Natural Iron Oxide Nanoparticles Produced by Aquatic Magnetotactic Bacteria as Ideal Nanozymes for Nano-Guided Biosensing Platforms—A Systematic Review
Abstract
1. Introduction
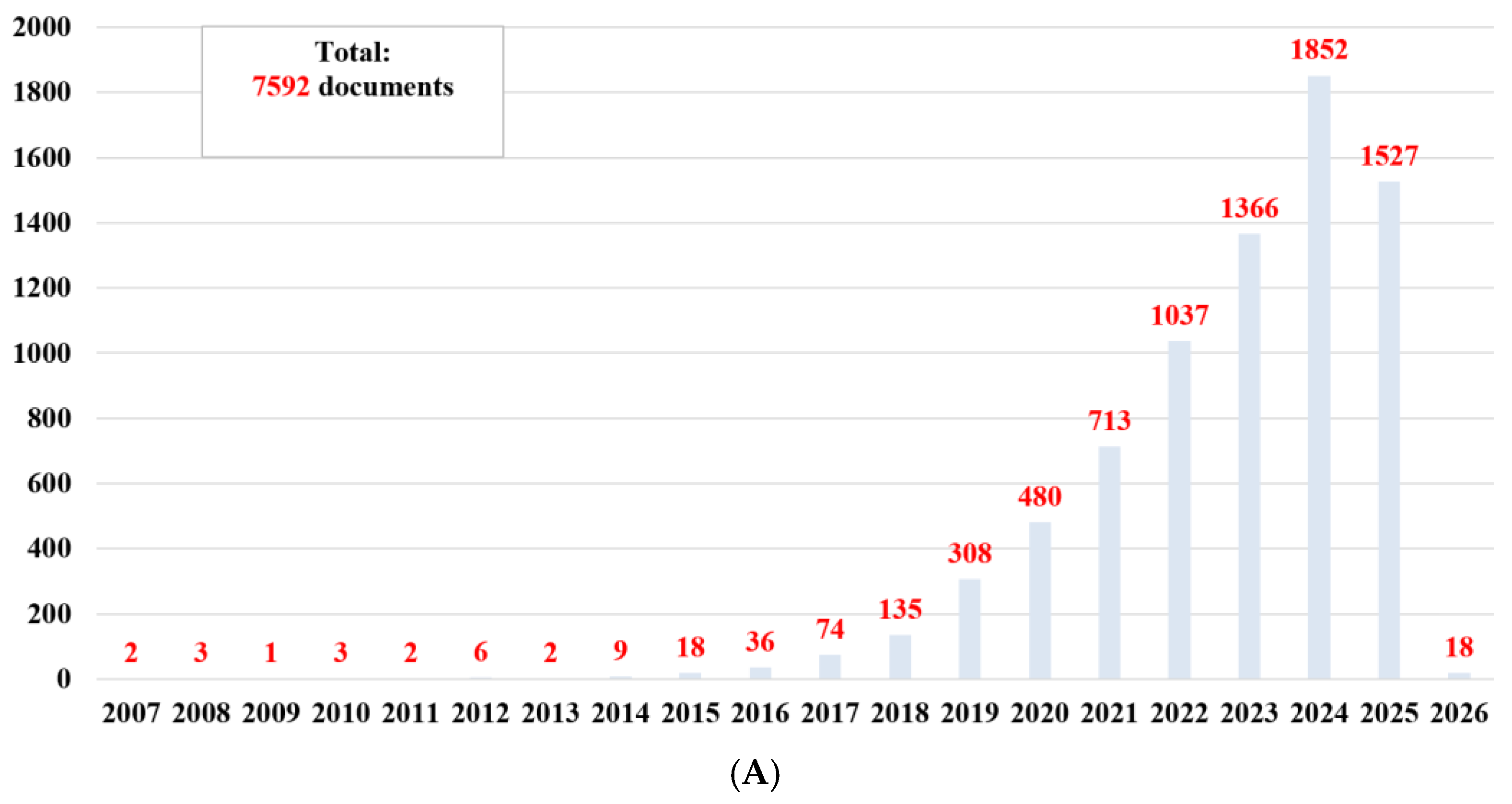
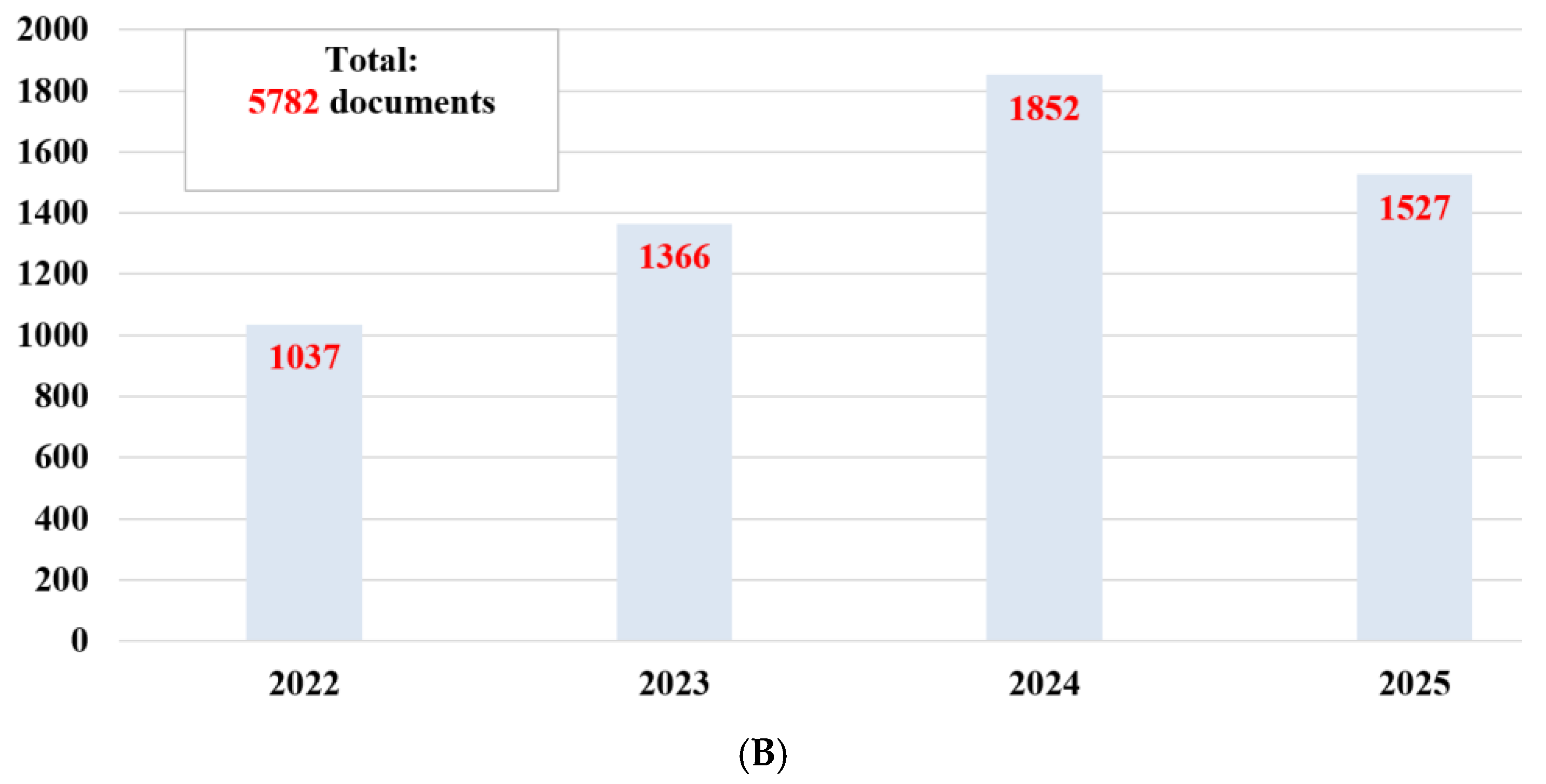
2. Information from Recent Bibliographic Studies
2.1. Data Collection
2.2. Data Analysis
2.2.1. Context Analysis
2.2.2. Content Analysis
3. Nanozymes: State of the Art
3.1. Natural Enzymes vs. Nanozymes
3.2. Factors Affecting the Activity of Nanozymes
3.3. Types of Nanozymes
4. Iron Oxide Nanoparticles
4.1. Synthesis Methods
4.2. Characterization Methods of Iron Oxide Nanoparticles
4.3. Sources of Iron Oxide Nanoparticles
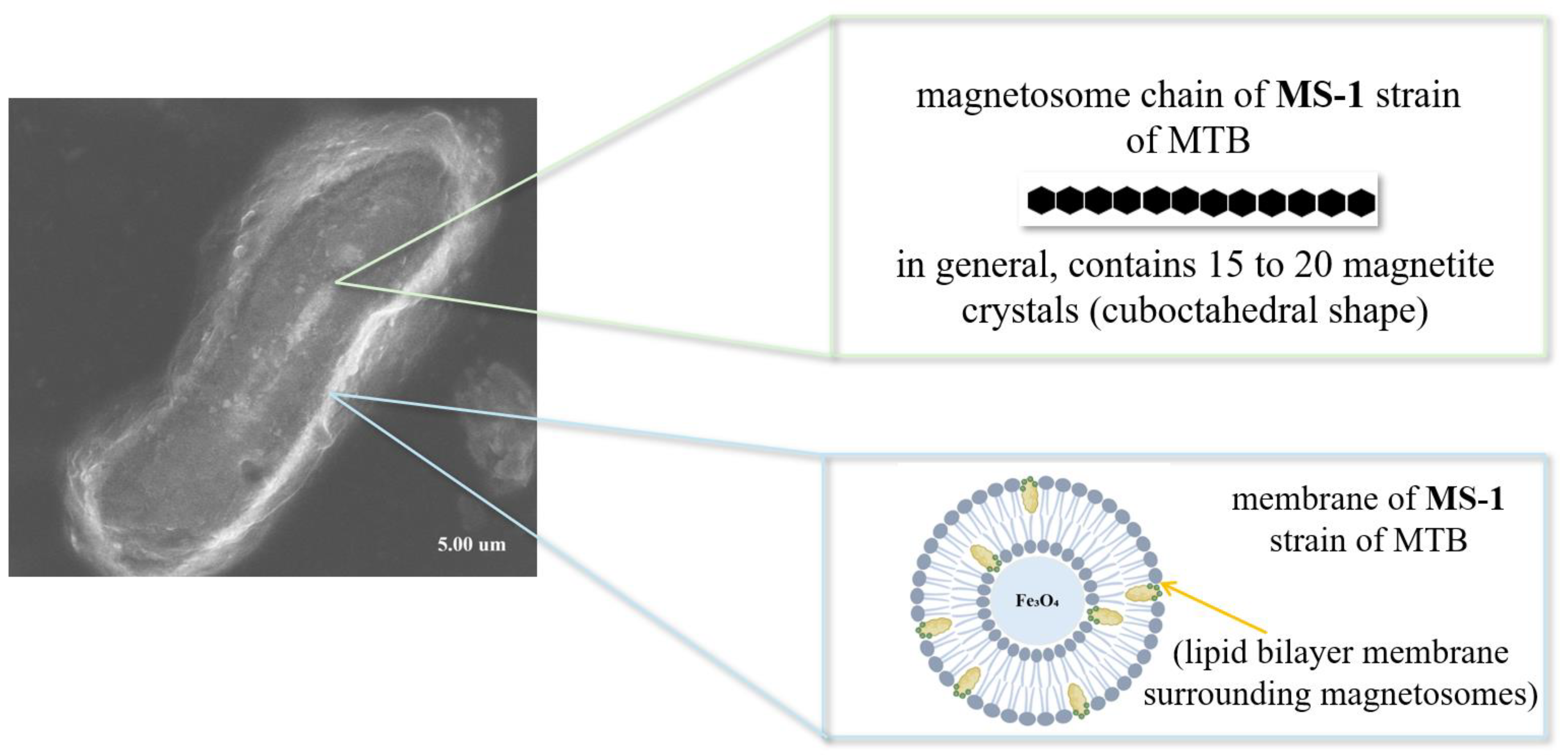
4.4. Magnetotactic Bacteria—Source of Naturally Synthesized Iron Oxide
4.4.1. Magnetosome Formation and Magnetic Crystals Biomineralization in MTB
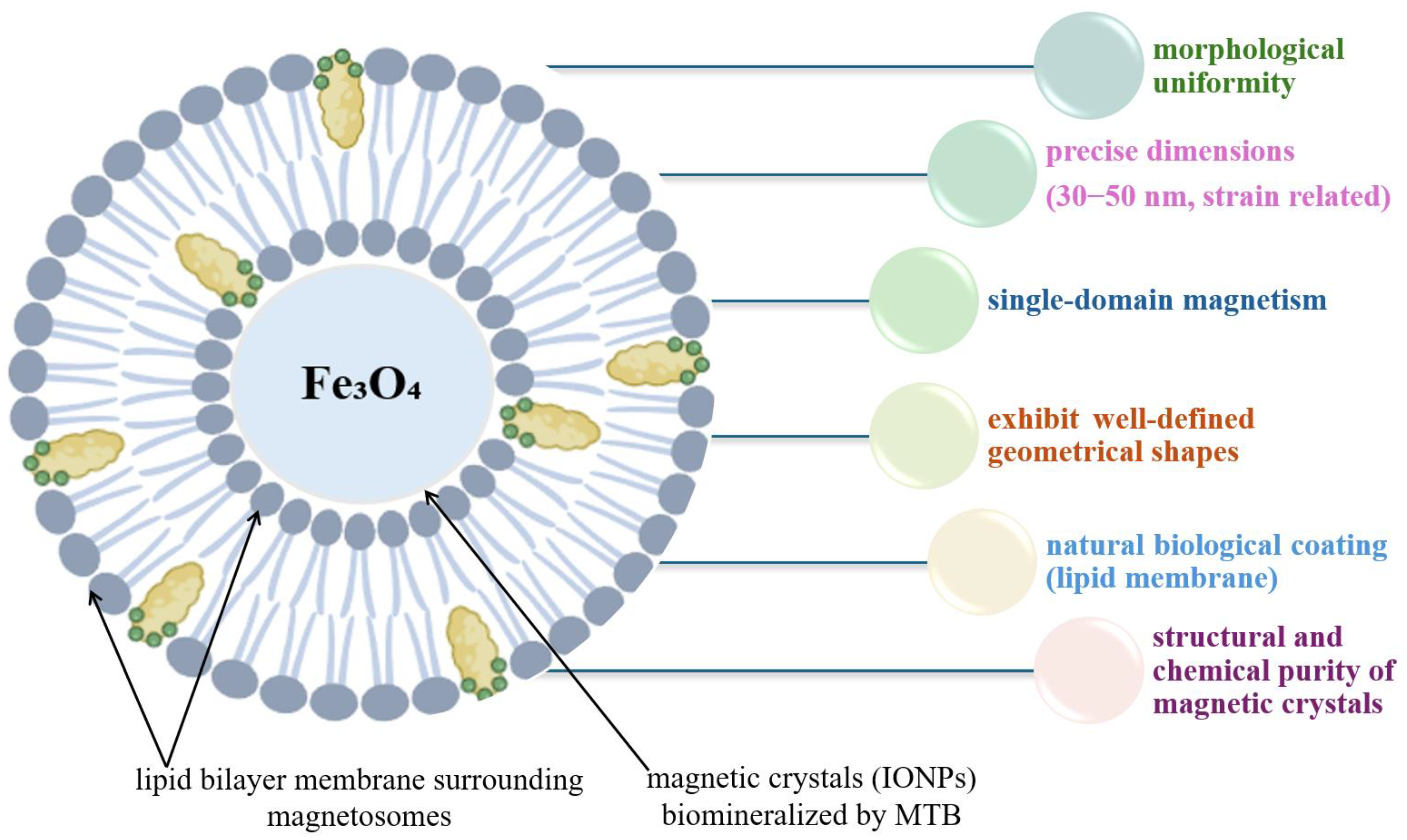
4.4.2. Advantages of Magnetite Crystals from MTB
5. Why Use Iron Oxide Nanoparticle-Based Biosensors Instead of Conventional Enzymatic Biosensors?
6. Iron Oxide-Based Nanozymes in a New Generation of Biosensors and Their Unique and Improved Catalytic Properties in the Presence of Various Added Nanomaterials
6.1. Using Iron Oxide NPs
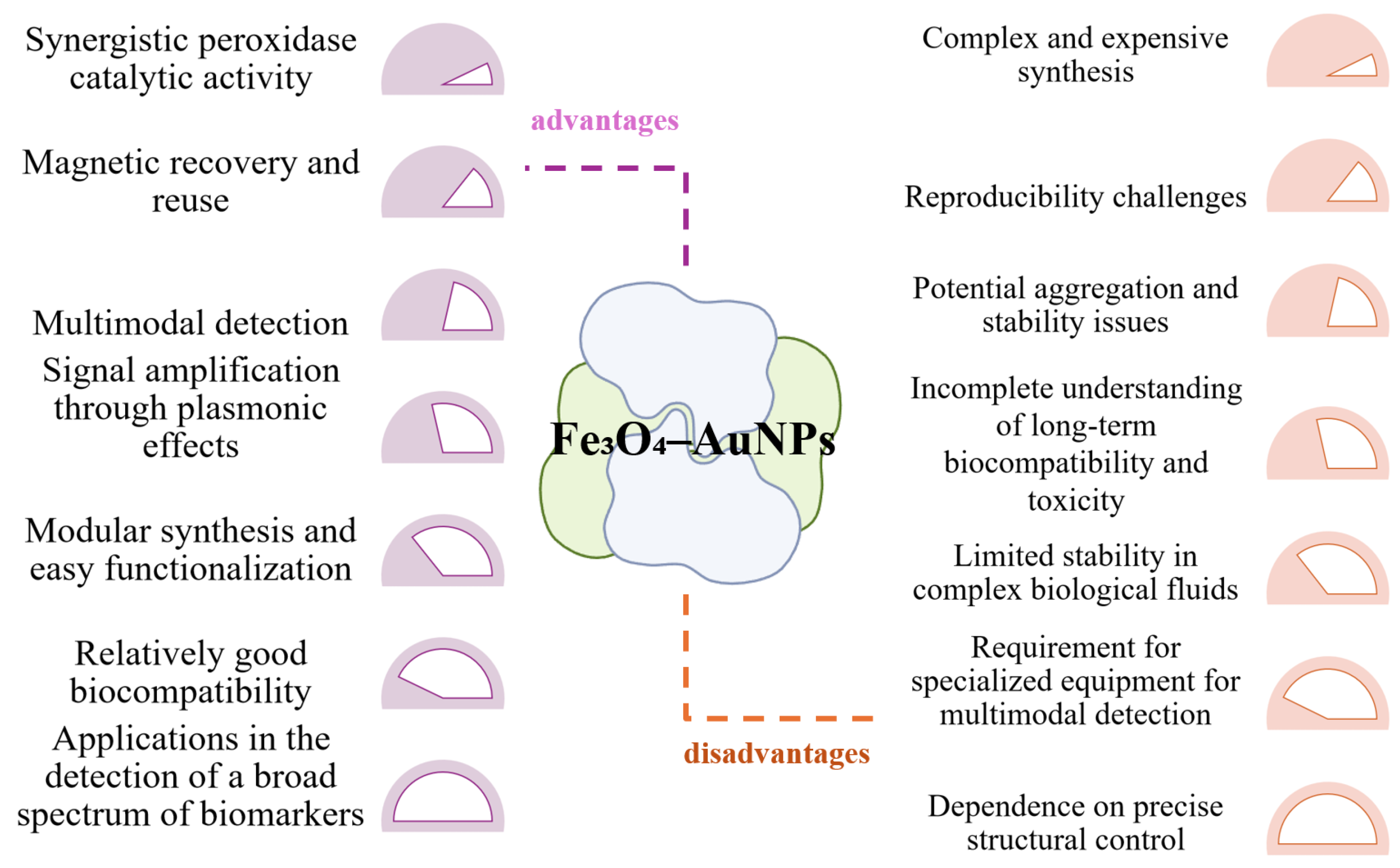
6.2. Using Hybrid Iron Oxide-Gold NPs
6.3. Using Hybrid Iron Oxide-Silver NPs
6.4. Using Hybrid Iron Oxide-Copper NPs
6.5. Using Hybrid Iron Oxide-Platinum NPs
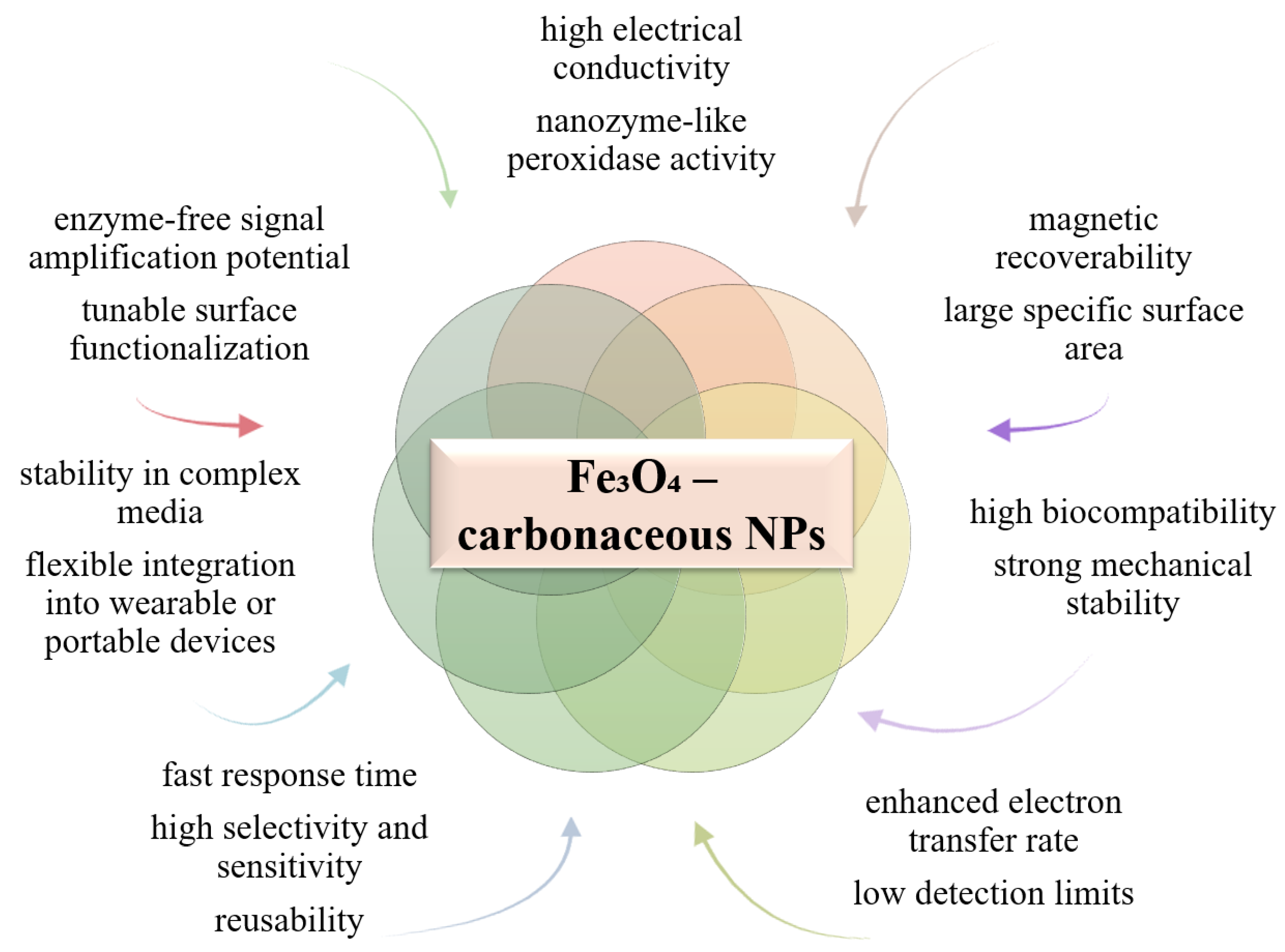
6.6. Using Hybrid Iron Oxide-Carbonaceous NPs
6.7. Using Hybrid Iron Oxide-Indium Tin Oxide NPs
7. Discussions and Future Research Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Hu, C.; Tan, Q.; Wu, S.; Shabala, S.; Yu, M.; Sun, X. Nanozymes as a Tool to Boost Agricultural Production: From Preparation to Application. Environ. Sci. Nano 2025, 12, 98–120. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- An, M.; He, M.-Q.; Lin, C.; Wu, Y.; Ai, Y.; Xin, H.; Liang, Q. Recent Progress of Nanozymes with Different Spatial Dimensions for Bioanalysis. Mater. Today Nano 2023, 22, 100330. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Zhang, Y.; Xia, H.; Wang, Y.; Ma, K.; Wang, J. Application of Magnetotactic Bacteria as Engineering Microrobots: Higher Delivery Efficiency of Antitumor Medicine. Chin. Chem. Lett. 2024, 35, 109420. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Qian, K. Nanozymes: Applications in Clinical Biomarker Detection. Interdiscip. Med. 2023, 1, e20230020. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Y.; Yu, L.; Qu, L.; Li, Z.; Zhang, L. A Co-Based MOF as Nanozyme with Enhanced Oxidase-like Activity for Highly Sensitive and Selective Colorimetric Differentiation of Aminophenol Isomers. Talanta 2023, 255, 124219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Kotha, S.; Fu, M.; Yang, Q.; Wang, H.; He, W.; Mao, X. Nanozyme Enabled Protective Therapy for Neurological Diseases. Nano Today 2024, 54, 102142. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotech 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Xu, D.; Wu, L.; Yao, H.; Zhao, L. Catalase-Like Nanozymes: Classification, Catalytic Mechanisms, and Their Applications—Xu—2022—Small—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202203400 (accessed on 21 May 2025).
- Lin, Z.; Yuan, J.; Niu, L.; Zhang, Y.; Zhang, X.; Wang, M.; Cai, Y.; Bian, Z.; Yang, S.; Liu, A. Oxidase Mimicking Nanozyme: Classification, Catalytic Mechanisms and Sensing Applications. Coord. Chem. Rev. 2024, 520, 216166. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Li, K.; Lin, Y. Superoxide Dismutase Nanozymes: Current Status and Future Perspectives on Brain Disease Treatment and Diagnosis. Chem. Commun. 2024, 60, 4140–4147. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y.; Zhao, D.; Wang, J.; Qiu, L. Transition-Metal-Oxide-Based Nanozymes for Antitumor Applications. Materials 2024, 17, 2896. [Google Scholar] [CrossRef]
- Wang, X.; Shu, C.; Wang, G.; Han, P.; Zheng, L.; Xu, L.; Chen, Y. Recent Progress of Noble Metal-Based Nanozymes: Structural Engineering and Biomedical Applications. Nanoscale 2025, 17, 10557–10580. [Google Scholar] [CrossRef]
- He, J.; Hou, Y.; Zhang, Z.; Zhang, J.; Yan, X.; Fan, K.; Liang, M. Carbon-Based Nanozymes: How Structure Affects Performance. Nano Biomed. Eng. 2024, 16, 28–47. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, H.; Chen, J.; Zhang, Y.; Mo, Q.; Zhang, P.; Wang, M.; Liu, H.; Bao, X.; Sun, Y.; et al. Silk-Based Hydrogel Incorporated with Metal-Organic Framework Nanozymes for Enhanced Osteochondral Regeneration. Bioact. Mater. 2023, 20, 221–242. [Google Scholar] [CrossRef]
- Sen, A.; Oswalia, J.; Yadav, S.; Vachher, M.; Nigam, A. Recent Trends in Nanozyme Research and Their Potential Therapeutic Applications. Curr. Res. Biotechnol. 2024, 7, 100205. [Google Scholar] [CrossRef]
- Singh, R.; Umapathi, A.; Patel, G.; Patra, C.; Malik, U.; Bhargava, S.K.; Daima, H.K. Nanozyme-Based Pollutant Sensing and Environmental Treatment: Trends, Challenges, and Perspectives. Sci. Total Environ. 2023, 854, 158771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.; Zhao, H.; Jiao, Y.; Chen, H.; Wang, P.; Song, T. Magnetotactic Bacteria-Mediated Integrated Magnetic Targeted Hyperthermia for in-Situ Deep-Seated Tumor. Colloids Surf. B Biointerfaces 2025, 252, 114658. [Google Scholar] [CrossRef] [PubMed]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.Á.; Phan, M.-H.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, 1902626. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Sharma, D. Self-Propelling Bacteria-Based Magnetic Nanoparticles (BacMags) for Targeted Magnetic Hyperthermia Therapy against Hypoxic Tumors. Nanoscale 2024, 16, 7892–7907. [Google Scholar] [CrossRef]
- Kuzajewska, D.; Wszołek, A.; Żwierełło, W.; Kirczuk, L.; Maruszewska, A. Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology 2020, 9, 102. [Google Scholar] [CrossRef]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef]
- Yadav, V.K.; Pramanik, S.; Alghamdi, S.; Atwah, B.; Qusty, N.F.; Babalghith, A.O.; Solanki, V.S.; Agarwal, N.; Gupta, N.; Niazi, P.; et al. Therapeutic Innovations in Nanomedicine: Exploring the Potential of Magnetotactic Bacteria and Bacterial Magnetosomes. Int. J. Nanomed. 2025, 20, 403–444. [Google Scholar] [CrossRef]
- Hossain, S.; Bahreini, B.; Pasteur, E.; Sadoh, A.; Bala, R.; M Ravindra, N. Magnetotactic Bacteria and Magnetosomes—An Overview. MSEIJ 2024, 8, 83–100. [Google Scholar] [CrossRef]
- Makela, A.V.; Schott, M.A.; Madsen, C.S.; Greeson, E.M.; Contag, C.H. Magnetic Particle Imaging of Magnetotactic Bacteria as Living Contrast Agents Is Improved by Altering Magnetosome Arrangement. Nano Lett. 2022, 22, 4630–4639. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Applications of Magnetotactic Bacteria and Magnetosome for Cancer Treatment: A Review Emphasizing on Practical and Mechanistic Aspects. Drug Discov. Today 2020, 25, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Gubieda, M.L.; Alonso, J.; García-Prieto, A.; García-Arribas, A.; Fernández Barquín, L.; Muela, A. Magnetotactic Bacteria for Cancer Therapy. J. Appl. Phys. 2020, 128, 070902. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Immobilisation of Lipase Enzyme onto Bacterial Magnetosomes for Stain Removal. Biotechnol. Rep. 2020, 25, e00422. [Google Scholar] [CrossRef]
- Jayaraman, J.; Sigamani, S.; Ramamurthy, D. Metal Biosorption by Magnetotactic Bacteria Isolated from Fresh Water Sediments and Characterization of Extracted Magnetosomes. Arch. Microbiol. 2021, 203, 5951–5962. [Google Scholar] [CrossRef]
- Oestreicher, Z.; Pérez-Guzmán, L.; Casillas-Ituarte, N.N.; Hostetler, M.R.; Mumper, E.; Bazylinski, D.A.; Lower, S.K.; Lower, B.H. Thermophilic Magnetotactic Bacteria from Mickey Hot Springs, an Arsenic-Rich Hydrothermal System in Oregon. ACS Earth Space Chem. 2022, 6, 530–540. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.-W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. Coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- Wu, L.; Gao, B.; Zhang, F.; Sun, X.; Zhang, Y.; Li, Z. A Novel Electrochemical Immunosensor Based on Magnetosomes for Detection of Staphylococcal Enterotoxin B in Milk. Talanta 2013, 106, 360–366. [Google Scholar] [CrossRef]
- Song, S.-J.; Mayorga-Martinez, C.C.; Vyskočil, J.; Častorálová, M.; Ruml, T.; Pumera, M. Precisely Navigated Biobot Swarms of Bacteria Magnetospirillum Magneticum for Water Decontamination. ACS Appl. Mater. Interfaces 2023, 15, 7023–7029. [Google Scholar] [CrossRef]
- Paul, N.L.; Carpa, R.; Ionescu, R.E.; Popa, C.O. The Biomedical Limitations of Magnetic Nanoparticles and a Biocompatible Alternative in the Form of Magnetotactic Bacteria. J. Funct. Biomater. 2025, 16, 231. [Google Scholar] [CrossRef]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef] [PubMed]
- Schwan, J.; Markert, S.; Rosenfeldt, S.; Schüler, D.; Mickoleit, F.; Schenk, A.S. Comparing the Colloidal Stabilities of Commercial and Biogenic Iron Oxide Nanoparticles That Have Potential In Vitro/In Vivo Applications. Molecules 2023, 28, 4895. [Google Scholar] [CrossRef]
- Mickoleit, F.; Pfister, F.; Friedrich, B.; Markert, S.; Kerpes, A.; Janko, C.; Lyer, S.; Alexiou, C.; Schüler, D.; Tietze, R. Assessing Cytotoxicity, Endotoxicity, and Blood Compatibility of Nanoscale Iron Oxide Magnetosomes for Biomedical Applications. ACS Appl. Nano Mater. 2024, 7, 1278–1288. [Google Scholar] [CrossRef]
- Alphandéry, E. Bio-Synthesized Iron Oxide Nanoparticles for Cancer Treatment. Int. J. Pharm. 2020, 586, 119472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, J.; Wang, Z.; Wang, L.; An, Y.; Lin, M.; Gao, Z.; Zhang, D. Biocompatibility of Fe3O4@Au Composite Magnetic Nanoparticles in Vitro and in Vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.-H.; Cho, S.; Kim, D.-M.; Lee, Y.; Ko, S.Y.; Hong, Y.; Choy, H.E.; Min, J.-J.; Park, J.-O.; et al. New Paradigm for Tumor Theranostic Methodology Using Bacteria-Based Microrobot. Sci. Rep. 2013, 3, 3394. [Google Scholar] [CrossRef]
- Buss, M.T.; Ramesh, P.; English, M.A.; Lee-Gosselin, A.; Shapiro, M.G. Spatial Control of Probiotic Bacteria in the Gastrointestinal Tract Assisted by Magnetic Particles. Adv. Mater. 2021, 33, 2007473. [Google Scholar] [CrossRef]
- Nuschke, A.; Sobey-Skelton, C.; Dawod, B.; Kelly, B.; Tremblay, M.-L.; Davis, C.; Rioux, J.A.; Brewer, K. Use of Magnetotactic Bacteria as an MRI Contrast Agent for In Vivo Tracking of Adoptively Transferred Immune Cells. Mol. Imaging Biol. 2023, 25, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Polash, S.A.; Aralappanavar, V.K.; Behera, B.K.; Bansal, V.; Shukla, R. Recent Progress and Prospect of Metal–Organic Framework-Based Nanozymes in Biomedical Application. Nanomaterials 2024, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, D.; Rizwan, M.S.; L’Huillier, B. Environmental, Social, and Governance Integration: The Case of Microfinance Institutions. Account. Financ. 2022, 62, 837–891. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Li, X.; Huo, M. Nanozymes-Recent Development and Biomedical Applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Tu, H.; Pan, C.; Chai, Y.; Chen, W. Advances in Colorimetric Biosensors of Exosomes: Novel Approaches Based on Natural Enzymes and Nanozymes. Nanoscale 2024, 16, 1005–1024. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, G.; Liu, W.; Li, T.; Wang, Y.; Zhou, M.; Liu, Y.; Wang, X.; Wei, H. Nanozymes for Nanohealthcare. Nat. Rev. Methods Primers 2024, 4, 36. [Google Scholar] [CrossRef]
- Zare, I.; Choi, D.; Zhang, J.; Yaraki, M.T.; Ghaee, A.; Nasab, S.Z.; Taheri-Ledari, R.; Maleki, A.; Rahi, A.; Fan, K.; et al. Modulating the Catalytic Activities of Nanozymes for Molecular Sensing. Nano Today 2024, 56, 102276. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The Nanozyme Revolution: Enhancing the Performance of Medical Biosensing Platforms. Adv. Mater. 2024, 36, 2300184. [Google Scholar] [CrossRef]
- Patil, P.D.; Karvekar, A.; Salokhe, S.; Tiwari, M.S.; Nadar, S.S. When Nanozymes Meet Enzyme: Unlocking the Dual-Activity Potential of Integrated Biocomposites. Int. J. Biol. Macromol. 2024, 271, 132357. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Y.; Wang, Y.; Han, B.; Lian, M. Progress in Antibacterial Applications of Nanozymes. Front. Chem. 2024, 12, 1478273. [Google Scholar] [CrossRef]
- Wang, P.; Min, D.; Chen, G.; Li, M.; Tong, L.; Cao, Y. Inorganic Nanozymes: Prospects for Disease Treatments and Detection Applications. Front. Chem. 2021, 9, 773285. [Google Scholar] [CrossRef]
- Thao, N.T.M.; Do, H.D.K.; Nam, N.N.; Tran, N.K.S.; Dan, T.T.; Trinh, K.T.L. Antioxidant Nanozymes: Mechanisms, Activity Manipulation, and Applications. Micromachines 2023, 14, 1017. [Google Scholar] [CrossRef]
- Deshwal, A.; Saxena, K.; Sharma, G.; Rajesh; Sheikh, F.A.; Seth, C.S.; Tripathi, R.M. Nanozymes: A Comprehensive Review on Emerging Applications in Cancer Diagnosis and Therapeutics. Int. J. Biol. Macromol. 2024, 256, 128272. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rai, N.; Tiwari, H.; Gupta, P.; Verma, A.; Kumar, R.; Kailashiya, V.; Salvi, P.; Gautam, V. Recent Advancements in the Formulation of Nanomaterials-Based Nanozymes, Their Catalytic Activity, and Biomedical Applications. ACS Appl. Bio Mater. 2023, 6, 3577–3599. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Shah, A.A.; Rahman, H.; Bilal, M.; Rajoka, M.S.R.; Khan, A.A.; Khurshid, M. Nanozymes for Medical Biotechnology and Its Potential Applications in Biosensing and Nanotherapeutics. Biotechnol. Lett. 2020, 42, 357–373. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Torres Castillo, N.E.; Melchor-Martínez, E.M.; Ochoa Sierra, J.S.; Ramírez-Torres, N.M.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldívar, R. Enzyme Mimics In-Focus: Redefining the Catalytic Attributes of Artificial Enzymes for Renewable Energy Production. Int. J. Biol. Macromol. 2021, 179, 80–89. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Gong, X.; Wang, Y.; Li, Y.F.; Huang, C.Z. Engineering Metal-Organic Frameworks-Based Nanozymes for Enhanced Biomimetic Catalytic Sensing. TrAC Trends Anal. Chem. 2024, 178, 117862. [Google Scholar] [CrossRef]
- Saini, N.; Choudary, R.; Chopra, D.S.; Singh, D.; Singh, N. Nanozymes: Classification, Synthesis and Challenges. Appl. Nanosci. 2023, 13, 6433–6443. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Hao, L.; Zhang, P.; Song, G.; Zhang, Y.; Wang, C.; Wang, Z.; Wu, Q. Enhancing Peroxidase Activity of NiCo2O4 Nanoenzyme by Mn Doping for Catalysis of CRISPR/Cas13a-Mediated Non-Coding RNA Detection. Int. J. Biol. Macromol. 2024, 283, 137594. [Google Scholar] [CrossRef]
- Isho, R.D.; Sher Mohammad, N.M.; Omer, K.M. Enhancing Enzymatic Activity of Mn@Co3O4 Nanosheets as Mimetic Nanozyme for Colorimetric Assay of Ascorbic Acid. Anal. Biochem. 2022, 654, 114818. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jin, B.; Li, H.; Wu, X.; Liu, Y.; Zhao, H.; Zhong, D.; Wang, L.; Chen, W.; Wen, M.; et al. Cold Nanozyme for Precise Enzymatic Antitumor Immunity. ACS Nano 2022, 16, 21491–21504. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, Y.; Yin, C.; Wang, L.; Huang, H.; Li, Y. A Novel and Facile Oxygen-Activated Time-Temperature Indicator with Wide Temperature Monitoring Range and Good Stability Based on the Laccase-like Nanozyme. Anal. Chim. Acta 2024, 1330, 343272. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dai, D.; Xiong, G.; Lan, S.; Zhang, C. Metal-Based Nanozymes with Multienzyme-Like Activities as Therapeutic Candidates: Applications, Mechanisms, and Optimization Strategy. Small 2023, 19, 2205870. [Google Scholar] [CrossRef]
- Fu, Q.; Wei, C.; Wang, M. Transition-Metal-Based Nanozymes: Synthesis, Mechanisms of Therapeutic Action, and Applications in Cancer Treatment. ACS Nano 2024, 18, 12049–12095. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, G.; Feng, W.; Wang, P.; Gao, J.; Liu, J.; Wang, M.; Wang, Q. Detection of Glucose Based on Noble Metal Nanozymes: Mechanism, Activity Regulation, and Enantioselective Recognition. Small 2023, 19, 2205924. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Ghazzy, A.; Nsairat, H.; Said, R.; Sibai, O.A.; AbuRuman, A.; Shraim, A.S.; Hunaiti, A.A. Magnetic Iron Oxide-Based Nanozymes: From Synthesis to Application. Nanoscale Adv. 2024, 6, 1611–1642. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yu, Y.; Dong, S.; Sun, X.; Mao, L. Carbon-Based Nanozymes: Design, Catalytic Mechanisms, and Environmental Applications. Anal. Bioanal. Chem. 2024, 416, 5949–5964. [Google Scholar] [CrossRef]
- Dong, C.; Ma, X.; Huang, Y.; Zhang, Y.; Gao, X. Carbon Dots Nanozyme for Anti-Inflammatory Therapy via Scavenging Intracellular Reactive Oxygen Species. Front. Bioeng. Biotechnol. 2022, 10, 943399. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon-Based Enzyme Mimetics for Electrochemical Biosensing. Micromachines 2023, 14, 1746. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Xue, R.; Liang, F.; Liu, Y.; Wang, Y.; Li, J.; Huang, Z. Synergistic Effect of Silver Nanoclusters and Graphene Oxide on Visible Light-Driven Oxidase-like Activity: Construction of a Sustainable Nanozyme for Total Antioxidant Capacity Detection. Talanta 2023, 259, 124565. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Qian, W.; Lei, F.; Chen, Z.; Wu, X.; Lin, Y.; Wang, F. Recent Advances in MOF-Based Nanozymes: Synthesis, Activities, and Bioapplications. Biosens. Bioelectron. 2024, 263, 116593. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Weng, W.; Xu, Y.; Zhou, Y.; Yi, Y.; Zhao, Y.; Zhu, G. Vanadium-Based Metal–Organic Frameworks with Peroxidase-like Activity as a Colorimetric Sensing Platform for Direct Detection of Organophosphorus Pesticides. Inorg. Chem. 2024, 63, 16442–16450. [Google Scholar] [CrossRef]
- Chen, M.; Qin, Y.; Peng, Y.; Mai, R.; Teng, H.; Qi, Z.; Mo, J. Advancing Stroke Therapy: The Potential of MOF-Based Nanozymes in Biomedical Applications. Front. Bioeng. Biotechnol. 2024, 12, 1363227. [Google Scholar] [CrossRef]
- Luo, J.; Gong, X.-Y.; Zhou, B.-Y.; Yang, L.; Yang, W.-C. Advances in Nanohydrolase-Based Pollutant Sensing. Trends Environ. Anal. Chem. 2024, 43, e00238. [Google Scholar] [CrossRef]
- Ghafori-Gorab, M.; Kashtiaray, A.; Karimi, M.; Aghamirza Moghim Aliabadi, H.; Bakhtiyar, F.; Daraei Ghadikolaei, F.; Mohajeri, M.; Maleki, A. Recent Advances on Biomedical Applications of Zirconium-Based Nanozymes: A Review. Chem. Eng. J. 2025, 505, 159464. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mandal, M.; Kumar Maiti, D. A Review on Zirconium-Based Metal–Organic Frameworks: Synthetic Approaches and Biomedical Applications. Mater. Adv. 2024, 5, 51–67. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, Z.; Lin, D.; Bao, Q.; Gao, Y.; Liu, Q.; Liu, A.; Lin, L.; Lin, X. Boosting the Oxidase-like Activity of Platinum Nanozyme in MBTH-TOOS Chromogenic System for Detection of Trypsin and Its Inhibitor. Talanta 2021, 234, 122647. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Kim, M.I. Recent Advances on Nanozyme-Based Electrochemical Biosensors for Cancer Biomarker Detection. TrAC Trends Anal. Chem. 2024, 177, 117815. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Liu, S.; Chen, Y.; Liang, D.; Liu, Y.; Chen, W.; Du, L.; Wu, C. Application of Nanozymes in Environmental Monitoring, Management, and Protection. Biosensors 2023, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kaushik, N.K.; Tiwari, S.K.; Singh, D.; Singh, B. Green Synthesis of Iron Nanoparticles: Sources and Multifarious Biotechnological Applications. Int. J. Biol. Macromol. 2023, 253, 127017. [Google Scholar] [CrossRef] [PubMed]
- Gareev, K.G. Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications. Magnetochemistry 2023, 9, 119. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Song, L.; Cui, X.; Zhou, J.; Jin, G.; Boccaccini, A.R.; Virtanen, S. Iron Oxide Nanoparticle-Based Nanocomposites in Biomedical Application. Trends Biotechnol. 2023, 41, 1471–1487. [Google Scholar] [CrossRef]
- Chaudhari, D.S.; Upadhyay, R.P.; Shinde, G.Y.; Gawande, M.B.; Filip, J.; Varma, R.S.; Zbořil, R. A Review on Sustainable Iron Oxide Nanoparticles: Syntheses and Applications in Organic Catalysis and Environmental Remediation. Green. Chem. 2024, 26, 7579–7655. [Google Scholar] [CrossRef]
- Gogoi, B. Synthesis and Characterisation of Transition Metal Iron Oxide Nanocomposite Crystals and Particles Using Wet Chemical Coprecipitation Method. Prot. Met. Phys. Chem. Surf. 2023, 59, 1200–1209. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef]
- Ogbezode, J.E.; Ezealigo, U.S.; Bello, A.; Anye, V.C.; Onwualu, A.P. A Narrative Review of the Synthesis, Characterization, and Applications of Iron Oxide Nanoparticles. Discov. Nano 2023, 18, 125. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Revathy, R.; Sajini, T.; Augustine, C.; Joseph, N. Iron-Based Magnetic Nanomaterials: Sustainable Approaches of Synthesis and Applications. Results Eng. 2023, 18, 101114. [Google Scholar] [CrossRef]
- Mohamed, A.; Atta, R.R.; Kotp, A.A.; Abo El-Ela, F.I.; Abd El-Raheem, H.; Farghali, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Green Synthesis and Characterization of Iron Oxide Nanoparticles for the Removal of Heavy Metals (Cd2+ and Ni2+) from Aqueous Solutions with Antimicrobial Investigation. Sci. Rep. 2023, 13, 7227. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Fatima, N.; Iqbal, J.; Chalgham, W.; Mumtaz, A.S.; El-Sheikh, M.A.; Tavafoghi, M. Oscillatoria Limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities. Molecules 2023, 28, 2091. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.N.; Diniz, F.B.; Rodrigues, A.R. Reaction between Fe3+ and Aniline in the Synthesis of PANI-γFe2O3 and PANI-Fe3O4 Nanocomposites: Mechanistic Studies and Evaluation of Parameters. Nano-Struct. Nano-Objects 2025, 42, 101477. [Google Scholar] [CrossRef]
- Eid, M.M. Characterization of Nanoparticles by FTIR and FTIR-Microscopy. In Handbook of Consumer Nanoproducts; Springer: Singapore, 2021; pp. 1–30. ISBN 978-981-15-6453-6. [Google Scholar]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Storozhuk, L.; LaGrow, A.P.; Panariello, L.; Maney, A.; Pal, S.; Kiefer, C.; Mertz, D.; Tung, L.D.; Lees, M.R.; et al. High Temperature Flow Synthesis of Iron Oxide Nanoparticles: Size Tuning via Reactor Engineering. Chem. Eng. J. 2023, 473, 144542. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shaaban, M.T.; Goda, A.; Morsi, H.H. Green Synthesis of Iron Oxide Nanoparticles Using Lyptolyngbya Foveolarum and Azospirillum Brasilense for Wastewater Treatment. Int. J. Environ. Sci. Technol. 2025, 22, 9933–9948. [Google Scholar] [CrossRef]
- Vaezi-Kakhki, A.; Asoodeh, A. Comparison of Different Methods for Synthesis of Iron Oxide Nanoparticles and Investigation of Their Cellular Properties, and Antioxidant Potential. Int. J. Pharm. 2023, 645, 123417. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent Trends in Preparation and Biomedical Applications of Iron Oxide Nanoparticles. J. Nanobiotechnol 2024, 22, 24. [Google Scholar] [CrossRef]
- Borth, K.W.; Galdino, C.W.; Teixeira, V.d.C.; Anaissi, F.J. Iron Oxide Nanoparticles Obtained from Steel Waste Recycling as a Green Alternative for Congo Red Dye Fast Adsorption. Appl. Surf. Sci. 2021, 546, 149126. [Google Scholar] [CrossRef]
- Ying, G.; Zhang, G.; Yang, J.; Hao, Z.; Xing, W.; Lu, D.; Zhang, S.; Yan, L. Biomineralization and Biotechnological Applications of Bacterial Magnetosomes. Colloids Surf. B Biointerfaces 2022, 216, 112556. [Google Scholar] [CrossRef]
- Strbak, O.; Hnilicova, P.; Gombos, J.; Lokajova, A.; Kopcansky, P. Magnetotactic Bacteria: From Evolution to Biomineralization and Biomedical Applications. Minerals 2022, 12, 1403. [Google Scholar] [CrossRef]
- Shibata, T.; Hattori, N.; Nishijo, H.; Kuroda, S.; Takakusaki, K. The Origins of Light-Independent Magnetoreception in Humans. Front. Hum. Neurosci. 2024, 18, 1482872. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ji, R.; Liu, J.; Ma, K.; Pan, Y.; Lin, W. Biomineralization in Magnetotactic Bacteria: From Diversity to Molecular Discovery-Based Applications. Cell Rep. 2024, 43. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.L.; Popa, C.O.; Ionescu, R.E. Updates on the Advantages and Disadvantages of Microscopic and Spectroscopic Characterization of Magnetotactic Bacteria for Biosensor Applications. Biosensors 2025, 15, 472. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; He, K.; Li, J.; Pan, Y.; Roberts, A.P.; Lin, W. Magnetotactic Bacteria and Magnetofossils: Ecology, Evolution and Environmental Implications. npj Biofilms Microbiomes 2022, 8, 43. [Google Scholar] [CrossRef]
- Lavorato, G.C.; de Almeida, A.A.; Vericat, C.; Fonticelli, M.H. Redox Phase Transformations in Magnetite Nanoparticles: Impact on Their Composition, Structure and Biomedical Applications. Nanotechnology 2023, 34, 192001. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, X.; Long, R.; Xie, M.; Kankala, R.K.; Wang, S.; Zhang, Y.S.; Liu, Y. Biomedical Applications of Magnetosomes: State of the Art and Perspectives. Bioact. Mater. 2023, 28, 27–49. [Google Scholar] [CrossRef]
- Jefremovas, E.M.; Gandarias, L.; Marcano, L.; Gacía-Prieto, A.; Orue, I.; Muela, A.; Fdez-Gubieda, M.L.; Barquín, L.F.; Alonso, J. Modifying the Magnetic Response of Magnetotactic Bacteria: Incorporation of Gd and Tb Ions into the Magnetosome Structure. Nanoscale Adv. 2022, 4, 2649–2659. [Google Scholar] [CrossRef]
- Shuwen, H.; Yifei, S.; Xinyue, W.; Zhanbo, Q.; Xiang, Y.; Xi, Y. Advances in Bacteria-Based Drug Delivery Systems for Anti-Tumor Therapy. Clin. Transl. Immunol. 2024, 13, e1518. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, T.; Chu, X.; Chen, C. Application of Mechanical Force Generated by Magnetic Nanoparticles under Magnetic Field in Tumor Treatment. J. Magn. Magn. Mater. 2025, 622, 172982. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I. Yield Cultivation of Magnetotactic Bacteria and Magnetosomes: A Review. J. Basic. Microbiol. 2017, 57, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.R.; Guo, F.F.; Jiang, W.; Li, Y.; Li, L.J. Large-Scale Production of Magnetosomes by Chemostat Culture of Magnetospirillum Gryphiswaldense at High Cell Density. Microb. Cell Fact. 2010, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Wang, J.; Guo, F.; Niu, W.; Jiang, W. Improved Methods for Mass Production of Magnetosomes and Applications: A Review. Microb. Cell Factories 2020, 19, 197. [Google Scholar] [CrossRef]
- Correa, T.; Presciliano, R.; Abreu, F. Why Does Not Nanotechnology Go Green? Bioprocess Simulation and Economics for Bacterial-Origin Magnetite Nanoparticles. Front. Microbiol. 2021, 12, 718232. [Google Scholar] [CrossRef]
- Ke, L.; Chen, Y.; Liu, P.; Liu, S.; Wu, D.; Yuan, Y.; Wu, Y.; Gao, M. Characteristics and Optimised Fermentation of a Novel Magnetotactic Bacterium, Magnetospirillum sp. ME-1. FEMS Microbiol. Lett. 2018, 365, fny052. [Google Scholar] [CrossRef]
- Yang, C.; Takeyama, H.; Matsunaga, T. Iron Feeding Optimization and Plasmid Stability in Production of Recombinant Bacterial Magnetic Particles by Magnetospirillum magneticum AMB-1 in Fed-Batch Culture. J. Biosci. Bioeng. 2001, 91, 213–216. [Google Scholar] [CrossRef]
- Heyen, U.; Schüler, D. Growth and Magnetosome Formation by Microaerophilic Magnetospirillum Strains in an Oxygen-Controlled Fermentor. Appl. Microbiol. Biotechnol. 2003, 61, 536–544. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, H.; Fan, K. Structure-Activity Mechanism of Iron Oxide Nanozymes. In Nanozymes: Design, Synthesis, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1422, pp. 1–35. ISBN 978-0-8412-9751-7. [Google Scholar]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes Eliminate Intracellular Reactive Oxygen Species in Magnetospirillum Gryphiswaldense MSR-1. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshni, N.; Singh, P.; Mahato, K. Magnetic Nanoparticle-Based Sensing Strategies for Clinical Analysis and Environmental Safety Assessment. In Surface Engineering and Functional Nanomaterials for Point-of-Care Analytical Devices; Purohit, B., Chandra, P., Eds.; Springer Nature: Singapore, 2023; pp. 67–102. ISBN 978-981-99-3025-8. [Google Scholar]
- Tanawish; Jahan, N.; Rasheed, K.; Iqbal, M.; Atif, M. Exploring the Advanced Synthesis Strategies and Biomedical Applications of Iron Oxide-Based Nanozymes: A Comprehensive Review. J. Clust. Sci. 2024, 35, 2637–2661. [Google Scholar] [CrossRef]
- Wang, M.; Jin, L.; Hang-Mei Leung, P.; Wang-Ngai Chow, F.; Zhao, X.; Chen, H.; Pan, W.; Liu, H.; Li, S. Advancements in Magnetic Nanoparticle-Based Biosensors for Point-of-Care Testing. Front. Bioeng. Biotechnol. 2024, 12. [Google Scholar] [CrossRef]
- Balaban Hanoglu, S.; Harmanci, D.; Ucar, N.; Evran, S.; Timur, S. Recent Approaches in Magnetic Nanoparticle-Based Biosensors of miRNA Detection. Magnetochemistry 2023, 9, 23. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Li, Y.; Zhang, Y.; Zhou, B.; Yang, L.; Yan, X.; Yang, L. Beyond Conventional Approaches: The Revolutionary Role of Nanoparticles in Breast Cancer. MedComm—Biomater. Appl. 2025, 4, e70012. [Google Scholar] [CrossRef]
- Govindaraj, M.; Srivastava, A.; Muthukumaran, M.K.; Tsai, P.-C.; Lin, Y.-C.; Raja, B.K.; Rajendran, J.; Ponnusamy, V.K.; Arockia Selvi, J. Current Advancements and Prospects of Enzymatic and Non-Enzymatic Electrochemical Glucose Sensors. Int. J. Biol. Macromol. 2023, 253, 126680. [Google Scholar] [CrossRef]
- Keles, G.; Sifa Ataman, E.; Taskin, S.B.; Polatoglu, İ.; Kurbanoglu, S. Nanostructured Metal Oxide-Based Electrochemical Biosensors in Medical Diagnosis. Biosensors 2024, 14, 238. [Google Scholar] [CrossRef]
- Valls-Chivas, Á.; Gómez, J.; Garcia-Peiro, J.I.; Hornos, F.; Hueso, J.L. Enzyme–Iron Oxide Nanoassemblies: A Review of Immobilization and Biocatalytic Applications. Catalysts 2023, 13, 980. [Google Scholar] [CrossRef]
- Bilge, S.; Dogan-Topal, B.; Gürbüz, M.M.; Ozkan, S.A.; Sınağ, A. Recent Trends in Core/Shell Nanoparticles: Their Enzyme-Based Electrochemical Biosensor Applications. Microchim. Acta 2024, 191, 240. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Kulabako, R.; Carrara, S. Electrochemical Sensors Modified with Iron Oxide Nanoparticles/Nanocomposites for Voltammetric Detection of Pb (II) in Water: A Review. Heliyon 2024, 10, e29743. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wei, X.; Zhang, D.; Huang, L.; Liu, H.; Fang, H. Immobilization of Enzyme Electrochemical Biosensors and Their Application to Food Bioprocess Monitoring. Biosensors 2023, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Ciobotaru, I.C.; Oprea, D.; Ciobotaru, C.C.; Enache, T.A. Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors. Biosensors 2023, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Adampourezare, M.; Hasanzadeh, M.; Hoseinpourefeizi, M.-A.; Seidi, F. Iron/Iron Oxide-Based Magneto-Electrochemical Sensors/Biosensors for Ensuring Food Safety: Recent Progress and Challenges in Environmental Protection. RSC Adv. 2023, 13, 12760–12780. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.M.F. Progress in Enzyme-Based Biosensors Using Optical Transducers. Microchim. Acta 2004, 148, 107–132. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Kaushik, A.; Khan, R.; Solanki, P.R.; Pandey, P.; Alam, J.; Ahmad, S.; Malhotra, B.D. Iron Oxide Nanoparticles–Chitosan Composite Based Glucose Biosensor. Biosens. Bioelectron. 2008, 24, 676–683. [Google Scholar] [CrossRef]
- Fredj, Z.; Singh, B.; Bahri, M.; Qin, P.; Sawan, M. Enzymatic Electrochemical Biosensors for Neurotransmitters Detection: Recent Achievements and Trends. Chemosensors 2023, 11, 388. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Wang, Q.; Li, J. Recent Progress in Biosensor Regeneration Techniques. Nanoscale 2024, 16, 2834–2846. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.H.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barceló, D. Biosensors as Useful Tools for Environmental Analysis and Monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef]
- Hazarika, K.K.; Darabdhara, G. Chapter 10—Iron and Iron Oxide Nanoparticles Used in Biosensors. In Materials and Components of Biosensors in Healthcare; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 201–227. ISBN 978-0-443-21676-3. [Google Scholar]
- Melo, A.F.A.A.; Singh, S.J.; Chinnamuthu, P.; Crespilho, F.N.; Rydzek, G. Magnetically Stimulated Bio- and Electrochemical Systems: State-of-the-Art, Applications, and Future Directions. ChemNanoMat 2023, 9, e202300192. [Google Scholar] [CrossRef]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite-Based Biosensors and Molecular Logic Gates: From Magnetite Synthesis to Application. Biosensors 2023, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Ratre, P.; Nazeer, N.; Kumari, R.; Thareja, S.; Jain, B.; Tiwari, R.; Kamthan, A.; Srivastava, R.K.; Mishra, P.K. Carbon-Based Fluorescent Nano-Biosensors for the Detection of Cell-Free Circulating MicroRNAs. Biosensors 2023, 13, 226. [Google Scholar] [CrossRef]
- Abdel-Karim, R. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Materials, Applications, Challenges, and Future Scope. Biomed. Mater. Devices 2024, 2, 759–777. [Google Scholar] [CrossRef]
- Bakshi, M.S. Iron Oxide Nanomaterials at Interfaces for Sustainable Environmental Applications. Acc. Mater. Res. 2024, 5, 1000–1012. [Google Scholar] [CrossRef]
- Arkhipova, V.I.; Mochalova, E.N.; Nikitin, M.P. Au-Based Bimetallic Nanoparticles: Current Biomedical Applications. J. Nanopart Res. 2024, 26, 214. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, X.; Xu, Y.; Zhu, L.; Cheng, J.; Qiao, Y.; Dai, S.; Zhu, J.; Jiang, N.; Wu, H.; et al. The Influence of Surface Charge on the Tumor-Targeting Behavior of Fe3O4 Nanoparticles for MRI. J. Mater. Chem. B 2022, 10, 646–655. [Google Scholar] [CrossRef]
- Bao, H.; Cheng, S.; Li, X.; Li, Y.; Yu, C.; Huang, J.; Zhang, Z. Functional Au Nanoparticles for Engineering and Long-Term CT Imaging Tracking of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis Treatment. Biomaterials 2022, 288, 121731. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, A.; Bainik, O.; Navyatha, B.; Santhosh, C.; Banoth, E.; Balaji, R.; Chandrasekar, N.; Kumar, S.; Daima, H.K. Emerging Trends, and Prospects in Nanozyme Engineering to Enhance Dual-Mode Sensing Applications. Coord. Chem. Rev. 2025, 540, 216768. [Google Scholar] [CrossRef]
- Tran, H.V.; Nguyen, N.D.; Le, A.-T.; Tran, L.T.; Le, T.D.; Huynh, C.D. Fe3O4@C Magnetite Nanocomposite: An Artificial Peroxidase Nanozyme for the Development of a Colorimetric Glucose Biosensor. New J. Chem. 2024, 48, 20007–20017. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Hasani, I.W.; Mahmoud Ragab, W. Promising Biomedical Applications Using Superparamagnetic Nanoparticles. Eur. J. Med. Res. 2025, 30, 441. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; González-González, R.B.; Iqbal, H.M.N. Bioremediation and Decontamination Potentials of Metallic Nanoparticles Loaded Nanohybrid Matrices—A Review. Environ. Res. 2022, 204, 112407. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.M.; El-Gebaly, R.H.; Abdelrahman, I.Y.; Rageh, M.M. Efficacy of Iron-Silver Bimetallic Nanoparticles to Enhance Radiotherapy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3647–3657. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Kazeminava, F.; Afrouz, M.; Abbaszadeh, M.; Mehr, N.T.; Shiran, J.A.; Gouda, C.; Adeli, M.; Kafil, H.S. A Review on the Impacts of Metal/Metal Nanoparticles on Characteristics of Hydrogels: Special Focus on Carbohydrate Polymers. Int. J. Biol. Macromol. 2023, 253, 126535. [Google Scholar] [CrossRef]
- González-Lavín, J.; Arenillas, A.; Rey-Raap, N. Revealing the Importance of Iron Aerogel Features as Electrocatalysts for the Oxygen Reduction Reaction. Gels 2025, 11, 154. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Sharaf, E.M.; Hassan, A.; AL-Salmi, F.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.; Fayad, E. Synergistic Antibacterial Activity of Compact Silver/Magnetite Core-Shell Nanoparticles Core Shell against Gram-Negative Foodborne Pathogens. Front. Microbiol. 2022, 13, 929491. [Google Scholar] [CrossRef]
- Wehbe, M.; Kadah El Habbal, R.; Kaj, J.; Karam, P. Synergistic Dual Antibacterial Activity of Magnetite Hydrogels Doped with Silver. Langmuir 2024, 40, 22865–22874. [Google Scholar] [CrossRef]
- Gizachew, D.G.; Jiru, E.B.; Tekle’Ab, T.; Bayisa, Y.M.; Bullo, T.A. Green Synthesis of Silver-Iron-Zinc Oxides Nanocomposite via Embelia Schimperia Leaf Extract for Photo-Degradation of Antibiotic Drug from Pharmaceutical Wastewater. Appl. Water Sci. 2024, 14, 210. [Google Scholar] [CrossRef]
- Nobile, C.; Cozzoli, P.D. Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials. Nanomaterials 2022, 12, 1729. [Google Scholar] [CrossRef]
- Lamichhane, N.; Sharma, S.; Parul; Verma, A.K.; Roy, I.; Sen, T. Iron Oxide-Based Magneto-Optical Nanocomposites for In Vivo Biomedical Applications. Biomedicines 2021, 9, 288. [Google Scholar] [CrossRef]
- Białas, K.; Moschou, D.; Marken, F.; Estrela, P. Electrochemical Sensors Based on Metal Nanoparticles with Biocatalytic Activity. Microchim. Acta 2022, 189, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Yang, H. Electrochemical Signal Enhancement via Redox Cycling Involving Iron Oxide Magnetic Particles (Adaptable, Reversible Redox Reservoirs) and Its Application in Sensitive Cu2+ Detection. J. Phys. Chem. C 2023, 127, 21561–21567. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, F.; Wang, Q.; Huang, Y. A Sensitive Electrochemical Biosensor for Quinolones Detection Based on Cu2+-Modulated Signal Amplification. Microchem. J. 2023, 190, 108636. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Demirbas, E. A Novel Comparative Study for Electrochemical Urea Biosensor Design: Effect of Different Ferrite Nanoparticles (MFe2O4, M: Cu, Co, Ni, Zn) in Urease Immobilized Composite System. Bioelectrochemistry 2023, 149, 108324. [Google Scholar] [CrossRef]
- Ayaz, S.; Üzer, A.; Dilgin, Y.; Apak, M.R. Fabrication of a Novel Optical Glucose Biosensor Using Copper(II) Neocuproine as a Chromogenic Oxidant and Glucose Dehydrogenase-Immobilized Magnetite Nanoparticles. ACS Omega 2023, 8, 47163–47172. [Google Scholar] [CrossRef]
- He, Y.; Wang, P.; Chen, X.; Li, Y.; Wei, J.; Cai, G.; Aoyagi, K.; Wang, W. Facile Preparation of Fe3O4@Pt Nanoparticles as Peroxidase Mimics for Sensitive Glucose Detection by a Paper-Based Colorimetric Assay. R. Soc. Open Sci. 2022, 9, 220484. [Google Scholar] [CrossRef]
- Madhavan, A.S.; Rajith, L. Glucose Mediated One-Pot Synthesis of RGO-Fe3O4 Hybrid Composite for Electrochemical Determination of Uric Acid. Discov. Chem. 2025, 2, 71. [Google Scholar] [CrossRef]
- Shakeel, N.; Perveen, R.; Imran Ahamed, M.; Ahmad, A.; Inamuddin. Cherry-like Pt@Fe3O4 Decorated MWCNT/PANI Nanohybrid Based Bioanode for Glucose Biofuel Cell Application. Fuel 2023, 341, 127579. [Google Scholar] [CrossRef]
- Văduva, M.; Nila, A.; Udrescu, A.; Cramariuc, O.; Baibarac, M. Nanocomposites Based on Iron Oxide and Carbonaceous Nanoparticles: From Synthesis to Their Biomedical Applications. Materials 2024, 17, 6127. [Google Scholar] [CrossRef] [PubMed]
- Puttaningaiah, C.H.; Prabhu, K. Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomaterials 2024, 14, 1890. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.K.; Tran, L.T.; Vu, T.A.; Tran, H.V. A Sensitive Electrochemical Sensor Based on Fe3O4 Magnetic Nanoparticles/Chitosan/Graphene Composite for Detection of Pb2+ in Aqueous Solutions. J. Appl. Electrochem. 2024, 54, 2595–2605. [Google Scholar] [CrossRef]
- Keerthanaa, M.R.; Panicker, L.R.; Narayan, R.; Kotagiri, Y.G. Biopolymer-Protected Graphene-Fe3O4 Nanocomposite Based Wearable Microneedle Sensor: Toward Real-Time Continuous Monitoring of Dopamine. RSC Adv. 2024, 14, 7131–7141. [Google Scholar] [CrossRef]
- Aydın, E.B.; Sezgintürk, M.K. Indium Tin Oxide (ITO): A Promising Material in Biosensing Technology. TrAC Trends Anal. Chem. 2017, 97, 309–315. [Google Scholar] [CrossRef]
- Silah, H.; Erkmen, C.; Demir, E.; Uslu, B. Modified Indium Tin Oxide Electrodes: Electrochemical Applications in Pharmaceutical, Biological, Environmental and Food Analysis. TrAC Trends Anal. Chem. 2021, 141, 116289. [Google Scholar] [CrossRef]
- Sachdeva, V.; Monga, A.; Vashisht, R.; Singh, D.; Singh, A.; Bedi, N. Iron Oxide Nanoparticles: The Precise Strategy for Targeted Delivery of Genes, Oligonucleotides and Peptides in Cancer Therapy. J. Drug Deliv. Sci. Technol. 2022, 74, 103585. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, S.K.; Govender, K.K.; Govender, P.P. Unveiling the Multifunctionality of Iron Oxide Nanoparticle: A Synergistic Experimental and Computational Investigation. Chem. Phys. Impact 2025, 10, 100845. [Google Scholar] [CrossRef]
- Huanan, G.; Shiqin, D.; Qiaoyan, W.; Qi, Z.; Hua, Y.; Dongxu, W. Rapid and Sensitive Smartphone Non-Enzymatic Colorimetric Assay for the Detection of Glucose in Food Based on Peroxidase-like Activity of Fe3O4@Au Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 122970. [Google Scholar] [CrossRef]
- Said, R.; Ghazzy, A.; Shakya, A.K.; Hunaiti, A.A. Iron Oxide Nanozymes as Versatile Analytical Tools: An Overview of Their Application as Detection Technique. Bioanalysis 2024, 16, 1261–1278. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Q.; Gu, J. Recent Advances in Colorimetric Sensors Based on Nanozymes with Peroxidase-like Activity. Analyst 2023, 148, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Saadatidizaji, Z.; Mahdavi, M.; Maleki, A.; Irani, M.; Zare, I. Recent Advances in Gold Nanoparticles-Based Biosensors for Tuberculosis Determination. Talanta 2024, 275, 126099. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar Sachan, R.S.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Kumar, G. Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Balusamy, S.R.; Madhusudanan, M.; Singh, H.; Amsath Haseef, H.M.; Mijakovic, I. Advanced Nanomaterials for Cancer Therapy: Gold, Silver, and Iron Oxide Nanoparticles in Oncological Applications. Adv. Healthc. Mater. 2025, 14, 2403059. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Voronova, A.; Batsalova, T.; Moten, D.; Naumkin, A.; Shtykova, E.; Volkov, V.; Teneva, I.; Dzhambazov, B. Evolution of Gold and Iron Oxide Nanoparticles in Conjugates with Methotrexate: Synthesis and Anticancer Effects. Materials 2023, 16, 3238. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Li, M.; Li, J. Advances in the Preparation and Biological Applications of Core@shell Nanocrystals Based on Quantum Dots and Noble Metal. RSC Adv. 2024, 14, 26308–26324. [Google Scholar] [CrossRef]
- Tiryaki, E.; Zorlu, T.; Alvarez-Puebla, R.A. Magnetic–Plasmonic Nanocomposites as Versatile Substrates for Surface–Enhanced Raman Scattering (SERS) Spectroscopy. Chem.—A Eur. J. 2024, 30, e202303987. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Wang, X.; Zhang, H.; Zheng, H.; Gu, N. Plasmonic/Magnetic Nanoarchitectures: From Controllable Design to Biosensing and Bioelectronic Interfaces. Biosens. Bioelectron. 2023, 219, 114744. [Google Scholar] [CrossRef]
- Griaznova, O.Y.; Belyaev, I.B.; Sogomonyan, A.S.; Zelepukin, I.V.; Tikhonowski, G.V.; Popov, A.A.; Komlev, A.S.; Nikitin, P.I.; Gorin, D.A.; Kabashin, A.V.; et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics 2022, 14, 994. [Google Scholar] [CrossRef]
- Caro, C.; Gámez, F.; Quaresma, P.; Páez-Muñoz, J.M.; Domínguez, A.; Pearson, J.R.; Pernía Leal, M.; Beltrán, A.M.; Fernandez-Afonso, Y.; De la Fuente, J.M.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Thakur, N.; Kumar, K.; Kumar, S.; Dutt, A.; Thakur, V.K.; Gutiérrez-Rodelo, C.; Thakur, P.; Navarrete, A.; Thakur, N. Catalyzing Innovation: Exploring Iron Oxide Nanoparticles—Origins, Advancements, and Future Application Horizons. Coord. Chem. Rev. 2024, 507, 215750. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Q.; Liu, L.; Su, X.; Lin, J.; Xu, G.; Luo, X. Mixed Self-Assembly of Polyethylene Glycol and Aptamer on Polydopamine Surface for Highly Sensitive and Low-Fouling Detection of Adenosine Triphosphate in Complex Media. ACS Appl. Mater. Interfaces 2017, 9, 31153–31160. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(Polyvinyl Alcohol)/Zeolite Electrospun Composite Nanofibrous Membrane for Adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. Chitosan-Based Nanocomposite Polymeric Membranes for Water Purification—A Review. Materials 2021, 14, 2091. [Google Scholar] [CrossRef]
- Wu, Q.; Hou, Q.; Wang, P.; Ding, C.; Lv, S. Antifouling Electrochemiluminescence Biosensor Based on Bovine Serum Albumin Hydrogel for the Accurate Detection of P53 Gene in Human Serum. ACS Appl. Mater. Interfaces 2023, 15, 44322–44330. [Google Scholar] [CrossRef]
- Yu, X.; Meng, W.; Li, Y.; Luo, X. A Low-Fouling Electrochemical Biosensor Based on BSA Hydrogel Doped with Carbon Black for the Detection of Cortisol in Human Serum. Anal. Chim. Acta 2024, 1307, 342645. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Maniti, O. Why Do Tethered-Bilayer Lipid Membranes Suit for Functional Membrane Protein Reincorporation? Appl. Sci. 2021, 11, 4876. [Google Scholar] [CrossRef]
- Penkauskas, T.; Preta, G. Biological Applications of Tethered Bilayer Lipid Membranes. Biochimie 2019, 157, 131–141. [Google Scholar] [CrossRef]
- Mickoleit, F.; Jörke, C.; Richter, R.; Rosenfeldt, S.; Markert, S.; Rehberg, I.; Schenk, A.S.; Bäumchen, O.; Schüler, D.; Clement, J.H. Long-Term Stability, Biocompatibility, and Magnetization of Suspensions of Isolated Bacterial Magnetosomes. Small 2023, 19, 2206244. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Whitaker, R.D.; Nap, R.J.; Paulsen, J.L.; Mathiyazhagan, V.; Doerrer, L.H.; Song, Y.-Q.; Hürlimann, M.D.; Szleifer, I.; Wong, J.Y. Stability of Superparamagnetic Iron Oxide Nanoparticles at Different pH Values: Experimental and Theoretical Analysis. Langmuir 2012, 28, 6246–6255. [Google Scholar] [CrossRef]
- Komkova, M.A.; Ibragimova, O.A.; Karyakina, E.E.; Karyakin, A.A. Catalytic Pathway of Nanozyme “Artificial Peroxidase” with 100-Fold Greater Bimolecular Rate Constants Compared to Those of the Enzyme. J. Phys. Chem. Lett. 2021, 12, 171–176. [Google Scholar] [CrossRef]
- Wang, J.; Xing, Y.; Ngatio, M.; Bies, P.; Xu, L.L.; Xing, L.; Zarea, A.; Makela, A.V.; Contag, C.H.; Li, J. Engineering Magnetotactic Bacteria as Medical Microrobots. Adv. Mater. 2025, 37, 2416966. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Marks, R.S.; Gheber, L.A. Nanolithography Using Protease Etching of Protein Surfaces. Nano Lett. 2003, 3, 1639–1642. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Marks, R.S.; Gheber, L.A. Manufacturing of Nanochannels with Controlled Dimensions Using Protease Nanolithography. Nano Lett. 2005, 5, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Aversa, I.F.S.; Covalcanti, M.H.S.; Pereira, T.M.; de Castro, A.A.; Tavano, O.L.; Coelho, Y.L.; da Silva, L.H.M.; Gorup, L.F.; Ramalho, T.C.; Virtuoso, L.S. Immobilization of Porcine Trypsin in Superparamagnetic Nanoparticles: Enzyme Activity and Stability. ACS Omega 2025, 10, 22970–22983. [Google Scholar] [CrossRef] [PubMed]
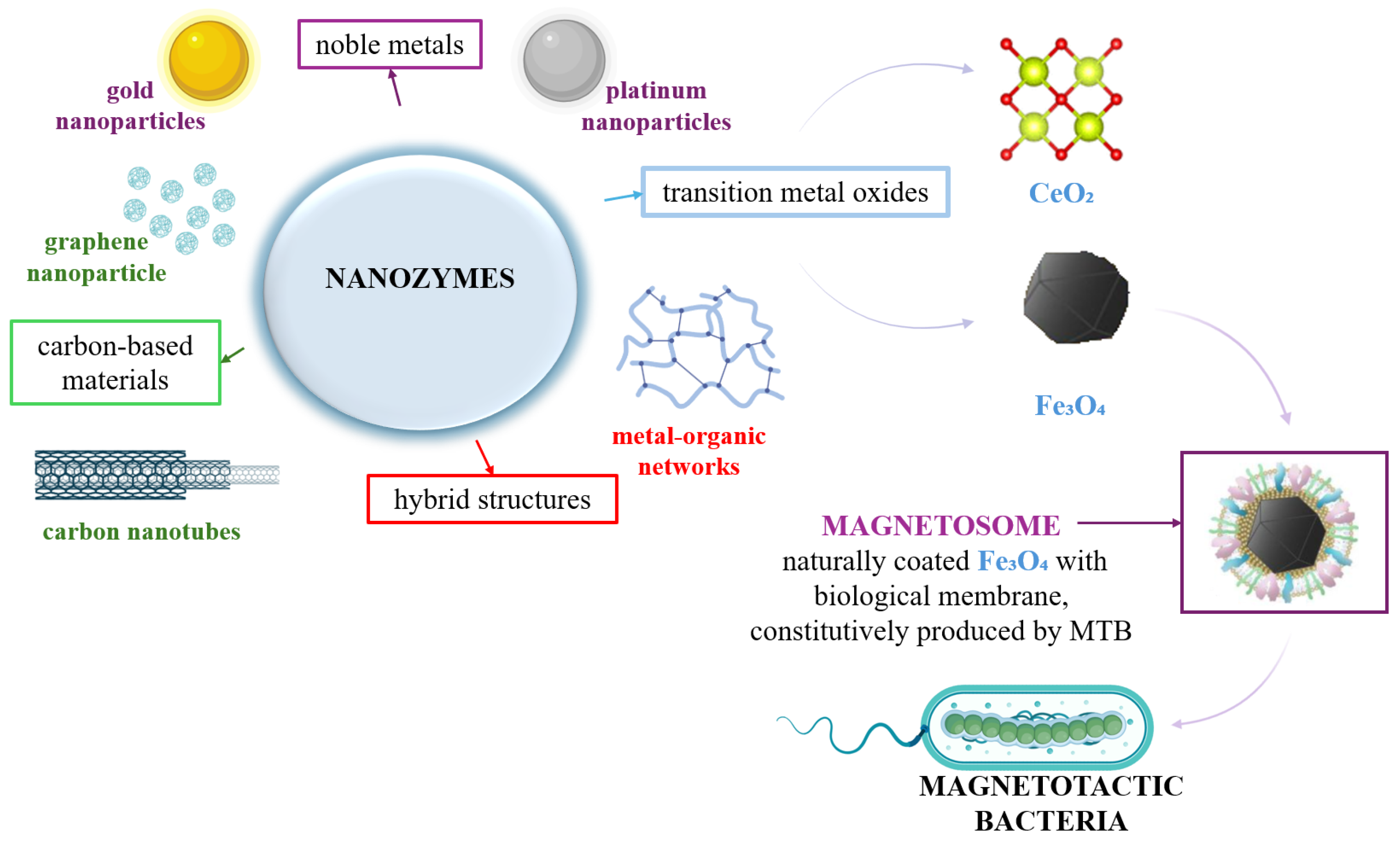


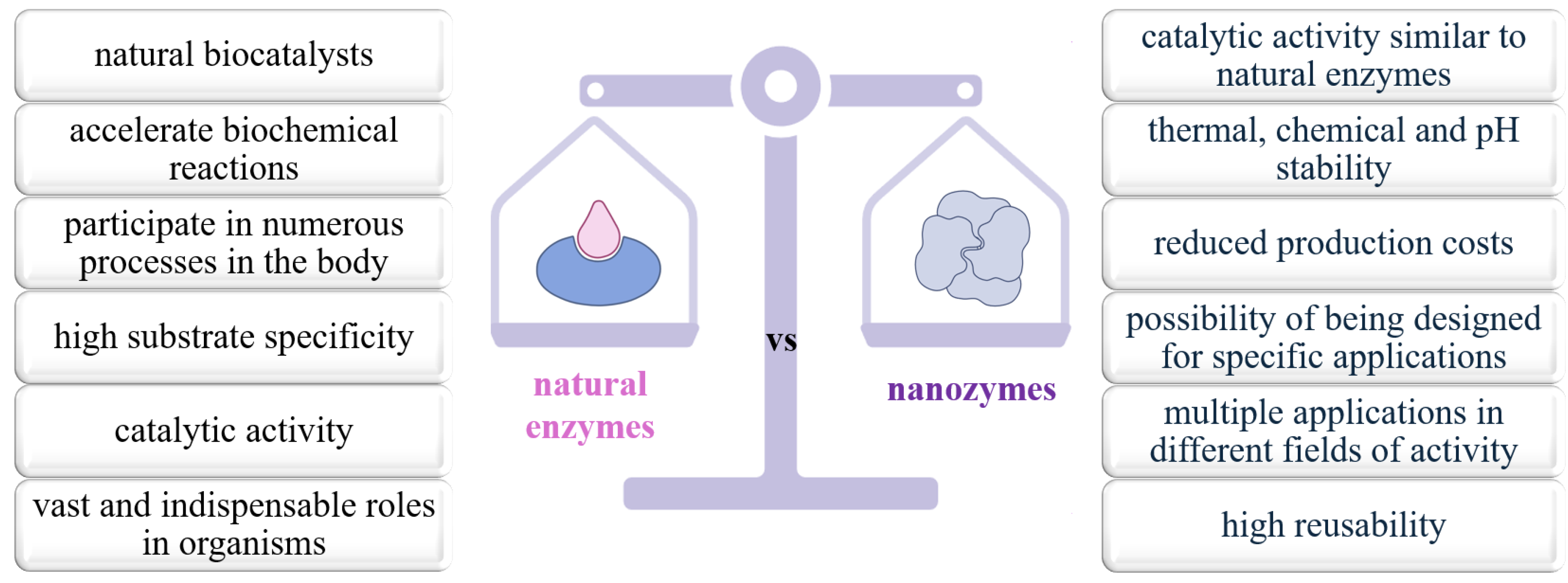









| Characteristic | Natural Enzymes | Nanozymes | Ref. |
|---|---|---|---|
| composition | proteins, composed of amino acid chains folded into specific three-dimensional structures | nanomaterials with enzyme-mimicking catalytic properties: - metal nanoparticles; - metal oxides; - carbon-based materials; - metal organic frameworks | [45] |
| specificity | - very high specificity; - active site highly selective | generally lower specificity can be improved through surface functionalization and ligand attachment | [46] |
| stability | sensitive to environmental factors: - prone to denaturation under extreme pH, temperature, or presence of proteases | high stability under harsh conditions: - including extreme pH and temperature; - resistant to proteolytic degradation | [47] |
| catalytic activity | - high specificity and efficiency under physiological conditions; - often limited to narrow pH and temperature ranges | - tunable activity, can function under a broader range of conditions; - activity can be enhanced through structural and surface modifications | [48] |
| production cost | - high, requires complex expression systems; - expensive production, purification, and storage conditions | - lower, synthesized through relatively simple chemical methods; - scalable production | [49] |
| reusability | - limited - often lose activity after a single use or over time | high, can retain activity over multiple cycles without activity loss | [50] |
| bio- compatibility | generally safe and non-toxic | varies, some nanozymes may exhibit cytotoxicity depending on composition and size, surface modifications can enhance biocompatibility | [45] |
| functional diversity | - limited to native biological roles, engineering is complex | broad, can be engineered to mimic various enzymatic activities (e.g., peroxidase, oxidase, catalase), multifunctional capabilities | [51] |
| environmental senstivity | - highly sensitive, activity can be affected by slight changes in environmental conditions | robust; maintain activity under a wide range of environmental conditions | [52] |
| storage and shelf-life | require specific storage conditions (e.g., low temperatures), limited shelf-life | stable at room temperature, longer shelf-life, easier storage and transportation | [53] |
| toxicity concerns | - minimal; - generally recognized as safe | - potential toxicity depending on material composition and accumulation; - requires thorough biocompatibility assessments | [45] |
| application scope | - widely used in biological systems, diagnostics, and therapeutics; - limitations in industrial applications due to stability issues | expanding applications in: - biosensing; - environmental remediation; - therapeutics, medicine; - industrial catalysis | [54] |
| design flexibility | - limited; - modifications can affect activity and stability | high, properties can be tailored (size, shape, composition, and surface modifications to suit specific applications) | [55] |
| regulatory approval | - many are approved for clinical use; - well-understood mechanisms; | - emerging field; - regulatory pathways are still being Established; - requires comprehensive safety and efficacy evaluations | [54] |
| scalability | - challenging; - complex production processes limit large-scale manufacturing | - highly scalable, chemical synthesis methods allow for mass production | [56] |
| variability | low—sequence-defined, reproducible | moderate to high—adjustable via engineering | [57,58] |
| environmental footprint | biodegradable, minimal environmental impact | depends on composition, some nanozymes may persist | [59] |
| clinical suitability | high, generally safe | variable, depends on toxicity and surface functionalization | [60] |
| Method | Advantages | Limitations | Ref. |
|---|---|---|---|
| co-precipitation | simple, cost-effective, high yield, scalable | - broad particle size distribution, - low crystallinity, aggregation | [90] |
| thermal decomposition | - excellent size and shape control, - high crystallinity | - high temperature, - toxic solvents, - expensive precursors | [91] |
| microemulsion | - fine control over particle size - shape, surfactant-stabilized | - expensive surfactants, - purification required, - poor scalability | [92] |
| sol-gel | - uniform particle size, - good chemical homogeneity, - low temperature process | - time-intensive, - solvent residues, - not always scalable | [91] |
| laser ablation | - high purity, - no chemical contamination, - precise control | - expensive equipment, - low yield, - not suitable for large-scale production | [90] |
| biological/ green synthesis | - environmentally friendly, - biocompatible particles, - mild reaction conditions | - limited reproducibility, - poor control over size and shape, - lower crystallinity | [93] |
| Technique | Main Parameters Assessed | Significance | Ref. |
|---|---|---|---|
| TEM | - particle size - morphology | - high-resolution imaging of shape - size distribution | [91,94] |
| SEM | - surface morphology - agglomeration | - analysis of topography - particle clustering | [91,94] |
| XRD | - crystal structure | - differentiates magnetite/maghemite, - estimates crystallinity | [95,96] |
| FTIR | - surface functional groups, - bonding interactions | - detection of organic coating agents, - surface coatings - bioconjugation | [97] |
| DLS | - hydrodynamic diameter, - polydispersity index | - size analysis in colloidal systems, - evaluates nanoparticle aggregation | [98] |
| Method Source | Description | Advantages | Limitations | Variability | Environmental Footprint | Clinical Suitability | Ref. |
|---|---|---|---|---|---|---|---|
| co-precipitation | - reaction between Fe2+ and Fe3+ salts in an alkaline medium | - simple, -cost-effective high yield | - limited control over size and shape | moderate | low, uses common salts, minimal waste | moderate, generally safe but requires purification for biomedical use | [102] |
| thermal decomposition | - high-temperature decomposition of iron organometallic precursors in solvents | - precise control of ize and monodispersity | - expensive, - requires specialized equipment | low | moderate, high energy consumption, organic solvents may be toxic | high, produces uniform nanoparticles suitable for clinical applications after proper surface modification | [90] |
| continuous flow synthesis | - scalable synthesis with controlled parameters (pH, temperature, flow rate) | - excellent size control, - suitable for industrial scaling | - complex system | low | moderate, energy consumption | high, scalable method compatible with biomedical applications after functionalization | [99] |
| physical methods | - methods like evaporation– condensation, laser ablation | - high purity | - low yield, - high cost | low | moderate, energy-intensive, solvent-free methods reduce chemical waste | moderate, nanoparticles may need surface modification for biocompatibility | [90] |
| green synthesis | - use of plant extracts or microorganisms for reduction of iron ions | - increased biocompatibility | - low reproducibility, - less control over nanoparticle properties | high | low, eco-friendly, uses renewable materials, minimal toxic byproducts | high, inherently biocompatible, ideal for biomedical applications | [100,101] |
| alternative sources | - use of iron-containing waste in chemical or electrochemical methods | - sustainable, -low-cost | - variable purity, - possible contamination | high | low to moderate, promotes recycling but potential contamination | moderate, requires purification for safe biomedical use | [103] |
| Nanozymes (Similar Sizes) | Substrate | Km (mM) | Vmax (M s−1) | Kcat (s−1) | pH | Temperature (°C) | Mimicking Activity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe3O4 (44 nm) synthetic | H2O2 | 54.6 | 1.8×10−8 | 7.4 | 30 | Peroxidase | [122] | |
| TMB | 0.374 | 2.6×10−8 | ||||||
| Fe3O4 (55 nm) synthetic | ABTS | 0.12–0.96 | 0.52–6.10 × 10−7 | 0.25–2.9 × 10−4 | RT (20–25 °C) | Peroxidase | [122] | |
| TMB | 0.24–0.71 | 0.42–2.4 × 10−7 | 0.2–1.14 × 10−4 | RT (20–25 °C) | Peroxidase | |||
| Fe3O4 (52 nm) magnetosome | H2O2 | 170.65 | 9.33 × 10−9 | 4 | 28 | Peroxidase | [122] | |
| TMB | 0.90 | 4.45 × 10−9 | ||||||
| Fe3O4 (40–45 nm) magnetosome (e.g., MSR-1) | TMB | 1.215 | 8.06 × 10−8 | 4–6 | 40–60 | Peroxidase | [125] | |
| H2O2 | 100.3 | 3.7 × 10−8 |
| Characteristics | Iron Oxide NP Based Biosensors | Conventional Enzymatic Biosensors | Ref. |
|---|---|---|---|
| sensitivity | - high, enables the detection of microRNAs via magnetically induced electrochemical amplification | - sensitive, but activity may fluctuate due to enzyme degradation in complex matrices and low analyte concentrations | [131,132] |
| specificity | - requires surface functionalization with aptamers, antibodies, or ligands potential for non-specific interactions | - high substrate specificity due to natural enzyme-substrate recognition | [133,134] |
| preconcentration capability | - enables magnetic preconcentration of analytes, enhance active surface area and signal-to-noise ratio | - lack inherent preconcentration, - signal depends entirely on substrate diffusion and enzymatic turnover | [135] |
| stability and durability | - stability across varying pH, temperature and environmental conditions, - magnetic separation allows reuse | - limited by enzyme denaturation, pH/temperature sensitivity, and short shelf-life | [136] |
| fabrication complexity | - synthesis involves multistep nanoparticle production and surface modifications, - requires precision in size, - dispersion, - chemical coating | - established immobilization methods (adsorption, entrapment) - require careful optimization for maximal enzyme activity | [136] |
| portability | - well-suited for integration into portable platforms | - commercial enzymatic devices exist (e.g., glucose test strips), - enzyme instability can limit field lifespan | [90] |
| biocompatibility | - generally biocompatible, - high concentrations may induce ROS and cytotoxic effects | - biodegradable enzymes pose minimal risk, - rare allergenic responses | [90] |
| cost and scalability | - cost-efficient and scalable synthesis, - including green chemistry routes | - enzyme purification - cold-chain logistics increase costs | [137] |
| limit of detection (LOD) | 0.60–0.90 ppb | - nanomolar (nM) to micromolar (µM) range, depending on the type of enzyme and the electrochemical or optical signaling method used | [138,139] |
| range | 10–100 ppb | a few nM/µM to hundreds of µM | [138,139] |
| response time | <30 s | a few seconds to a few minutes | [140,141,142] |
| regeneration cycles | 7 | varies, some require active regeneration | [143,144] |
| sample matrix | various applications in complex environments (blood, serum, urine, environmental water, food samples) | applications in complex environments, enzyme stability may be affected (blood, plasma, urine, saliva, food extracts, buffer solutions) | [140,145] |
| Hybrid NP Systems | Composition | Detection Mode | Target Analyte | Enhancement Mechanism | Ref. |
|---|---|---|---|---|---|
| Au-Fe3O4 | Fe3O4 + Au (core–shell, composites) | colorimetric, electrochemical, SERS | glucose, H2O2, biomolecules | synergistic peroxidase-like catalysis, plasmonic amplification, magnetic separation | [154,185,189] |
| Ag-Fe3O4 | Fe3O4 + Ag (core–shell, hydrogel, composites) | optical (plasmonic, SERS) | various bacteria, contaminants | bactericidal effect, SERS amplification, magnetic control | [157,159,164,165] |
| Cu-Fe3O4 | Fe3O4 doped with CuO/Cu2O, composites | electrochemical, optical | glucose, urea | electrocatalysis, magnetic separation | [170,171,172,173] |
| Pt-Fe3O4 | Fe3O4 + Pt (thin layer, core–shell) | electrochemical, colorimetric | glucose, H2O2, nitrite, other redox analytes | synergistic peroxidase-like activity, strong Pt electrocatalysis, magnetic recovery of sensor | [174,176] |
| C-Fe3O4 | Fe3O4 + Graphene/CNTs/ amorphous carbon | electrochemical, colorimetric | Pb2+, H2O2, glucose | conductivity, peroxidase-like, rapid immobilization | [177,178,179,180] |
| ITO-Fe3O4 | Fe3O4 immobilized on ITO electrodes | electrochemical, optoelectronic | biomarkers, enzymes, H2O2 | synergy of Fe3O4 (magnetic immobilization and peroxidase) with ITO (conductivity and transparency) | [181,182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, N.L.; Popa, C.O.; Ionescu, R.E. Natural Iron Oxide Nanoparticles Produced by Aquatic Magnetotactic Bacteria as Ideal Nanozymes for Nano-Guided Biosensing Platforms—A Systematic Review. Biosensors 2025, 15, 590. https://doi.org/10.3390/bios15090590
Paul NL, Popa CO, Ionescu RE. Natural Iron Oxide Nanoparticles Produced by Aquatic Magnetotactic Bacteria as Ideal Nanozymes for Nano-Guided Biosensing Platforms—A Systematic Review. Biosensors. 2025; 15(9):590. https://doi.org/10.3390/bios15090590
Chicago/Turabian StylePaul, Natalia Lorela, Catalin Ovidiu Popa, and Rodica Elena Ionescu. 2025. "Natural Iron Oxide Nanoparticles Produced by Aquatic Magnetotactic Bacteria as Ideal Nanozymes for Nano-Guided Biosensing Platforms—A Systematic Review" Biosensors 15, no. 9: 590. https://doi.org/10.3390/bios15090590
APA StylePaul, N. L., Popa, C. O., & Ionescu, R. E. (2025). Natural Iron Oxide Nanoparticles Produced by Aquatic Magnetotactic Bacteria as Ideal Nanozymes for Nano-Guided Biosensing Platforms—A Systematic Review. Biosensors, 15(9), 590. https://doi.org/10.3390/bios15090590







