Exploration and Analysis of GaN-Based FETs with Varied Doping Concentration in Nano Regime for Biosensing Application
Abstract
1. Introduction
2. Device Architecture
3. Results and Discussion
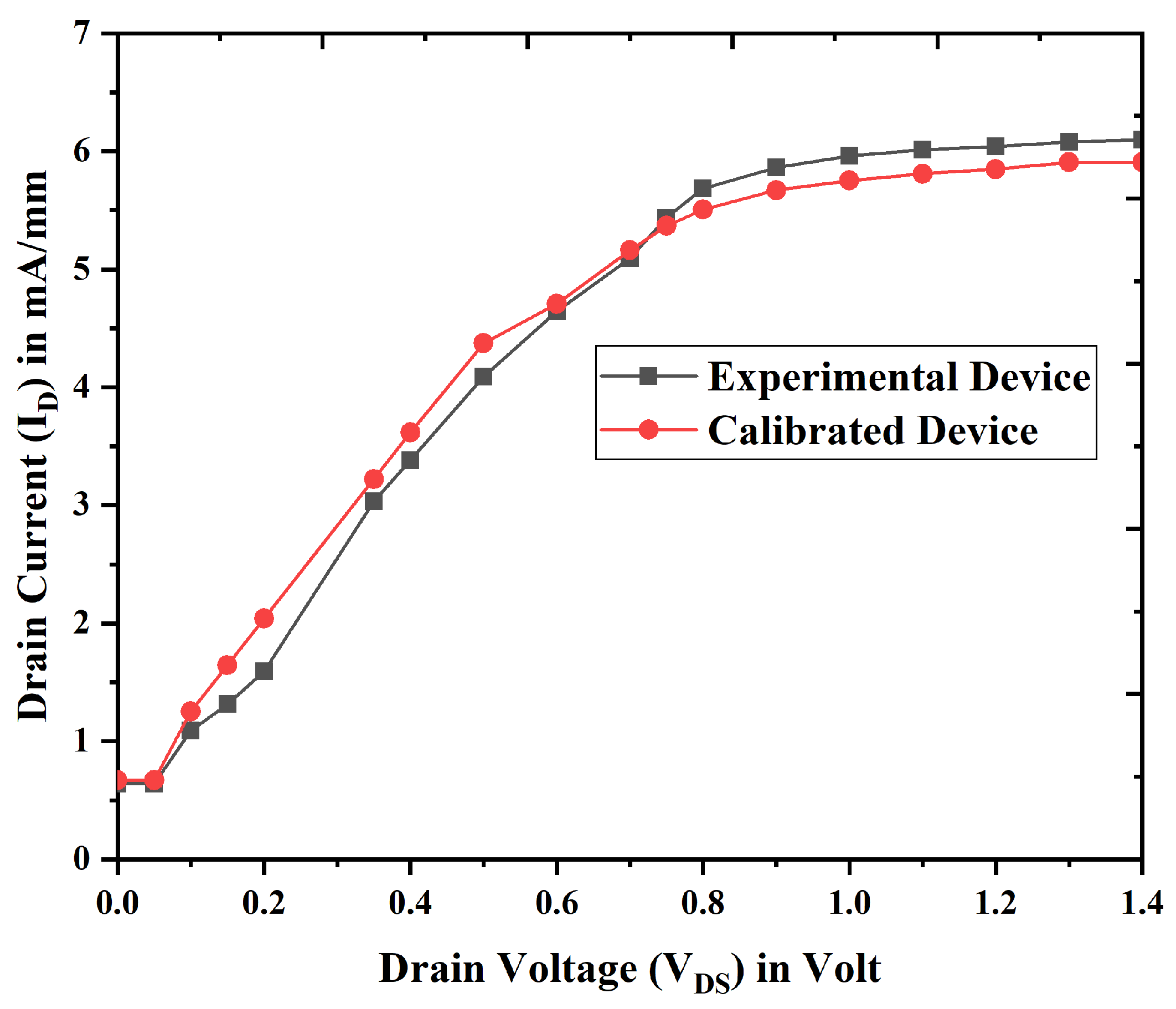
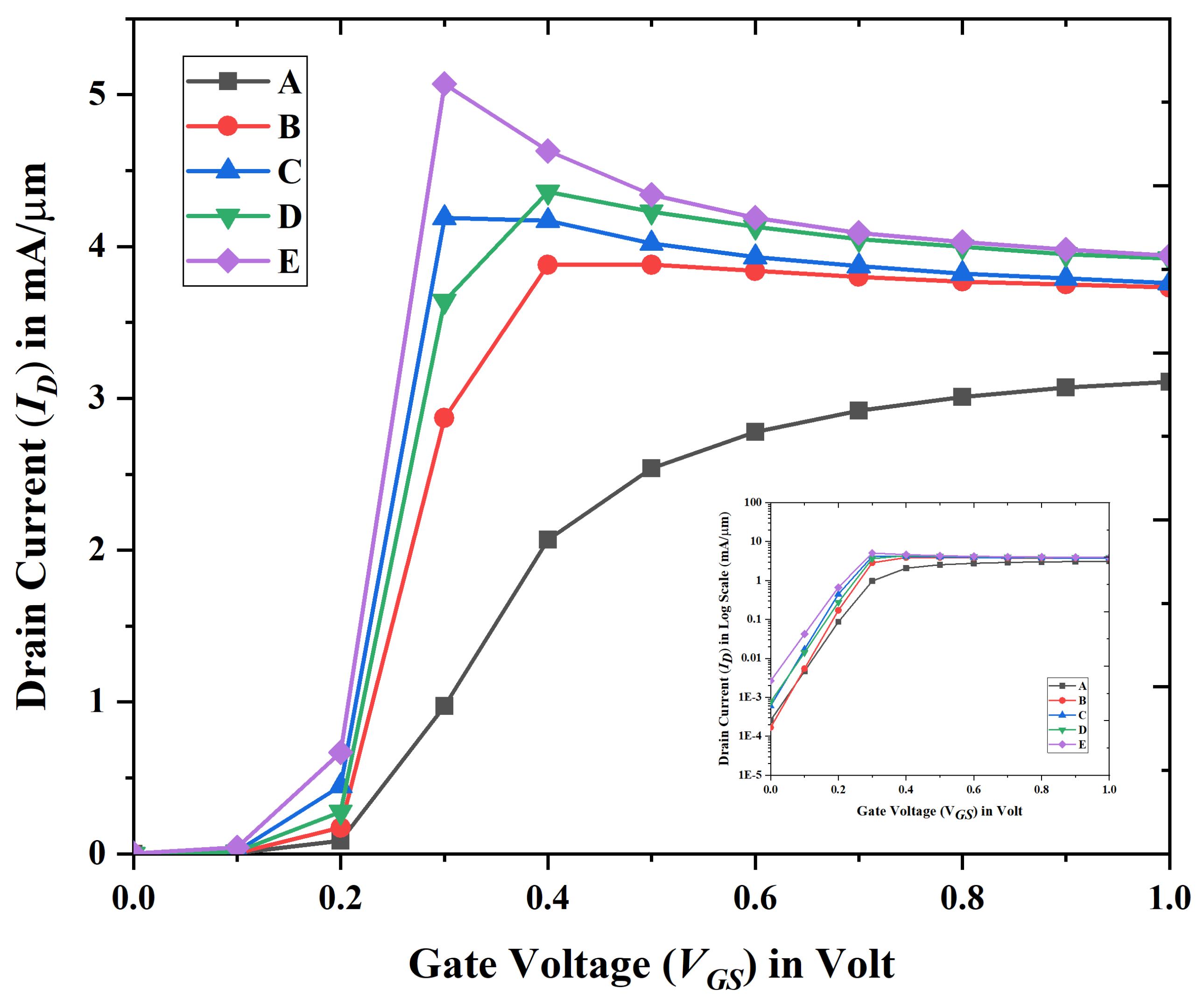
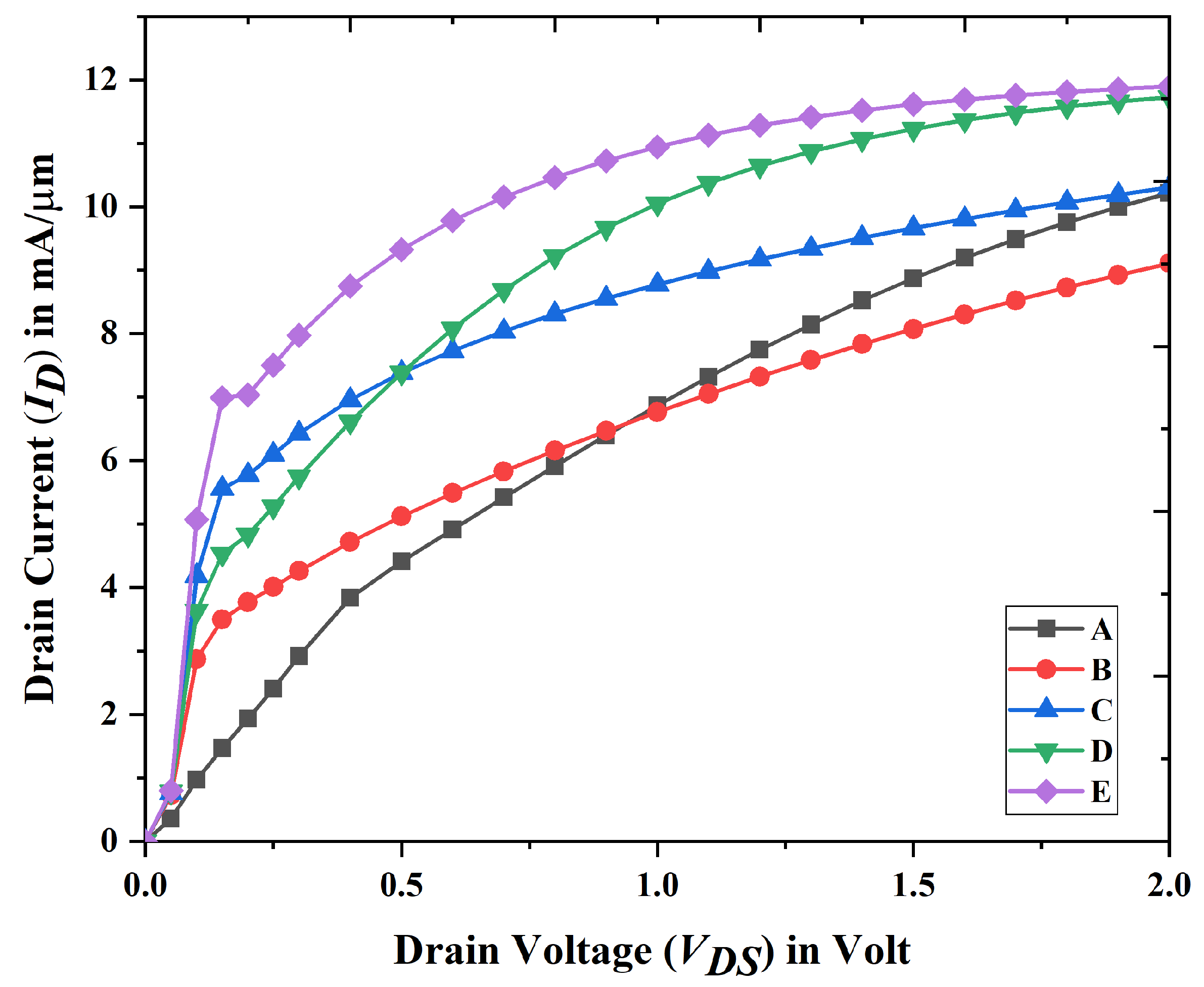
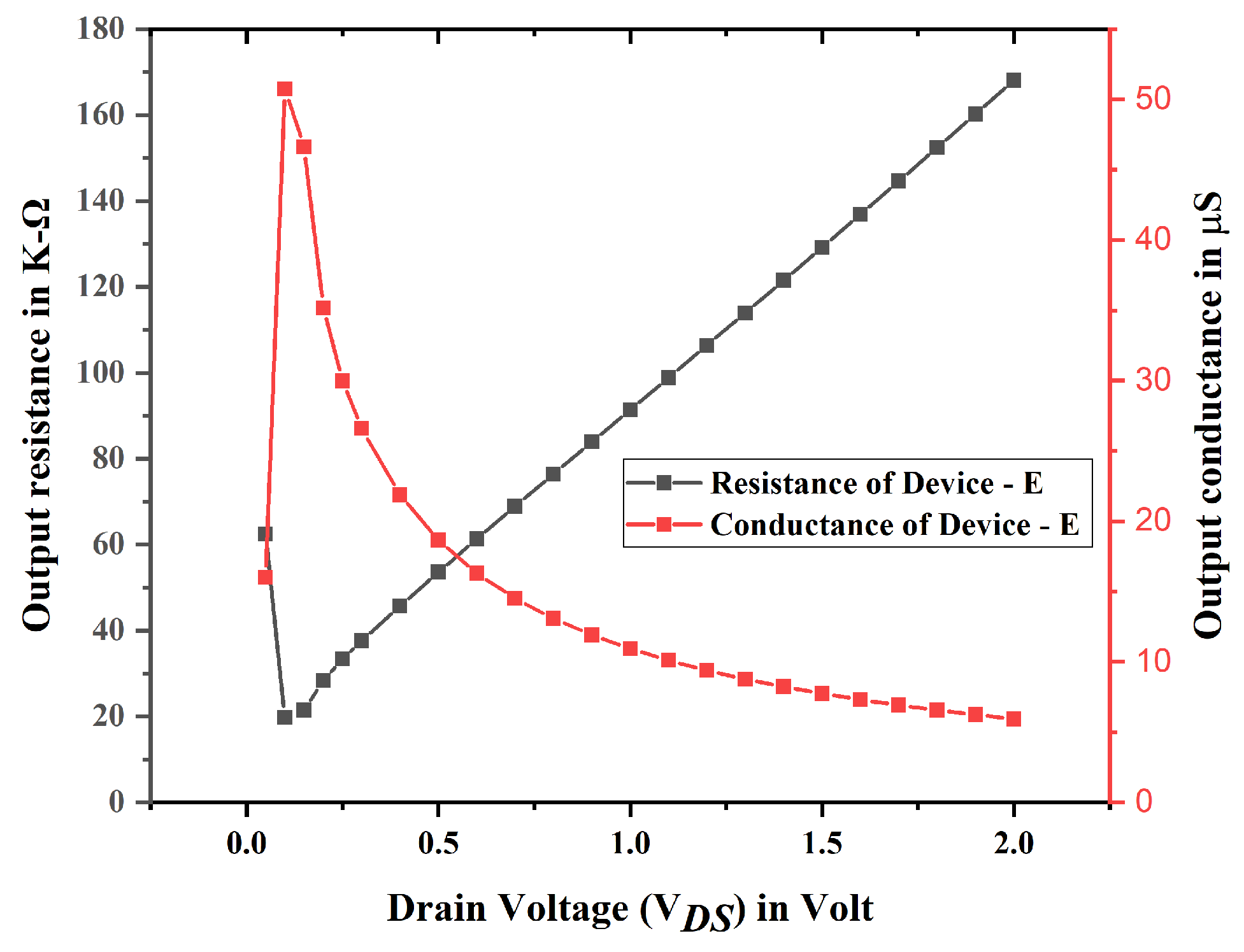
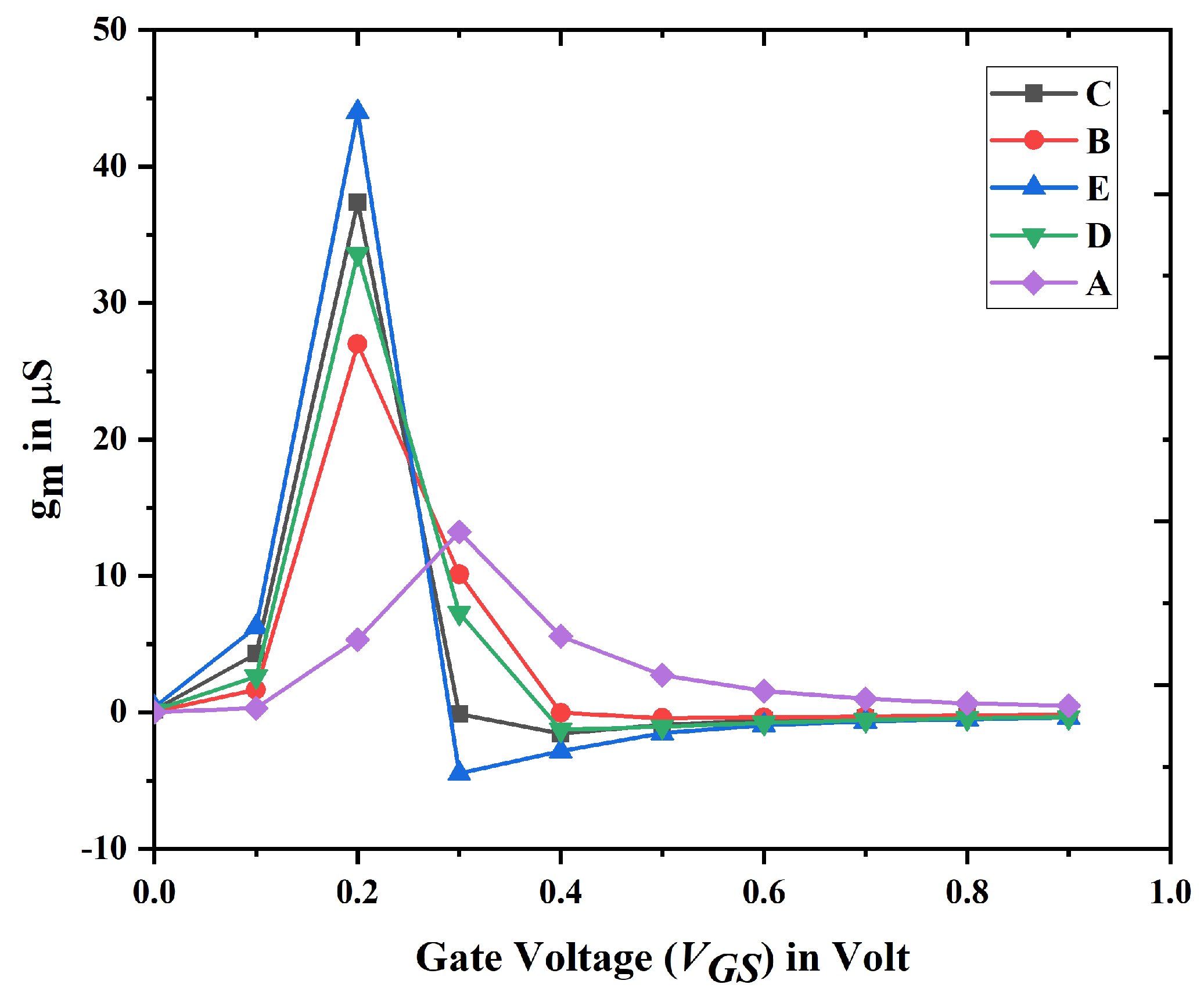
3.1. Electrical Characteristics and Analysis of Nanobiosensor
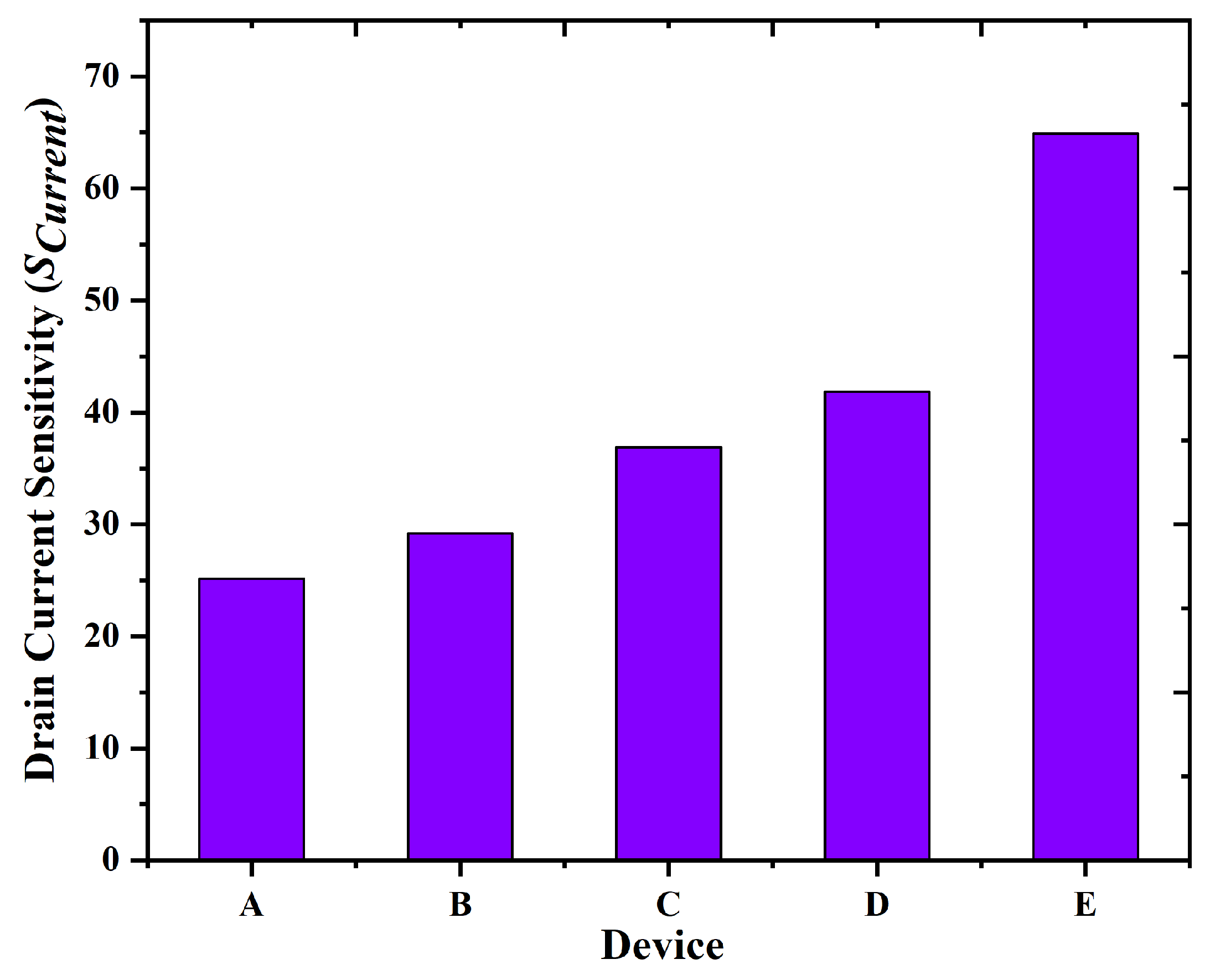
3.2. Sensitivity and Limit of Detection (LOD) Analysis
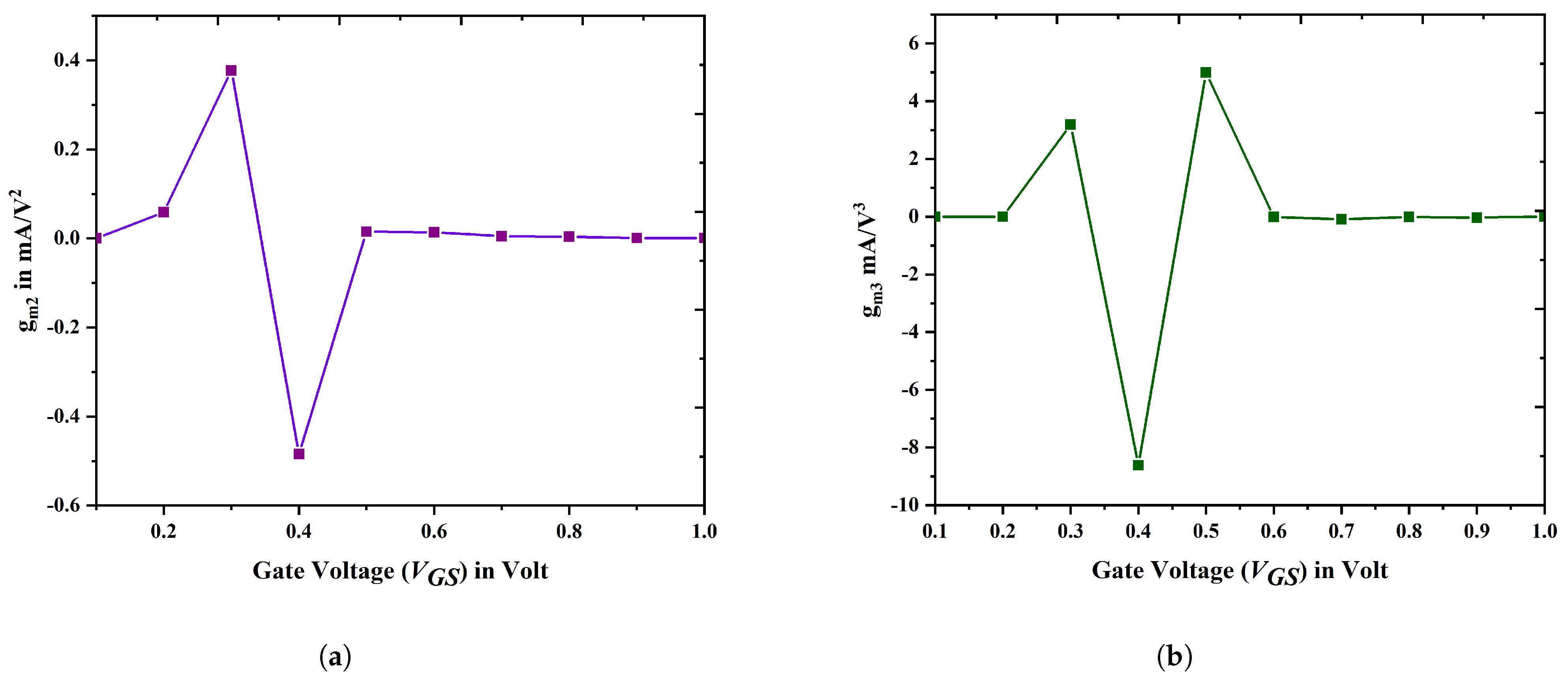
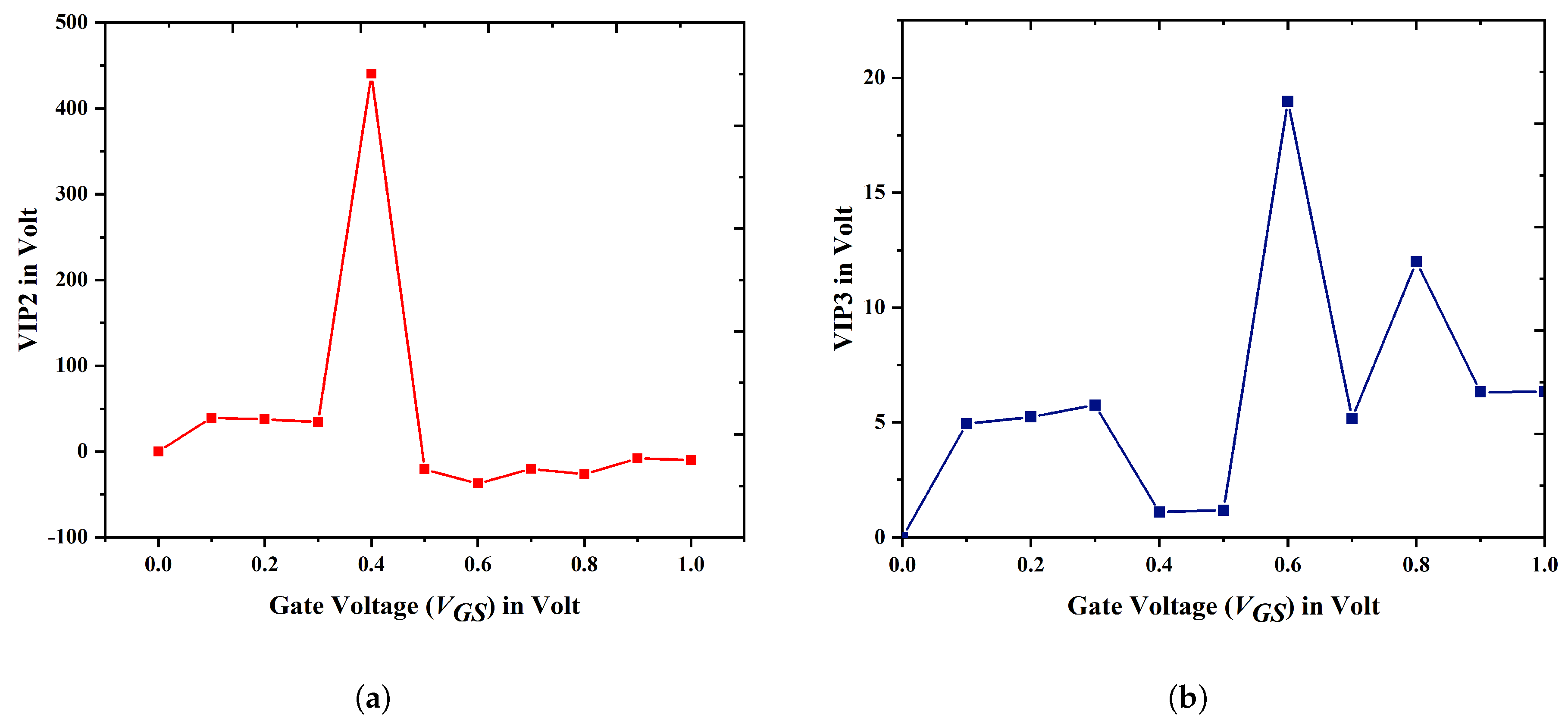
3.3. Linearity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham-Nguyen, L.; Fenouillet-Beranger, C.; Ghibaudo, G.; Skotnicki, T.; Cristoloveanu, S. Mobility enhancement by CESL strain in short-channel ultrathin SOI MOSFETs. Solid State Electron. 2010, 54, 123–130. [Google Scholar] [CrossRef]
- Riyadi, M.A.; Saad, I.; Ismail, R. Investigation of Short Channel Effect on Vertical Structures in Nanoscale Mosfet. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2009, 7, 175–180. [Google Scholar] [CrossRef][Green Version]
- Seoane, N.; García-Loureiro, A.; Kalna, K. Nanowire Field-Effect Transistor (FET); MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Mohsenifar, S.; Shahrokhabadi, M. Gate stack high-κ materials for Si-based MOSFETs past, present, and futures. Terminology 2015, 2, 5. [Google Scholar]
- Pantić, D.; Prijić, Z.; Pavlović, Z. Process design and optimization of the channel doping profile in power VDMOSFETs. Microelectron. J. 1996, 27, 191–200. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Zhang, R.; Chen, B.; Cheng, R. The past and future of multi-gate field-effect transistors: Process challenges and reliability issues. J. Semicond. 2021, 42, 23102. [Google Scholar] [CrossRef]
- Chhabra, A.; Kumar, A.; Chaujar, R. Sub-20 nm GaAs junctionless FinFET for biosensing application. Vacuum 2019, 160, 467–471. [Google Scholar] [CrossRef]
- Kumar, M.J.; Singh, T.V. Quantum confinement effects in strained silicon mosfets. Int. J. Nanosci. 2008, 7, 81–84. [Google Scholar] [CrossRef]
- Khiangte, L.; Dhar, R.S. Development of tri-layered s-Si/s-SiGe/s-Si channel heterostructure-on-insulator MOSFET for enhanced drive current. Phys. Status Solidi (B) 2018, 255, 1800034. [Google Scholar] [CrossRef]
- Colinge, J.P. Multi-gate soi mosfets. Microelectron. Eng. 2007, 84, 2071–2076. [Google Scholar] [CrossRef]
- Karbalaei, M.; Dideban, D.; Heidari, H. A sectorial scheme of gate-all-around field effect transistor with improved electrical characteristics. Ain Shams Eng. J. 2021, 12, 755–760. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Kumar, N.; Sigroha, D.; Kishore, A.; Pathak, V.; Rahman, Z.U. A novel approach to investigate the impact of hetero-high-K gate stack on SiGe Junctionless Gate-All-Around (JL-GAA) MOSFET. Silicon 2022, 14, 1005–1012. [Google Scholar] [CrossRef]
- Goel, A.; Rewari, S.; Verma, S.; Gupta, R. High-K spacer dual-metal gate stack underlap junctionless gate all around (HK-DMGS-JGAA) MOSFET for high frequency applications. Microsyst. Technol. 2020, 26, 1697–1705. [Google Scholar] [CrossRef]
- Moore, M.; Agarwal, S.; Anku, A.; Belyansky, M.; Chandra, V.; Datta, S.; Engelmann, U.; Gao, W.; Iwai, H.; Jeong, J.; et al. International Roadmap for Devices and Systems; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- Daga, M.; Mishra, G.P. Subthreshold performance improvement of underlapped FinFET using workfunction modulated dual-metal gate technique. Silicon 2021, 13, 1541–1548. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Wang, Y.; Dai, P.; Li, T.; Gao, X.; Wang, Y.; Fan, C. Enhanced sensing of nucleic acids with silicon nanowire field effect transistor biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Acharya, D.P.; Mahapatra, K. A read disturbance free differential read SRAM cell for low power and reliable cache in embedded processor. AEU Int. J. Electron. Commun. 2017, 74, 192–197. [Google Scholar] [CrossRef]
- Colinge, J.P. Multiple-gate soi mosfets. Solid-State Electron. 2004, 48, 897–905. [Google Scholar] [CrossRef]
- Kannan, N.; Kumar, M.J. Dielectric-modulated impact-ionization MOS transistor as a label-free biosensor. IEEE Electron Device Lett. 2013, 34, 1575–1577. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, M.; Chaujar, R. Ultralow-power dielectric-modulated nanogap-embedded sub-20- nm TGRC-MOSFET for biosensing applications. J. Comput. Electron. 2018, 17, 1807–1815. [Google Scholar] [CrossRef]
- Saha, A.; Dhar, R.S.; Ghosal, S. A Comparative Study of GaN and Si-Based SOI FinFET. In Proceedings of the International Conference on Computational Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–355. [Google Scholar]
- Kondaveeti, G.S.; Kumar, R.A.; Karumuri, S.R. Design and Performance Analysis of N+ Pocket-Doped Vertical DMDGDP TFET with T-Shape channel for Sensitivity Improvement—As a Biosensor. IEEE Sens. J. 2024, 99, 1–10. [Google Scholar]
- Kumar, R.A.; Kondavitee, G.S.; Wadhwa, G.; Karumuri, S.R. A Novel T-shape channel with an inverted-T nano-cavity Label-free detection using Si: HfO 2 ferroelectric DGDM-JLTFET as a biosensor—A Simulation Study. IEEE Sens. J. 2024, 99, 1–10. [Google Scholar]
- Klein, P.; Binari, S.; Ikossi-Anastasiou, K.; Wickenden, A.; Koleske, D.; Henry, R.; Katzer, D. Investigation of traps producing current collapse in AlGaN/GaN high electron mobility transistors. Electron. Lett. 2001, 37, 661–662. [Google Scholar] [CrossRef]
- Meneghesso, G.; Verzellesi, G.; Pierobon, R.; Rampazzo, F.; Chini, A.; Mishra, U.K.; Canali, C.; Zanoni, E. Surface-related drain current dispersion effects in AlGaN-GaN HEMTs. IEEE Trans. Electron Devices 2004, 51, 1554–1561. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, B.; Pahuja, H.; Jambagi, S.G.; Chhabra, V.A. Design and performance analysis of operational transconductance amplifier using FinFET. In Proceedings of the 2018 8th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 11–12 January 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 14–15. [Google Scholar]
- Rahin, V.B.; Rahin, A.B. A low-voltage and low-power two-stage operational amplifier using FinFET transistors. Int. Acad. J. Sci. Eng. 2016, 3, 80–95. [Google Scholar]
- Nguyen, H.V.; Kim, Y. 32 nm FinFET-based 0.7-to-1.1 V digital voltage sensor with 50 mV resolution. In Proceedings of the 2012 IEEE International Conference on IC Design & Technology, Austin, TX, USA, 30 May–1 June 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–4. [Google Scholar]
- Verma, G.; Negi, H.; Saxena, A.K. Design and Simulation of FinFET based Temperature Sensor. In Proceedings of the 2019 International Conference on Cutting-Edge Technologies in Engineering (ICon-CuTE), Uttar Pradesh, India, 14–16 November; IEEE: Piscataway, NJ, USA, 2019; pp. 147–150. [Google Scholar]
- Hashim, Y. Investigation of FinFET as a temperature nano-sensor based on channel semiconductor type. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 226, p. 12123. [Google Scholar]
- Sze, S.M.; Li, Y.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hisamoto, D.; Lee, W.C.; Kedzierski, J.; Takeuchi, H.; Asano, K.; Kuo, C.; Anderson, E.; King, T.J.; Bokor, J.; Hu, C. FinFET-a self-aligned double-gate MOSFET scalable to 20 nm. IEEE Trans. Electron Devices 2000, 47, 2320–2325. [Google Scholar]
- Barik, R.; Dhar, R.S.; Hussein, M.I. Exploration of underlap induced high-k spacer with gate stack on strain channel cylindrical nanowire FET for enriched performance. Sci. Rep. 2024, 14, 2902. [Google Scholar] [CrossRef]
- Karbalaei, M.; Dideban, D.; Heidari, H. Impact of high-k gate dielectric with different angles of coverage on the electrical characteristics of gate-all-around field effect transistor: A simulation study. Results Phys. 2020, 16, 102823. [Google Scholar] [CrossRef]
- Barik, R.; Dhar, R.S.; Awwad, F.; Hussein, M.I. Evolution of type-II hetero-strain cylindrical-gate-all-around nanowire FET for exploration and analysis of enriched performances. Sci. Rep. 2023, 13, 11415. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dhar, R.S.; Banerjee, A.; Gupta, V. Design and Analysis of High-K wrapped GaN Gate All Around FET as High-Frequency Device in IOT systems. IEEE Access 2025, 13, 78833–78849. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, N.; Tripathi, S.K.; Tripathi, M.; Chaujar, R. Performance evaluation of linearity and intermodulation distortion of nanoscale GaN-SOI FinFET for RFIC design. AEU Int. J. Electron. Commun. 2020, 115, 153052. [Google Scholar] [CrossRef]
- Rajawat, V.S.; Choudhary, B.; Kumar, A. Performance assessment of high-k soi gan finfet with different fin aspect ratio for rf/wireless applications. Wirel. Pers. Commun. 2024, 136, 867–882. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, L.; Cheng, X.; Shen, L.; Liu, X.; Liu, S.; Tian, Y.; Feng, J.; Yu, Y. Substrate Biasing Effect in a High-Voltage Monolithically-Integrated GaN-on-SOI Half Bridge with Partial Recessed-Gate HEMTs. IEEE Trans. Electron Devices 2023, 70, 2975–2980. [Google Scholar] [CrossRef]
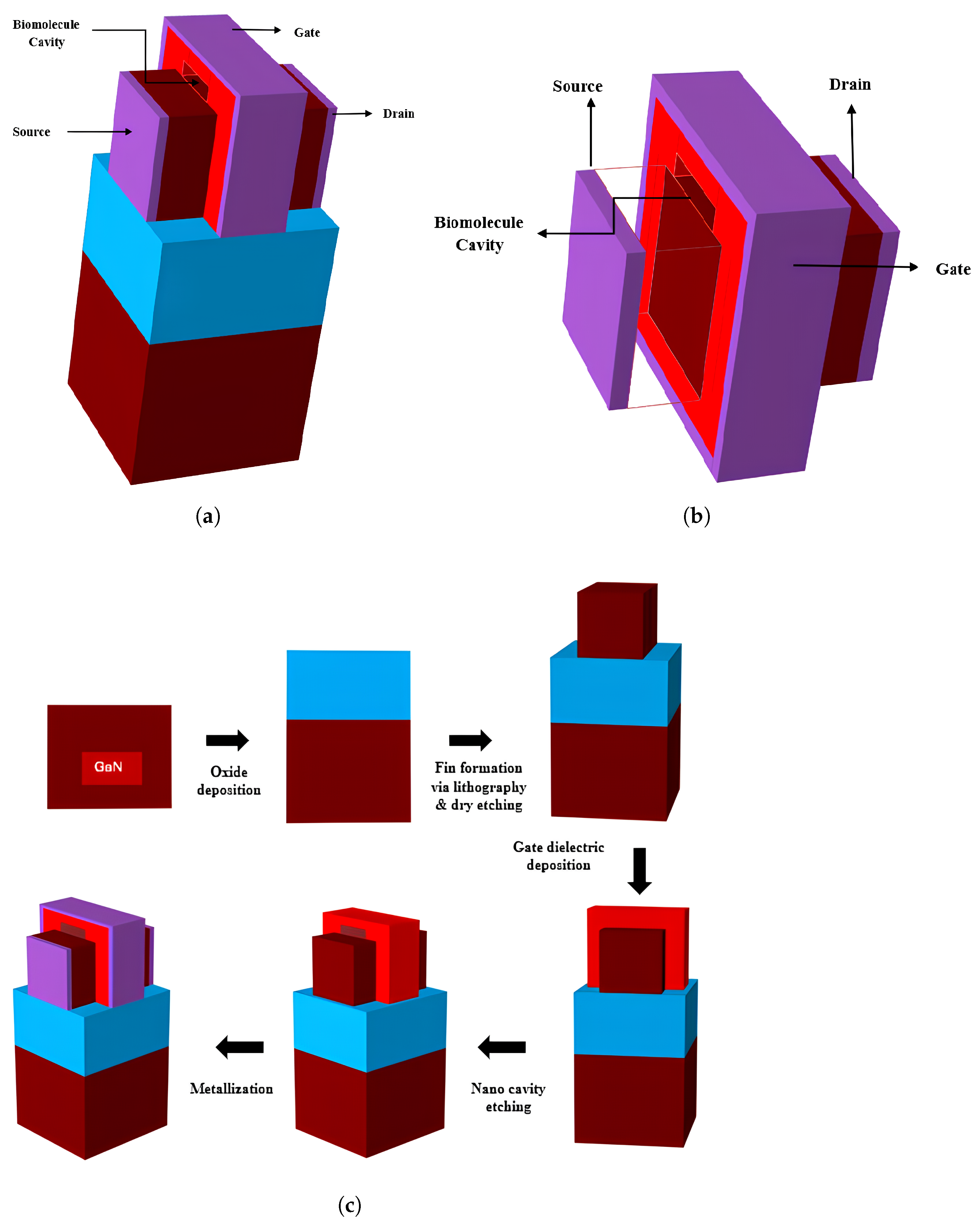





| Device | Specification of Device | Channel Doping | Channel Length | Source and Drain Length |
|---|---|---|---|---|
| A | TGFinFET | Gaussian | 14 nm | 10 nm |
| B | GAAFET | Gaussian | 14 nm | 10 nm |
| C | GAAFET | Uniform ( atoms/CC) | 14 nm | 10 nm |
| D | Nano Biosensor (GAA) | Gaussian | 10 nm | 8 nm |
| E | Nano Biosensor (GAA) | Uniform ( atoms/CC) | 10 nm | 8 nm |
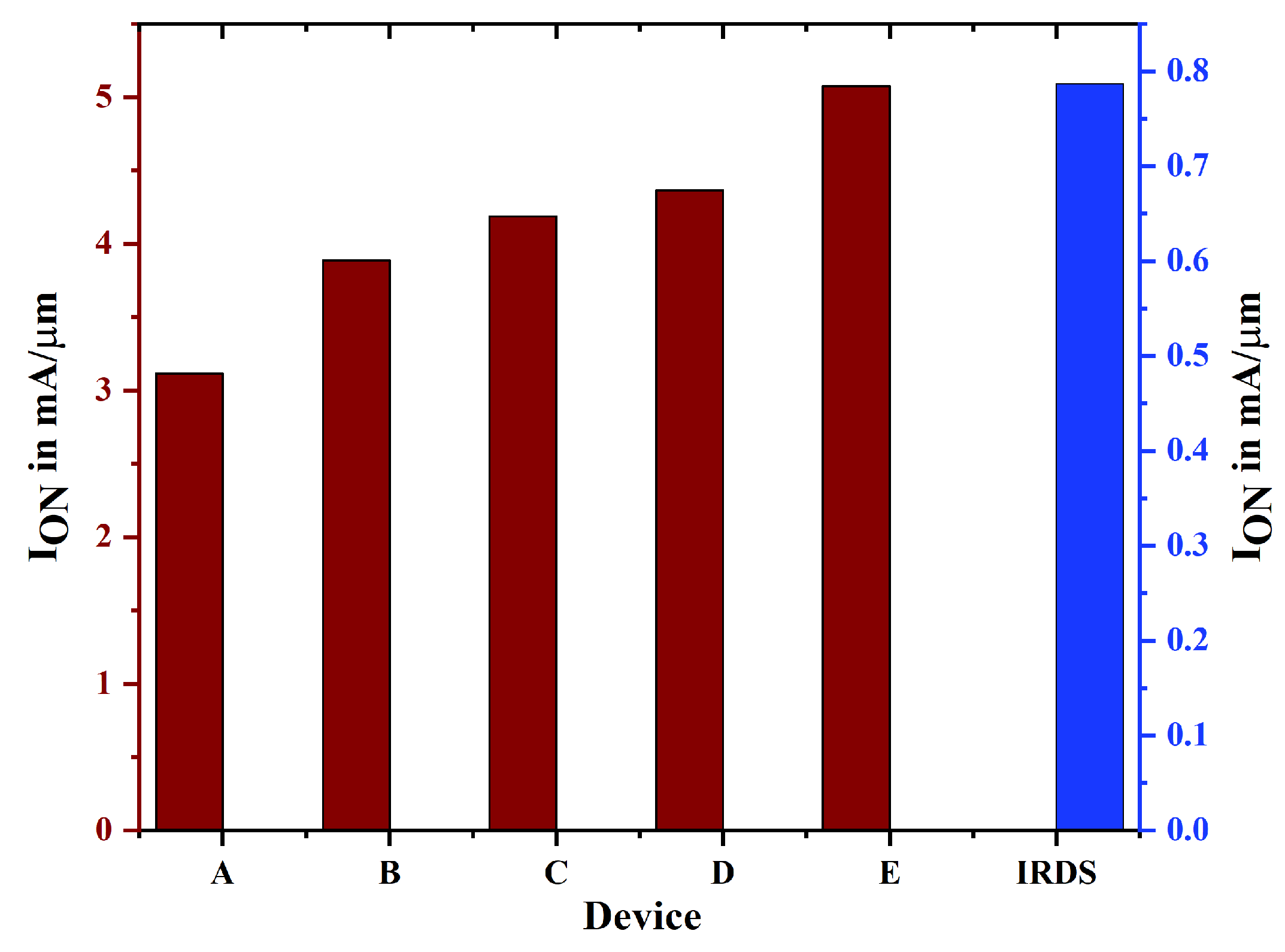
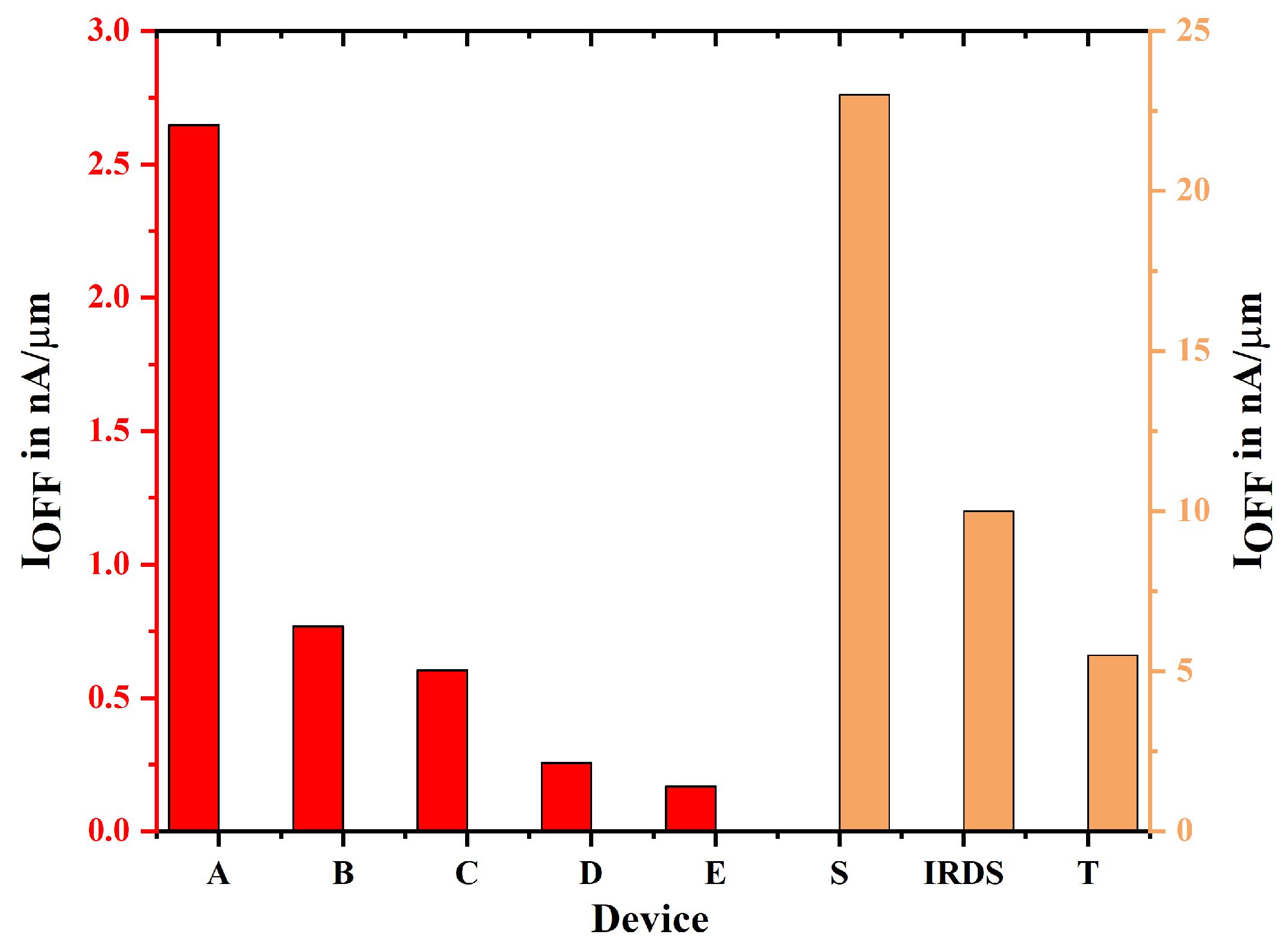
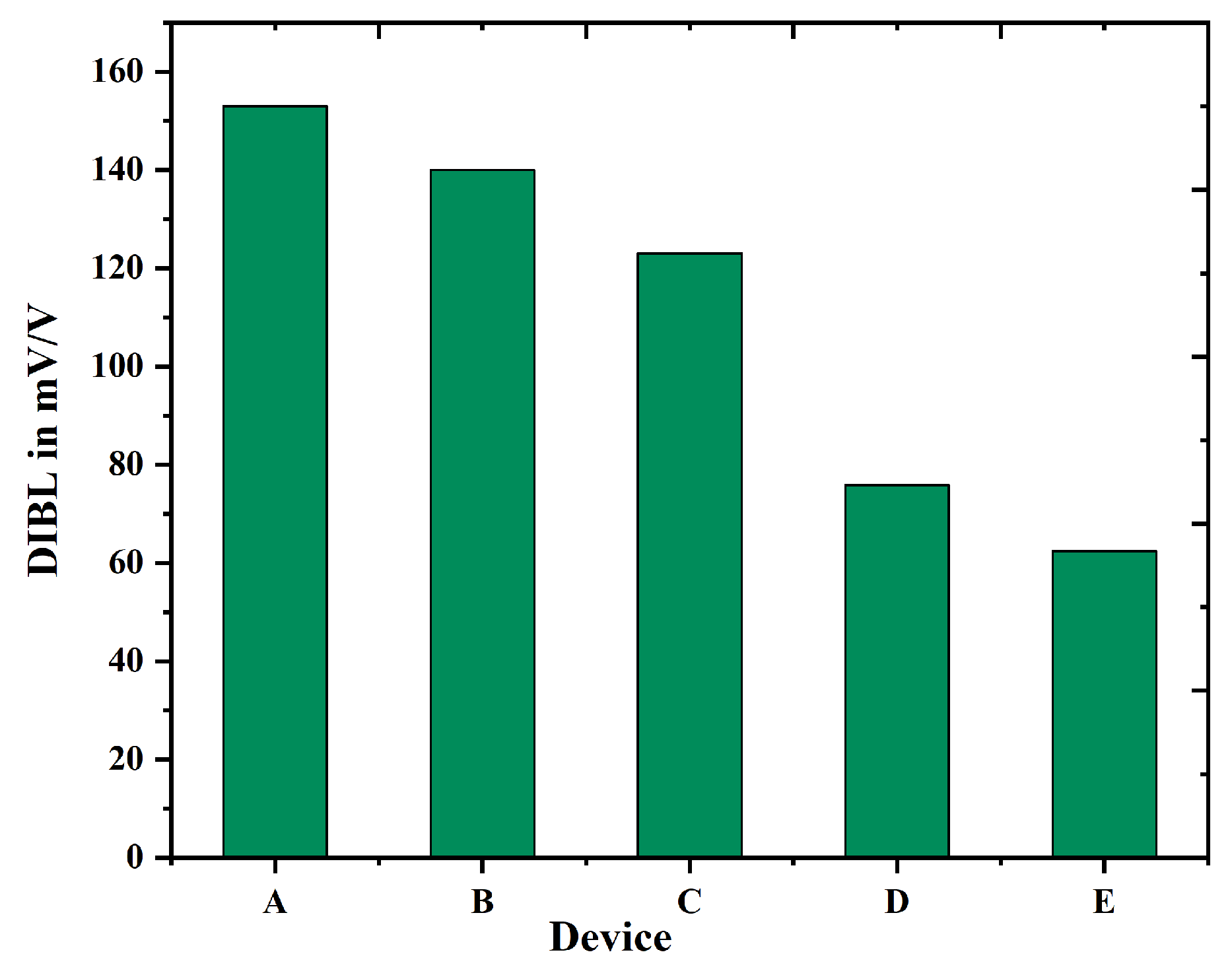
| Device Abbreviation | (mA/m) | (nA/m) | Switching Ratio (nA/m) | Subthreshold Voltage | DIBL |
|---|---|---|---|---|---|
| A | 3.113 | 2.647 | 1.176 | 77 | 153 |
| B | 3.884 | 0.767 | 5.06 | 77.5 | 140 |
| C | 4.186 | 0.602 | 6.953 | 72 | 123 |
| D | 4.364 | 0.257 | 16.98 | 70.9 | 75.9 |
| E | 5.072 | 0.169 | 30.01 | 64.6 | 62.4 |
| Q [33] | 2.5 | 0.11 | 21.34 | 66 | Not Given |
| R [34] | 1.2 | 1.2 | 1 | 71 | Not Given |
| S [13] | 0.037 | 23 | 0.0016 | 0.07 | Not Given |
| T [35] | 1.57 | 5.5 | 28.54 | 79 | Not Given |
| IRDS 2025 [14] | 0.787 | 10 | 0.787 | 72 | Not Given |
| P [36] | 4.46 | 4.86 | 9.18 | 65.03 | Not Given |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Singh, S.; Dhar, R.S.; Ghosh, K.; Seteikin, A.Y.; Banerjee, A.; Samusev, I.G. Exploration and Analysis of GaN-Based FETs with Varied Doping Concentration in Nano Regime for Biosensing Application. Biosensors 2025, 15, 613. https://doi.org/10.3390/bios15090613
Saha A, Singh S, Dhar RS, Ghosh K, Seteikin AY, Banerjee A, Samusev IG. Exploration and Analysis of GaN-Based FETs with Varied Doping Concentration in Nano Regime for Biosensing Application. Biosensors. 2025; 15(9):613. https://doi.org/10.3390/bios15090613
Chicago/Turabian StyleSaha, Abhishek, Sneha Singh, Rudra Sankar Dhar, Kajjwal Ghosh, A. Y. Seteikin, Amit Banerjee, and I. G. Samusev. 2025. "Exploration and Analysis of GaN-Based FETs with Varied Doping Concentration in Nano Regime for Biosensing Application" Biosensors 15, no. 9: 613. https://doi.org/10.3390/bios15090613
APA StyleSaha, A., Singh, S., Dhar, R. S., Ghosh, K., Seteikin, A. Y., Banerjee, A., & Samusev, I. G. (2025). Exploration and Analysis of GaN-Based FETs with Varied Doping Concentration in Nano Regime for Biosensing Application. Biosensors, 15(9), 613. https://doi.org/10.3390/bios15090613






