Li, Q.; Zhan, S.; Li, L.; Yin, Z.-Z.; Zhang, L. A highly selective and sensitive trimethoprim sensor based on surface molecularly imprinted nanocavities coordinated polydopamine/gold nanorods-functionalized acupuncture needle microelectrode. Microchem. J. 2024, 200, 110503. [Google Scholar] [CrossRef]
- Song, J.; Fan, X.; Ying Ee, L.; Lin, X.; Zhang, D.; Yau Li, S.F.; Huang, M. Novel dopamine-derived carbon modified fe-based metal–organic framework enhanced aptasensor rendering ultrasensitive electrochemical detection of oxytetracycline. Chem. Eng. J. 2024, 499, 156373. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Chen, L.; Liu, Y.; Zhao, F.; Zeng, B. A novel ratiometric electrochemical sensor based on bifunctional copper-coordinated polydopamine molecular imprinting for the detection of chlorpromazine. Sens. Actuators B: Chem. 2025, 440, 137910. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Abdurexit, A.; Jamal, R.; Abdiryim, T.; Li, J.; Yang, H.; Zhang, G.; Su, E.; Chen, J.; et al. Molecularly imprinted electrochemical sensor based on nitrogen-doped hollow mesoporous carbon spheres with PEDOT for selective and ultrasensitive detection of chlorpromazine. Biosens. Bioelectron. 2025, 287, 117708. [Google Scholar] [CrossRef]
- Prasanna, S.B.; Bahajjaj, A.A.A.; Lee, Y.H.; Lin, Y.C.; Dhawan, U.; Sakthivel, R.; Chung, R.J. Highly responsive and sensitive non-enzymatic electrochemical sensor for the detection of β-NADH in food, environmental and biological samples using AuNP on polydopamine/titanium carbide composite. Food Chem. 2023, 426, 136609. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tawanda Tavengwa, N. Extraction and electrochemical sensing of pesticides in food and environmental samples by use of polydopamine-based materials. Chemosphere 2021, 266, 129222. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Su, M.; Yang, X.; Ning, Z.; Liu, H.; Wang, S.; Wang, L. Electrochemical analysis of harmful dichlorvos insecticides based on a high-performance composite sensor. J. Chem. Technol. Biotechnol. 2024, 99, 872–879. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Huang, W.; Jin, Z.; Wei, X.; Li, J. Ultrasensitive detection of atrazine by Schottky junction photoelectrochemical aptamer sensor based on signal amplification by cascade catalysis of CRISPR/Cas12a and G-quadruplex/hemin DNAzyme. Microchim. Acta 2025, 192, 376. [Google Scholar] [CrossRef]
- Miao, J.; Liu, A.; Wu, L.; Yu, M.; Wei, W.; Liu, S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal. Chim. Acta 2020, 1095, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gou, W.; Zeng, W.; Chen, W.; Jiang, J.; Lu, J. Determination of Perfluorooctanesulfonic acid in water by polydopamine molecularly imprinted /Gold nanoparticles sensor. Microchem. J. 2023, 187, 108378. [Google Scholar] [CrossRef]
- Jiao, S.; Tang, J.; Li, X.; Zhai, Y.; Li, M. A sensor based on Mo, N, S-doped interconnected porous carbon spheres material for simultaneous determination of hydroquinone and catechol. Microchem. J. 2025, 211, 113065. [Google Scholar] [CrossRef]
- Fu, D.; Chen, T.; Liu, H.; Cheng, Y.; Zong, H.; Li, A.; Liu, J. Specific sensing of resorcin based on the hierarchical porous nanoprobes constructed by cuttlefish-derived biomaterials through differential pulse voltammetry. Anal. Chim. Acta 2021, 1188, 339203. [Google Scholar] [CrossRef]
- Selvi, S.V.; Krishnapandi, A.; Damastuti, R.; Prasannan, A.; Liang, S.-T.; Hong, P.-D.; Kim, S.-C. Effectively Reinforced α-Bi2O3 MPs/PDA-RGO Sensor for Selective Modality Sensing of a Hazardous Phenolic Compound. J. Agric. Food Chem. 2023, 71, 20563–20574. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Kim, M.; Kang, R.; Lim, I.; Jang, Y. Rapid and simple on-site salmonella detection in food via direct sample loading using a lipopolysaccharide-imprinted polymer. J. Nanobiotechnol. 2025, 23, 279. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, E.; Rekas, A.; Moran-Mirabal, J. Simple and Inexpensive Fabrication of High Surface-Area Paper-Based Gold Electrodes for Electrochemical and Surface-Enhanced Raman Scattering Sensing. ACS Appl. Mater. Interfaces 2023, 15, 55183–55192. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Jiang, T.; Liang, R.; Qin, W. Polymeric membrane ion-selective electrodes with anti-biofouling properties by surface modification of silver nanoparticles. Sens. Actuators B: Chem. 2021, 328, 129014. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Mollica, F.; Lucernati, R.; Montalti, M.; Amorati, R. Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles. Antioxidants 2023, 12, 1511. [Google Scholar] [CrossRef]
- Khaleel, S.; Gao, Z.; Camus, A.; Isasmendi Ramirez, O.J.; Cavarroc, M.; Santato, C. Melanin granules extracted from Sepia ink: A nanoscale study of charge carrier transport. J. Phys. Mater. 2025, 8, 035002. [Google Scholar] [CrossRef]
- Bisht, N.; Dwivedi, N.; Khosla, A.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Review—Recent Advances in Polydopamine-based Electrochemical Biosensors. J. Electrochem. Soc. 2022, 169, 107505. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pane, A.; Vicenzi, S.; Mattioli, C.; Mordini, D.; Menichetti, A.; Montalti, M. Melanin-Related Materials in Electrochemical Sensors for Monitoring the Environment and Food. Biosensors 2025, 15, 631. https://doi.org/10.3390/bios15090631
Pane A, Vicenzi S, Mattioli C, Mordini D, Menichetti A, Montalti M. Melanin-Related Materials in Electrochemical Sensors for Monitoring the Environment and Food. Biosensors. 2025; 15(9):631. https://doi.org/10.3390/bios15090631
Chicago/Turabian StylePane, Agata, Silvia Vicenzi, Chiara Mattioli, Dario Mordini, Arianna Menichetti, and Marco Montalti. 2025. "Melanin-Related Materials in Electrochemical Sensors for Monitoring the Environment and Food" Biosensors 15, no. 9: 631. https://doi.org/10.3390/bios15090631
APA StylePane, A., Vicenzi, S., Mattioli, C., Mordini, D., Menichetti, A., & Montalti, M. (2025). Melanin-Related Materials in Electrochemical Sensors for Monitoring the Environment and Food. Biosensors, 15(9), 631. https://doi.org/10.3390/bios15090631





