Portable Point-of-Care Device for Dual Detection of Glucose-6-Phosphate Dehydrogenase Deficiency and Hemoglobin in Low-Resource Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
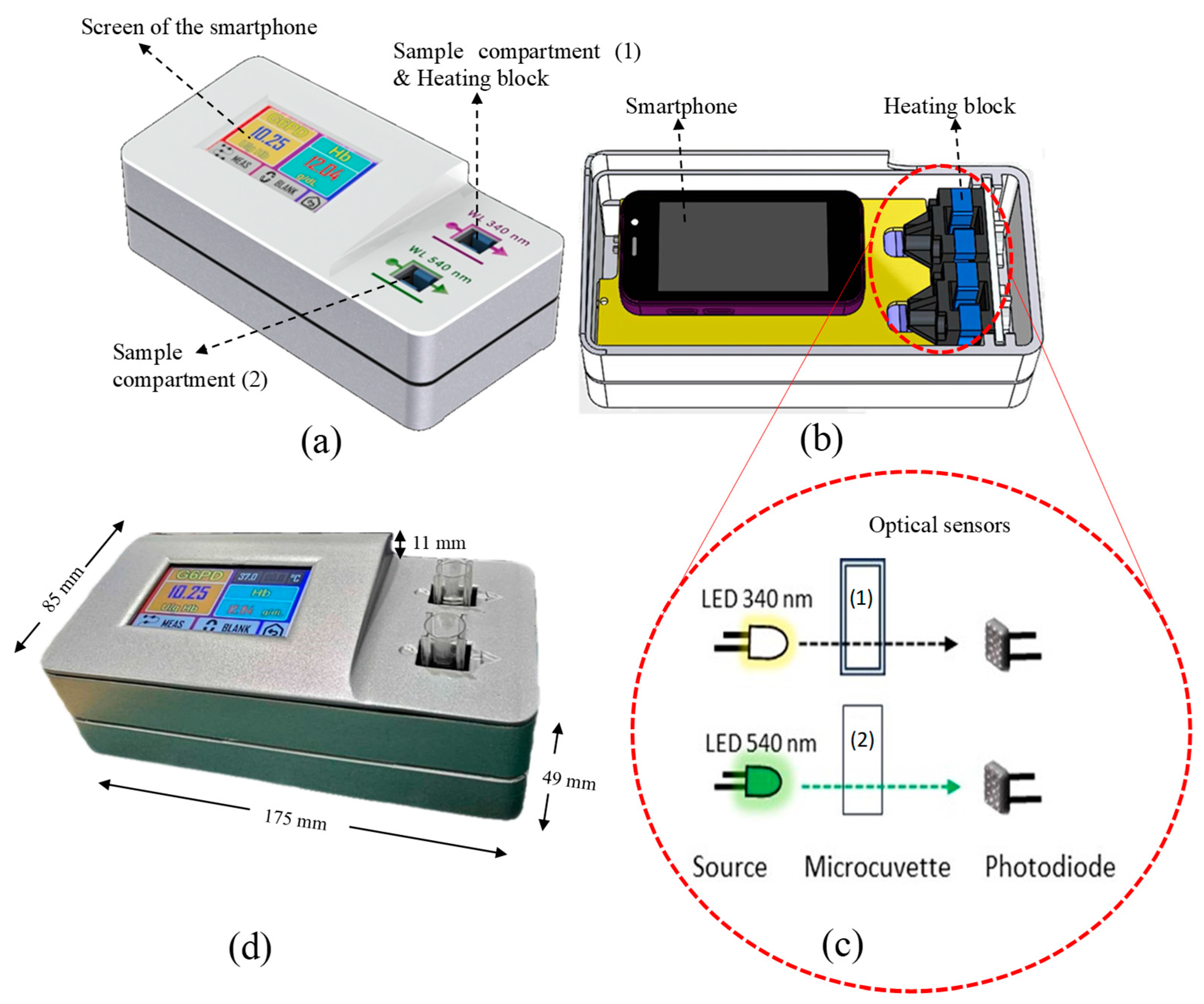
2.2. Instrumentation and Measurement Platform of MyG6PD
2.3. Determination of Hemoglobin and G6PD Enzyme Activity
2.3.1. Hemoglobin
2.3.2. G6PD Activity
2.4. MyG6PD Assay Performance
2.4.1. Linearity
Hemoglobin
G6PD
2.4.2. Limit of Quantification
2.4.3. Precision
2.4.4. Accuracy
measured value by spectrophotometer] × 100
2.5. Clinical Application of MyG6PD
2.5.1. Correlation Analysis
2.5.2. Stability Analysis
measured value on day 1] × 100
3. Results
3.1. MyG6PD Assay Performance
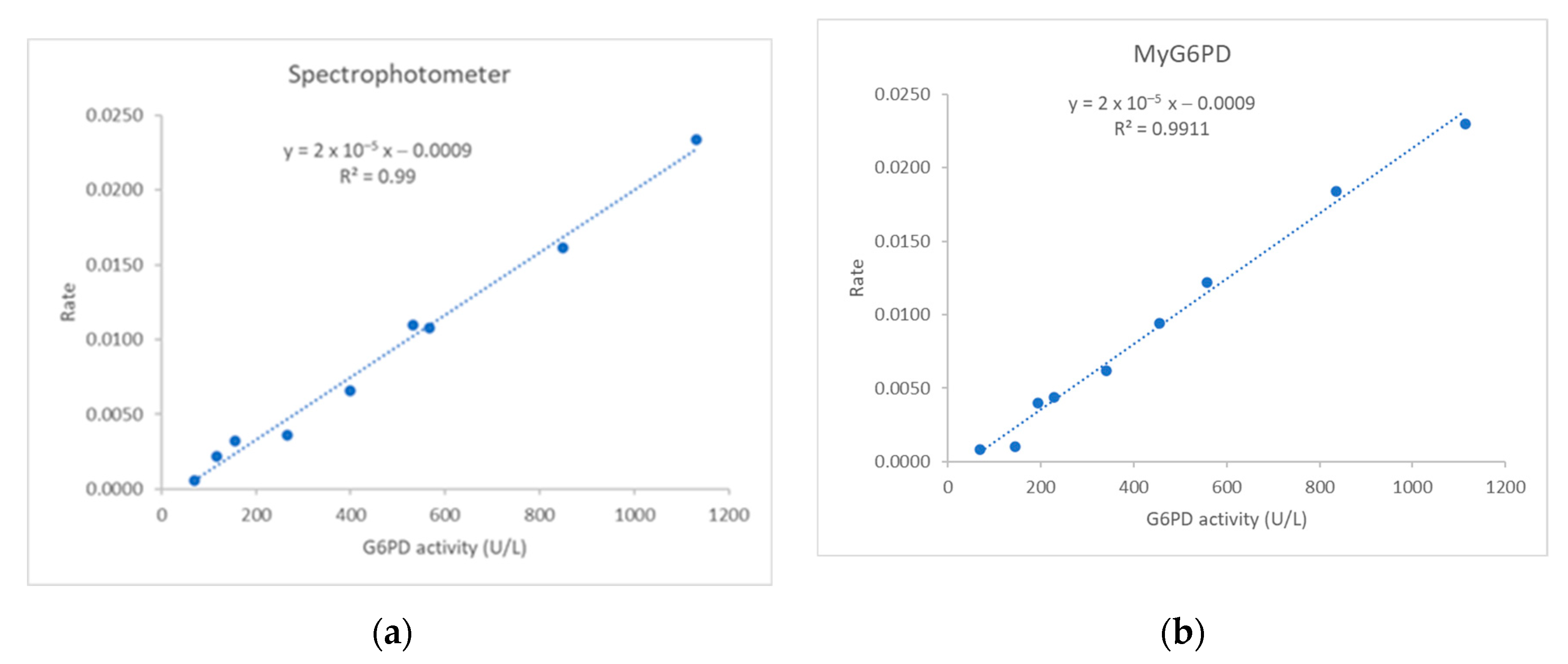
3.1.1. Linearity
Hemoglobin
G6PD
3.1.2. Limit of Quantification
3.1.3. Precision and Accuracy
Hemoglobin
G6PD
3.2. Clinical Application of MyG6PD
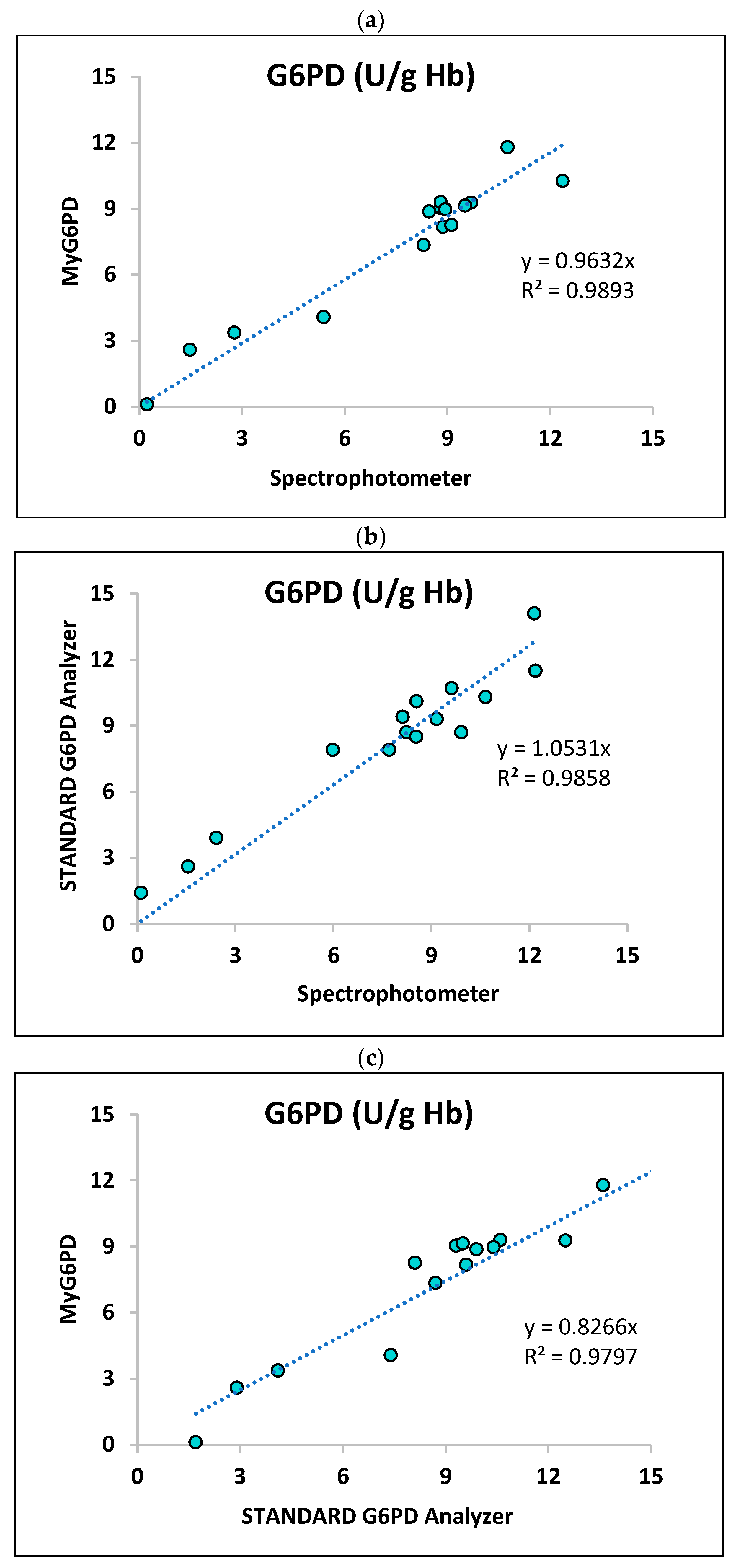
3.2.1. Correlation Analysis
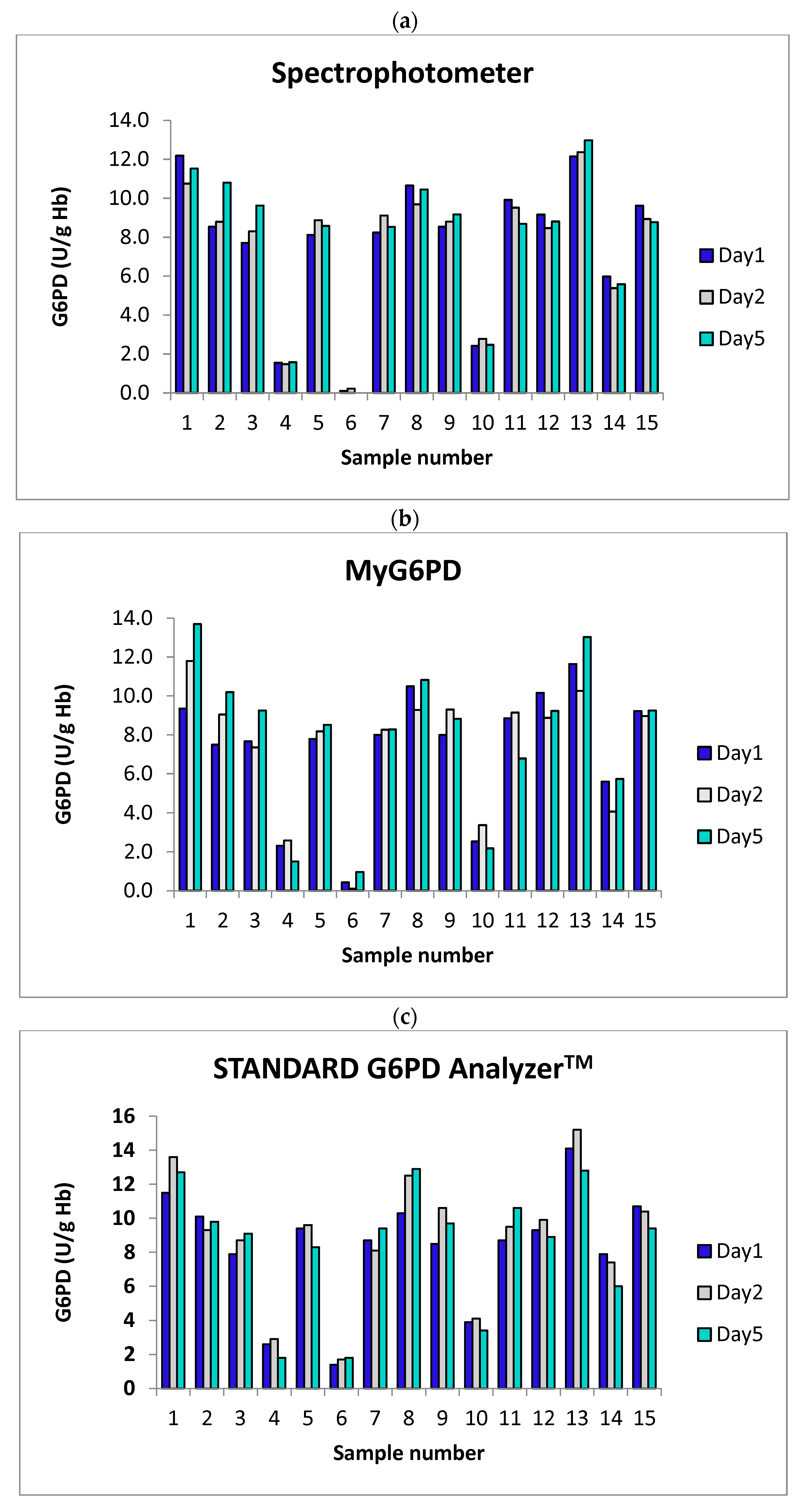
3.2.2. Stability Analysis
4. Discussion
4.1. Analytical Performance
4.2. Clinical and Operational Relevance
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FST | Fluorescent spot test |
| g/dL | Grams per deciliter |
| G6P | D-glucose-6-phosphate disodium salt hydrate |
| G6PD | Glucose-6-phosphate dehydrogenase |
| Hb | Hemoglobin |
| L | Liter |
| LOQ | Limit of quantification |
| mM | Millimolar |
| mmoL | Millimoles |
| NADP+ | β-Nicotinamide adenine dinucleotide phosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NH | Neonatal hyperbilirubinemia |
| nM | Nanometer |
| °C | Degrees Celsius |
| PADs | Paper-based analytical devices |
| pH | Potential of hydrogen |
| POC | Point-of-care |
| PPP | Pentose phosphate pathway |
| RBCs | Red blood cells |
| TCF | Temperature correction factor |
| Tris-HCl | Tris (hydroxymethyl) aminomethane hydrochloride |
| U/L | Units per litre |
| vs | Versus |
| WHO | World Health Organization |
References
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Screening for G6PD Deficiency; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Howes, R.E.; Piel, F.B.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Hogg, M.M.; Battle, K.E.; Padilla, C.D.; Baird, J.K.; et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: A geostatistical model-based map. PLoS Med. 2012, 9, e1001339. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L. Glucose 6-phosphate dehydrogenase deficiency: From genotype to phenotype. Haematologica 2006, 91, 1303–1306. [Google Scholar] [PubMed]
- Nkhoma, E.T.; Poole, C.; Vannappagari, V.; Hall, S.A.; Beutler, E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol. Dis. 2009, 42, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Domingo, G.J.; Satyagraha, A.W.; Anvikar, A.; Baird, K.; Bancone, G.; Bansil, P.; Carter, N.; Cheng, Q.; Culpepper, J.; Eziefula, C.; et al. G6PD testing in support of treatment and elimination of malaria: Recommendations for evaluation of G6PD tests. Malar. J. 2013, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Al-Bedaywi, R.R.R.; Salameh, K.M.K.; Abedin, S.; Viswanathan, B.; Khedr, A.A.; Habboub, L.H.M. Glucose-6-phosphate dehydrogenase deficiency and neonatal indirect hyperbilirubinemia: A retrospective cohort study among 40,305 consecutively born babies. J. Perinatol. 2024, 44, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K.; Dewi, M.; Subekti, D.; Elyazar, I.; Satyagraha, A.W. Noninferiority of glucose-6-phosphate dehydrogenase deficiency diagnosis by a point-of-care rapid test vs the laboratory fluorescent spot test demonstrated by copper inhibition in normal human red blood cells. Transl. Res. J. Lab. Clin. Med. 2015, 165, 677–688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riskin, A.; Bravdo, Y.; Habib, C.; Maor, I.; Mousa, J.; Shahbarat, S.; Shahak, E.; Shalata, A. The Genetics of Glucose-6-Phosphate-Dehydrogenase (G6PD) and Uridine Diphosphate Glucuronosyl Transferase 1A1 (UGT1A1) Promoter Gene Polymorphism in Relation to Quantitative Biochemical G6PD Activity Measurement and Neonatal Hyperbilirubinemia. Children 2023, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Blume, K.G.; Kaplan, J.C.; Löhr, G.W.; Ramot, B.; Valentine, W.N. International Committee for Standardization in Haematology: Recommended screening test for glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Br. J. Haematol. 1979, 43, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Nantakomol, D.; Paul, R.; Palasuwan, A.; Day, N.P.; White, N.J.; Imwong, M. Evaluation of the phenotypic test and genetic analysis in the detection of glucose-6-phosphate dehydrogenase deficiency. Malar. J. 2013, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, D.A.; Ley, B.; Howes, R.E.; Adu, P.; Alam, M.S.; Bansil, P.; Boum, Y., 2nd; Brito, M.; Charoenkwan, P.; Clements, A.; et al. Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003084. [Google Scholar] [CrossRef] [PubMed]
- Zailani, M.A.H.; Raja Sabudin, R.Z.A.; Ithnin, A.; Alauddin, H.; Sulaiman, S.A.; Ismail, E.; Othman, A. Population screening for glucose-6-phosphate dehydrogenase deficiency using quantitative point-of-care tests: A systematic review. Front. Genet. 2023, 14, 1098828. [Google Scholar] [CrossRef] [PubMed]
- LaRue, N.; Kahn, M.; Murray, M.; Leader, B.T.; Bansil, P.; McGray, S.; Kalnoky, M.; Zhang, H.; Huang, H.; Jiang, H.; et al. Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am. J. Trop. Med. Hyg. 2014, 91, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Quantitative Point-of-Care G6PD Tests for Radical Cure of Plasmodium Vivax Malaria. Available online: https://www.path.org/our-impact/resources/quantitative-point-of-care-g6pd-tests-for-radical-cure-of-plasmodium-vivax-malaria (accessed on 30 July 2025).
- Bancone, G.; Gornsawun, G.; Chu, C.S.; Porn, P.; Pal, S.; Bansil, P.; Domingo, G.J.; Nosten, F. Validation of the quantitative point-of-care CareStart biosensor for assessment of G6PD activity in venous blood. PLoS ONE 2018, 13, e0196716. [Google Scholar] [CrossRef] [PubMed]
- Kaewarsa, P.; Laiwattanapaisal, W.; Palasuwan, A.; Palasuwan, D. A new paper-based analytical device for detection of Glucose-6-phosphate dehydrogenase deficiency. Talanta 2017, 164, 534–539. [Google Scholar] [CrossRef] [PubMed]
- International Committee for Standardization in Haematology. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard EP 6/2: 1977) and specifications for international haemiglobincyanide reference preparation (ICSH standard EP 6/3: 1977). J. Clin. Pathol. 1978, 31, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Bancone, G.; von Seidlein, L.; Thriemer, K.; Richards, J.S.; Domingo, G.J.; Price, R.N. Methods for the field evaluation of quantitative G6PD diagnostics: A review. Malar. J. 2017, 16, 361. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Myburgh, J.; Bansil, P.; Hann, A.; Robertson, L.; Gerth-Guyette, E.; Ambler, G.; Bizilj, G.; Kahn, M.; Zobrist, S.; et al. Reference and point-of-care testing for G6PD deficiency: Blood disorder interference, contrived specimens, and fingerstick equivalence and precision. PLoS ONE 2021, 16, e0257560. [Google Scholar] [CrossRef] [PubMed]


| Hemoglobin Concentration (g/L) | Precision (%CV) | Accuracy (%) | |||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| MyG6PD | 2.4 | 0.9 | 1.2 | +2.0 | +2.3 |
| 10.3 | 1.2 | 1.7 | +1.2 | +0.9 | |
| 20.7 | 1.8 | 2.1 | −0.19 | −1.8 | |
| Spectrophotometer | 2.4 | 0.7 | 1.1 | NA | NA |
| 10.3 | 0.9 | 0.9 | NA | NA | |
| 20.7 | 1.2 | 1.0 | NA | NA | |
| G6PD Concentration (U/L) | Precision (%CV) | Accuracy (%) | |||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| MyG6PD | 1409 ± 423 | 9.03 | 12.8 | −9.68 | −9.4 |
| 451 ± 135 | 9.95 | 7.2 | +11.67 | −12.8 | |
| 110 ± 72 | 14.29 | 9.8 | +11.56 | +13.3 | |
| Spectrophotometer | 1409 ± 423 | 5.54 | 10.7 | NA | NA |
| 451 ± 135 | 8.68 | 7.6 | NA | NA | |
| 110 ± 72 | 11.17 | 7.8 | NA | NA | |
| % Mean deviation from Day 1 (SD) value | Spectrophotometer | MyG6PD | STANDARD G6PD AnalyzerTM | |||
| Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | |
| 9.7 ± 1.3 | 10.0 ± 1.9 | 10.3 ± 2.0 | 11.4 ± 3.1 | 11.9 ± 2.3 | 10.9 ± 2.3 | |
| G6PD Activity (U/g Hb) | Day 1 | Day 2 | Day 5 | |
|---|---|---|---|---|
| MyG6PD | 11.8 ± 3.5 | 12.5 | 8.7 | 10.9 |
| 4.0 ± 1.2 | 3.2 | 3.9 | 4.6 | |
| 0.9 ± 0.6 | 2.2 | 1.1 | 1.9 | |
| STNADARD G6PD AnalyzerTM | 11.8 ± 3.5 | 8.5 | 12.5 | 8.3 |
| 4.0 ± 1.2 | 2.0 | 2.5 | 2.0 | |
| 0.9 ± 0.6 | 1.3 | 1.4 | 0.9 | |
| Spectrophotometer | 11.8 ± 3.5 | 15.1 | 9.5 | 10.8 |
| 4.0 ± 1.2 | 4.9 | 3.6 | 3.2 | |
| 0.9 ± 0.6 | 1.0 | 1.3 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, R.O.; Youngvises, N.; Pochairach, R.; Phompradit, P.; Na-Bangchang, K. Portable Point-of-Care Device for Dual Detection of Glucose-6-Phosphate Dehydrogenase Deficiency and Hemoglobin in Low-Resource Settings. Biosensors 2025, 15, 577. https://doi.org/10.3390/bios15090577
Taha RO, Youngvises N, Pochairach R, Phompradit P, Na-Bangchang K. Portable Point-of-Care Device for Dual Detection of Glucose-6-Phosphate Dehydrogenase Deficiency and Hemoglobin in Low-Resource Settings. Biosensors. 2025; 15(9):577. https://doi.org/10.3390/bios15090577
Chicago/Turabian StyleTaha, Rehab Osman, Napaporn Youngvises, Runtikan Pochairach, Papichaya Phompradit, and Kesara Na-Bangchang. 2025. "Portable Point-of-Care Device for Dual Detection of Glucose-6-Phosphate Dehydrogenase Deficiency and Hemoglobin in Low-Resource Settings" Biosensors 15, no. 9: 577. https://doi.org/10.3390/bios15090577
APA StyleTaha, R. O., Youngvises, N., Pochairach, R., Phompradit, P., & Na-Bangchang, K. (2025). Portable Point-of-Care Device for Dual Detection of Glucose-6-Phosphate Dehydrogenase Deficiency and Hemoglobin in Low-Resource Settings. Biosensors, 15(9), 577. https://doi.org/10.3390/bios15090577





