CH3COOAg with Laccase-like Activity for Differentiation and Detection of Aminoglycoside Antibiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of CH3COOAg
2.3. An Investigation of the Laccase-like Activity of CH3COOAg
2.4. Steady-State Kinetic Analysis
2.5. Free Radical Scavenging Experiments
2.6. Construction of CH3COOAg-Based Sensor Array for AG Recognition
2.7. CH3COOAg-Based Colorimetric Sensor for Kanamycin (KAN) Detection
3. Results and Discussion
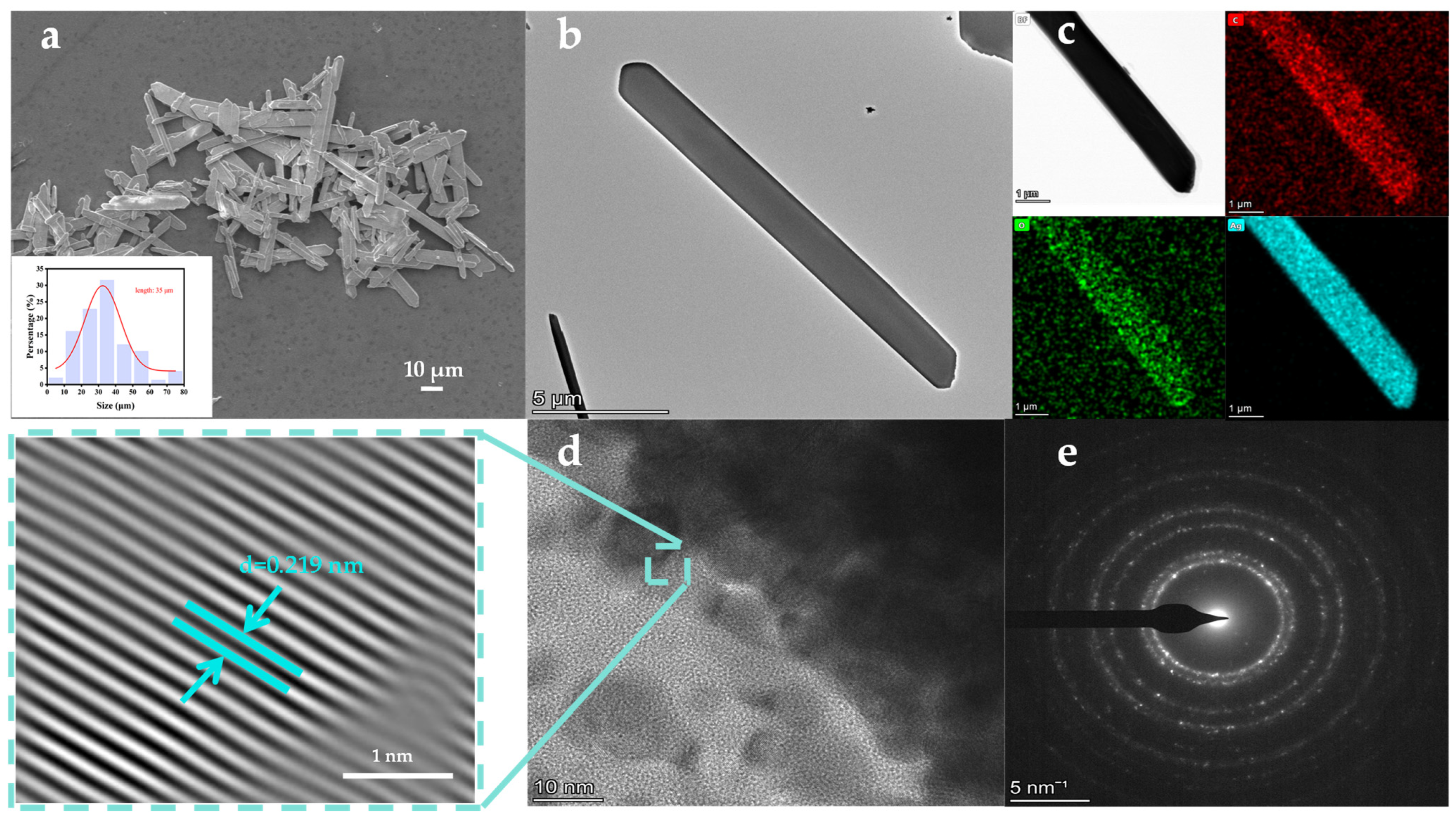
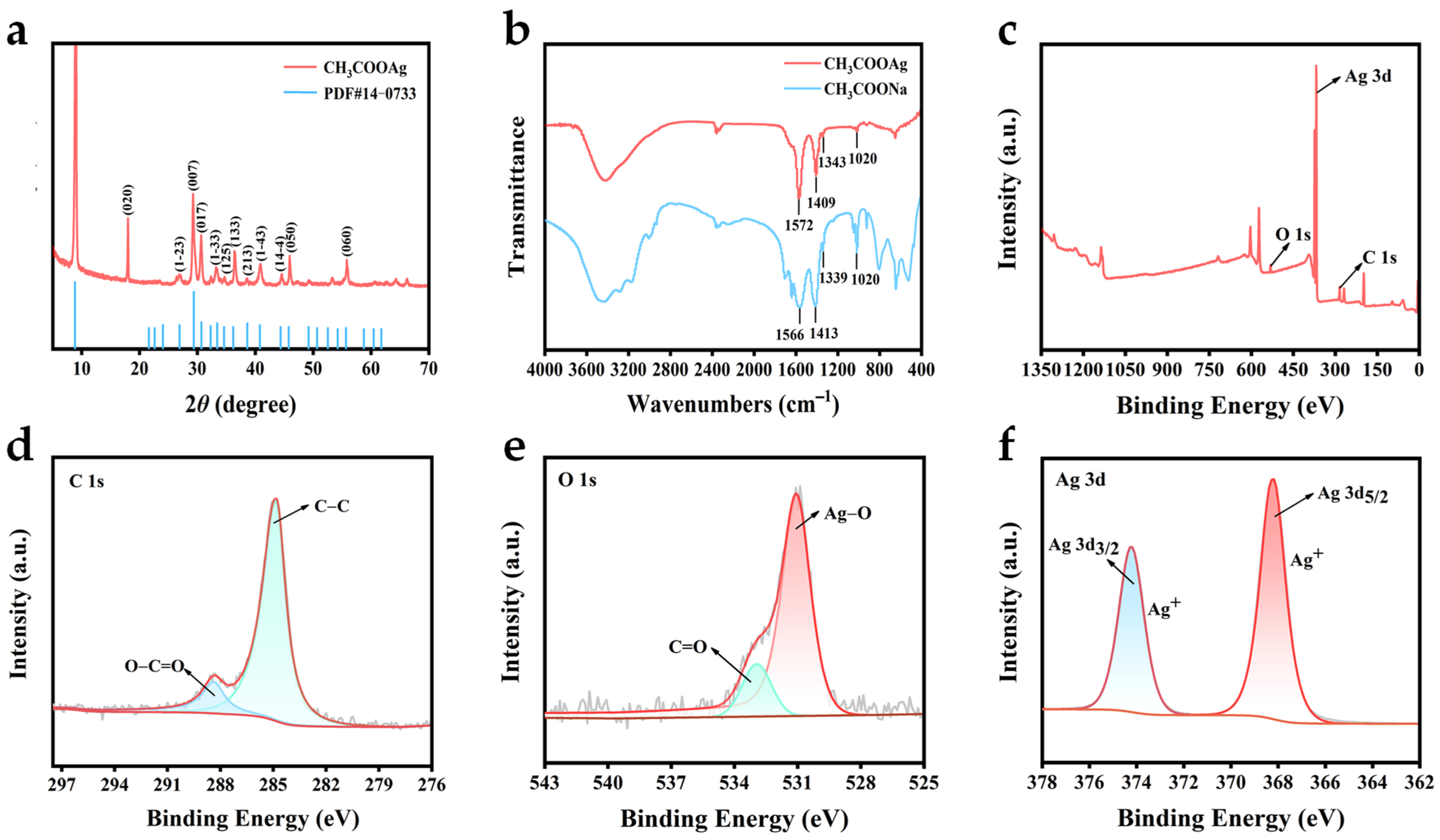
3.1. Characterization of CH3COOAg
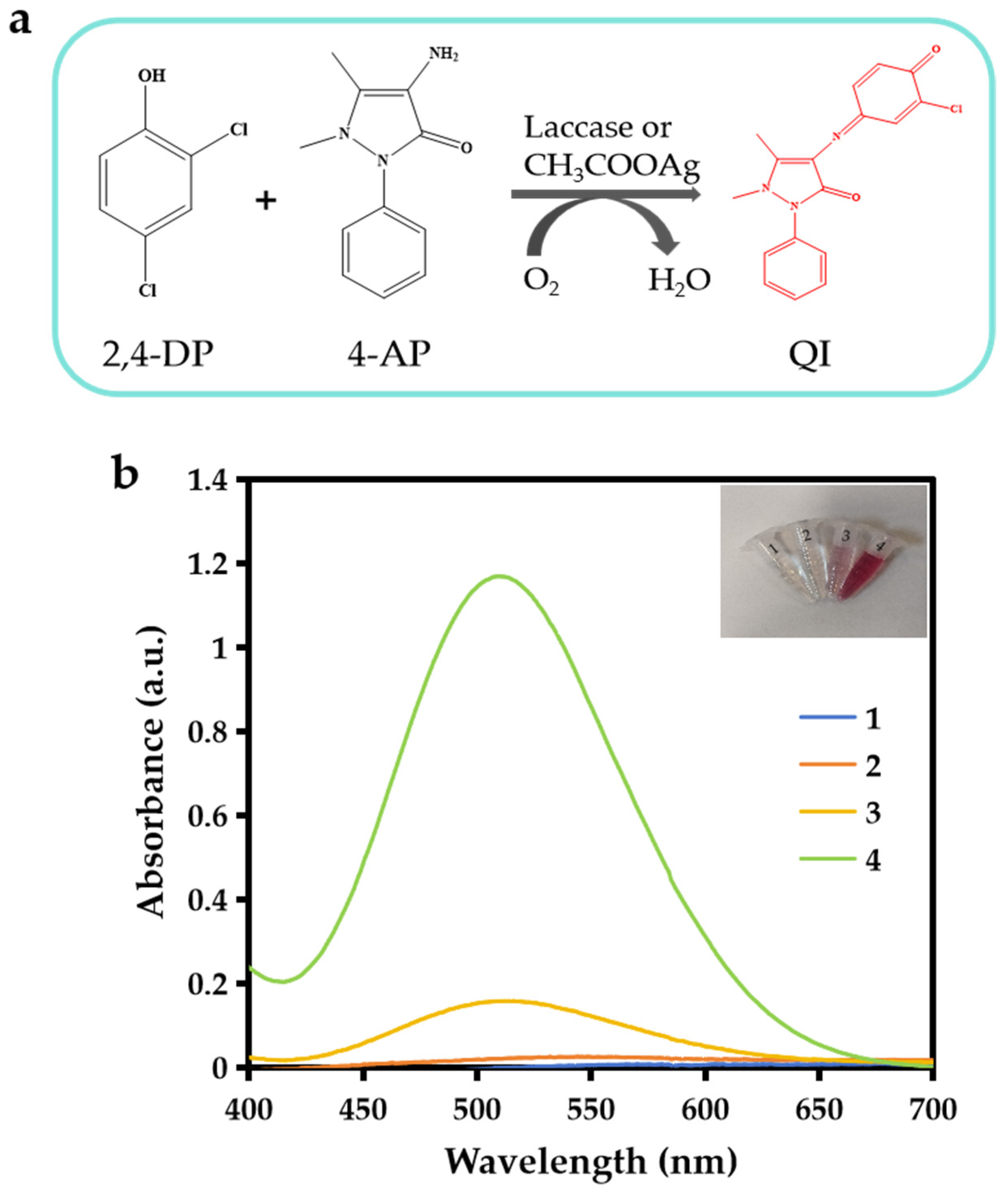
3.2. The Catalytic Activity of CH3COOAg and Laccase
3.3. Catalytic Kinetics of CH3COOAg and Laccase
3.4. Catalytic Mechanism
3.5. Effect of AGs on Laccase-like Activity of CH3COOAg
3.6. Mechanism of Inhibitory Effect of AGs on Laccase-like Activity of CH3COOAg
3.6.1. Zeta Potential Analysis
3.6.2. Enzyme Kinetics
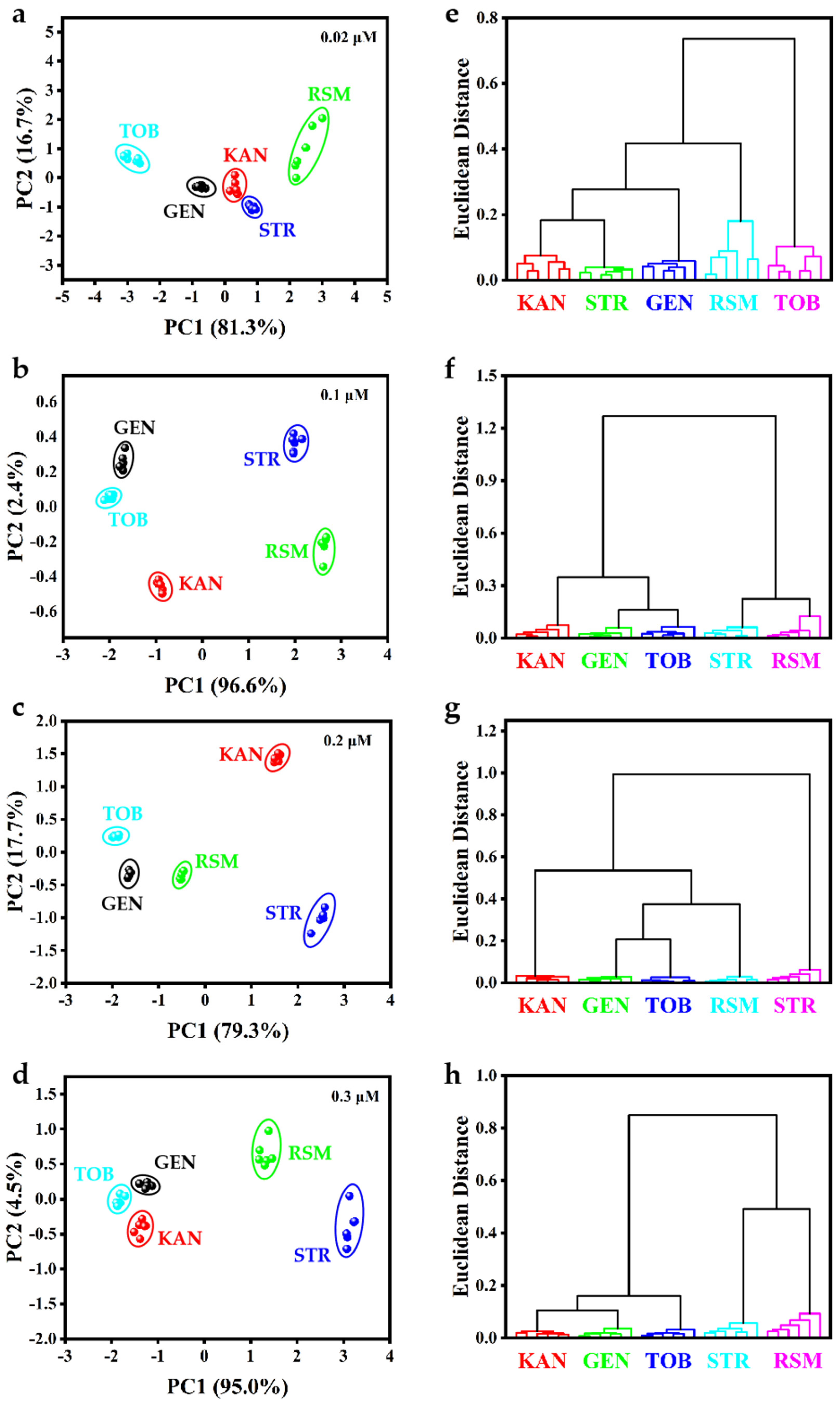
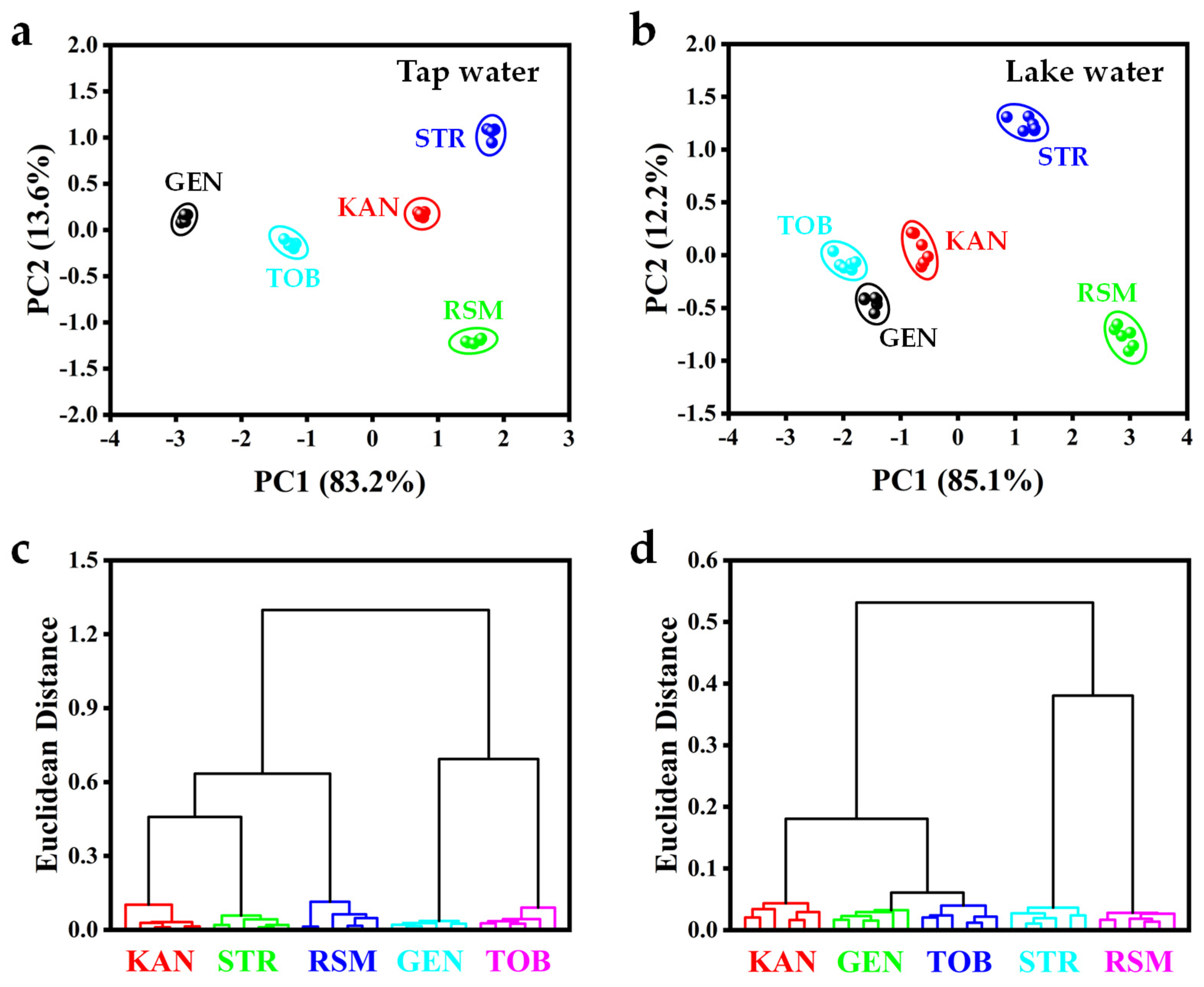
3.7. CH3COOAg-Based Sensor Array for AG Recognition
3.8. Detection of KAN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowacka-Kozak, E.; Gajda, A.; Gbylik-Sikorska, M. Analysis of Aminoglycoside Antibiotics: A Challenge in Food Control. Molecules 2023, 28, 4595. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lai, X.; Du, G.; Xiang, Y. Identification of Aminoglycoside Antibiotics in Milk Matrix with a Colorimetric Sensor Array and Pattern Recognition Methods. Anal. Chim. Acta 2018, 1034, 153–160. [Google Scholar] [CrossRef]
- McKeating, K.S.; Couture, M.; Dinel, M.-P.; Garneau-Tsodikova, S.; Masson, J.-F. High Throughput LSPR and SERS Analysis of Aminoglycoside Antibiotics. Analyst 2016, 141, 5120–5126. [Google Scholar] [CrossRef]
- Mikolasch, A.; Lindequist, U.; Witt, S.; Hahn, V. Laccase-Catalyzed Derivatization of Aminoglycoside Antibiotics and Glucosamine. Microorganisms 2022, 10, 626. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Li, Y.; Ni, C.; Wang, L. Colorimetric Sensor Arrays for the Detection and Identification of Antibiotics. Anal. Methods 2019, 11, 2836–2854. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent Advances in Aptamer-Based Sensors for Aminoglycoside Antibiotics Detection and Their Applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Su, P.; Chen, X.; He, Z.; Yang, Y. Preparation of Polyclonal Antibody and Development of a Biotin-Streptavidin-Based ELISA Method for Detecting Kanamycin in Milk and Honey. Chem. Res. Chin. Univ. 2017, 33, 876–881. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Mitiku, B.A.; Tamrat Admasu, H. Veterinary Drug Residues in Food Products of Animal Origin and Their Public Health Consequences: A Review. Vet. Med. Sci. 2024, 10, e70049. [Google Scholar] [CrossRef]
- Fu, X.; Wan, P.; Li, P.; Wang, J.; Guo, S.; Zhang, Y.; An, Y.; Ye, C.; Liu, Z.; Gao, J.; et al. Mechanism and Prevention of Ototoxicity Induced by Aminoglycosides. Front. Cell. Neurosci. 2021, 15, 692762. [Google Scholar] [CrossRef] [PubMed]
- Rozenblat, D.; Serret-Larmande, A.; Maillard, A.; Arrestier, R.; Benghanem, S.; Charpentier, J.; Darmon, M.; Das, V.; Dépret, F.; Donay, J.L.; et al. Impact of Aminoglycosides on Survival Rate and Renal Outcomes in Patients with Urosepsis: A Multicenter Retrospective Study. Ann. Intensive Care 2025, 15, 52. [Google Scholar] [CrossRef]
- Childs-Kean, L.M.; Shaeer, K.M.; Varghese Gupta, S.; Cho, J.C. Aminoglycoside Allergic Reactions. Pharmacy 2019, 7, 124. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Med. Chem. Commun. 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Li, M.; Huang, R.; Liao, X.; Zhou, Z.; Zou, L.; Liu, B. An Inner Filter Effect-Based Fluorescent Aptasensor for Sensitive Detection of Kanamycin in Complex Samples Using Gold Nanoparticles and Graphene Oxide Quantum Dots. Anal. Methods 2023, 15, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, Y.; Zhang, J.; Jin, Y.; Zhao, W.; Zhang, Y.; Huang, J.; Wang, P.; Wu, C.; et al. Optimization and Application of Parallel Solid-Phase Extraction Coupled with Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry for the Determination of 11 Aminoglycoside Residues in Honey and Royal Jelly. J. Chromatogr. A 2018, 1542, 28–36. [Google Scholar] [CrossRef]
- Yue, F.; Liu, M.; Bai, M.; Hu, M.; Li, F.; Guo, Y.; Vrublevsky, I.; Sun, X. Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk. Biosensors 2022, 12, 626. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kang, H.-S. Multi-Residue Determination of Twenty Aminoglycoside Antibiotics in Various Food Matrices by Dispersive Solid Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Food Control 2021, 130, 108374. [Google Scholar] [CrossRef]
- Guliy, O.I.; Zaitsev, B.D.; Semyonov, A.P.; Alsowaidi, A.K.M.; Teplykh, A.A.; Karavaeva, O.A.; Borodina, I.A. Microbial Acoustic Sensor Test-System Based on a Piezoelectric Resonator with a Lateral Electric Field for Kanamycin Detection in Liquid. Ultrasonics 2022, 120, 106651. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Yan, C.-X.; Fan, X.-H.; Wang, H.-Y.; Wang, Y.-L.; Peng, D.-P. Development of Enzyme-Linked Immunosorbent Assay and Colloidal Gold-Based Immunochromatographic Assay for the Rapid Detection of Gentamicin in Chicken Muscle and Milk. Chin. J. Anal. Chem. 2022, 50, 100142. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive Lateral Flow Immunoassay of an Antibiotic Neomycin in Foodstuffs. J. Food Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef]
- Deng, T.; He, H.; Chen, H.; Peng, X.; Li, H.; Yan, X.; Lei, Y.; Luo, L. Dual-Ligand Lanthanide Metal-Organic Framework Based Ratiometric Fluorescent Platform for Visual Monitoring of Aminoglycoside Residues in Food Samples. Talanta 2024, 276, 126200. [Google Scholar] [CrossRef] [PubMed]
- Sethu, N.; Krishnakumar, S.; Mitra, V.; Tagad, C.; Vyas, R. Design and Development of a Novel Colorimetric Assay and a Portable Optical System for the Detection of Aminoglycoside Antibiotics. Sens. Actuators Rep. 2023, 5, 100151. [Google Scholar] [CrossRef]
- Gao, Z.; Cheng, Y.; Long, C.; Tang, W.; Liu, Q.; Chen, X. Dual-Nanozyme Cascade for System-Wide Specific Colorimetric Detection of Aminoglycoside Antibiotics. Anal. Chem. 2025, 97, 6136–6144. [Google Scholar] [CrossRef]
- Koyappayil, A.; Kim, H.T.; Lee, M.-H. ‘Laccase-like’ Properties of Coral-like Silver Citrate Micro-Structures for the Degradation and Determination of Phenolic Pollutants and Adrenaline. J. Hazard. Mater. 2021, 412, 125211. [Google Scholar] [CrossRef]
- Jin, Z.; Yim, W.; Retout, M.; Housel, E.; Zhong, W.; Zhou, J.; Strano, M.S.; Jokerst, J.V. Colorimetric Sensing for Translational Applications: From Colorants to Mechanisms. Chem. Soc. Rev. 2024, 53, 7681–7741. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Liu, Y.; Khan, M.A.; Zhao, H.; Cao, H.; Ye, D. Identification of Multiple Foodborne Pathogens Using Single-Atom Nanozyme Colorimetric Sensor Arrays and Machine Learning. Chem. Eng. J. 2025, 511, 162115. [Google Scholar] [CrossRef]
- Jing, W.; Yang, Y.; Shi, Q.; Xu, J.; Xing, G.; Dai, Y.; Liu, F. Nanozymes Sensor Array for Discrimination and Intelligent Sensing of Phenolic Acids in Food. Food Chem. 2024, 450, 139326. [Google Scholar] [CrossRef]
- Li, M.; Jia, L.; Zhao, X.; Zhang, L.; Zhao, D.; Xu, J.; Zhao, T. Machine Learning-Assisted Ratiometric Fluorescence Sensor Array for Recognition of Multiple Quinolones Antibiotics. Food Chem. 2025, 478, 143722. [Google Scholar] [CrossRef]
- Che, H.; Tian, X.; Guo, F.; Nie, Y.; Dai, C.; Li, Y.; Lu, L. Enhancement of the Peroxidase Activity of G-C3N4 with Different Morphologies for Simultaneous Detection of Multiple Antibiotics. Anal. Chem. 2023, 95, 12550–12556. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Min, H.; Li, T.; Yan, B. Fluorescence Array Sensor Based on Lanthanide Complex for Pattern Recognition Detection of Fluoroquinolone Antibiotics. Talanta 2024, 280, 126719. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.-Q.; Li, J.-X.; Chen, G.-Y.; Luo, M.-L.; Yang, F.-Q. Construction of Pyrimidine Derivatives-Copper Enzyme Mimics as Colorimetric Sensing Elements for Efficient Detection of Phenolic Compounds and Hydrogen Peroxide. J. Hazard. Mater. 2024, 480, 136294. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Zhou, H.; Zhao, Y.; Cheng, Y.; Wu, Y.; Zhang, J.; Chen, J.; Zhang, S. Machine Learning-Assisted Photoluminescent Sensor Array Based on Gold Nanoclusters for the Discrimination of Antibiotics with Test Paper. Talanta 2024, 266, 125122. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Wu, X.; Liu, W.; Peng, Z.; Dong, Y.; Huang, Z.; Zhang, L.; Liang, Y. Fluorescent Sensor Array Based on Janus Silica Nanoflakes to Realize Pattern Recognition of Multiple Aminoglycoside Antibiotics and Heavy Metal Ions. Sens. Actuators B Chem. 2023, 378, 133154. [Google Scholar] [CrossRef]
- Xia, J.; Li, Z.; Ding, Y.; Shah, L.A.; Zhao, H.; Ye, D.; Zhang, J. Construction and Application of Nanozyme Sensor Arrays. Anal. Chem. 2024, 96, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Tang, Y.; Wu, Z.; Zhou, J.; Tao, H.; Wu, Y. A Colorimetric Sensor Array Based on Bimetallic CeCo-MOF with Triple-Enzyme-Mimic Activities for Highly Sensitive Detection of Tetracycline Antibiotics. Chem. Eng. J. 2024, 500, 157234. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Su, X. Versatile Laccase-Mimicking Enzyme for Dye Decolorization and Tetracyclines Identification upon a Colorimetric Array Sensor. J. Hazard. Mater. 2025, 483, 136683. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Wang, Y.; Wang, Y.; Wu, P. Recent Progress on the Rational Design of Laccase Mimics. Chem.—Asian J. 2025, 20, e202401942. [Google Scholar] [CrossRef]
- Mekonnen, M.L.; Abda, E.M.; Csáki, A.; Fritzsche, W. Frontiers in Laccase Nanozymes-Enabled Colorimetric Sensing: A Review. Anal. Chim. Acta 2025, 1337, 343333. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various Types and Applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Yu, J.; Zhang, X.; Lu, G.-P. Iron Single-Atom Anchored N-Doped Carbon as a ‘Laccase-like’ Nanozyme for the Degradation and Detection of Phenolic Pollutants and Adrenaline. J. Hazard. Mater. 2022, 425, 127763. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-Pot Synthesis of Active Copper-Containing Carbon Dots with Laccase-like Activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chai, L.; Gui, J.; Liu, Y.; Liu, M.; Liu, X.; Zhang, Y.; Yao, S. Copper-Manganese Bimetallic Oxide with Excellent Laccase-like Activity for Colorimetric Detection of Formaldehyde via the Specific Aldimine Condensation Reaction. Talanta 2025, 293, 128151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Construction of Biomimetic Nanozyme with High Laccase- and Catecholase-like Activity for Oxidation and Detection of Phenolic Compounds. J. Hazard. Mater. 2022, 429, 128404. [Google Scholar] [CrossRef]
- Chai, T.-Q.; Wang, J.-L.; Chen, G.-Y.; Chen, L.-X.; Yang, F.-Q. Tris-Copper Nanozyme as a Novel Laccase Mimic for the Detection and Degradation of Phenolic Compounds. Sensors 2023, 23, 8137. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, F.; Yuan, Q.; Liu, J.; Li, Y.; Liang, H. Robust Magnetic Laccase-Mimicking Nanozyme for Oxidizing o-Phenylenediamine and Removing Phenolic Pollutants. J. Environ. Sci. 2020, 88, 103–111. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a Bioinspired Laccase-Mimicking Nanozyme for the Degradation and Detection of Phenolic Pollutants. Appl. Catal. B Environ. 2019, 254, 452–462. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, C.; Shi, L.; Zhu, X.; Liu, W.; Li, B.; Jin, Y. Multifunctional Manganese–Nucleotide Laccase-Mimicking Nanozyme for Degradation of Organic Pollutants and Visual Assay of Epinephrine via Smartphone. Anal. Chem. 2024, 96, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, X.-Y.; Zheng, Q.-H.; Zhang, Y.; Yao, L.; Xu, Q.-X.; Chen, J.-C.; He, S.-B.; Chen, W. Construction of Platinum Nanozyme by Using Carboxymethylcellulose with Improved Laccase-like Activity for Phenolic Compounds Detection. Sens. Actuators B Chem. 2023, 393, 134165. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.-L.; Xiong, J.; Yuan, X.; Liu, S.-L.; Zong, M.-H.; Lou, W.-Y. Multivalent Ce-MOFs as Biomimetic Laccase Nanozyme for Environmental Remediation. Chem. Eng. J. 2022, 450, 138220. [Google Scholar] [CrossRef]
- Xia, L.; Han, J.; Huang, X.; Niu, X.; Lin, X.; Wu, Y. Colorimetric Sensor and Visual Enzyme Sheets for Sensitive Detection of Dimethoate Residue in Vegetables Based on Laccase-like Activity of Coral-like Silver Citrate. Food Control 2024, 158, 110252. [Google Scholar] [CrossRef]
- Huang, L.; Tang, Y.; Wang, J.; Niu, X.; Zhou, J.; Wu, Y. Cubic Ag2O Nanoparticles as Robust Laccase Mimetics in a Smartphone-Assisted Colorimetric Sensor for Rapid and Ultrasensitive Detection of Kanamycin in Environment. Sens. Actuators B Chem. 2023, 391, 134052. [Google Scholar] [CrossRef]
- Niu, X.; He, H.; Ran, H.; Wu, Z.; Tang, Y.; Wu, Y. Rapid Colorimetric Sensor for Ultrasensitive and Highly Selective Detection of Fumonisin B1 in Cereal Based on Laccase-Mimicking Activity of Silver Phosphate Nanoparticles. Food Chem. 2023, 429, 136903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Huang, X.; Xia, L.; Tao, H.; Wu, Y. Coral-like Silver Citrate as Robust Laccase Mimetics in a Novel Colorimetric Aptasensor for Sensitive and Highly Selective Detection of Histamine in Food. Food Control 2024, 165, 110645. [Google Scholar] [CrossRef]
- Lazareva, A.; Daugulis, O. Direct Palladium-Catalyzed Ortho-Arylation of Benzylamines. Org. Lett. 2006, 8, 5211–5213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hou, Y.; Lin, M.; Yang, Q. Construction of Extracellular Peptide Laccase-Mimic Nanozyme for the Detection and Degradation of Phenols Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134687. [Google Scholar] [CrossRef]
- Wang, J.-L.; Chen, G.-Y.; Chai, T.-Q.; Chen, L.-X.; Chen, H.; Yang, F.-Q. Construction of Mn-Decorated Zeolitic Imidazolate Framework-90 Nanostructure as Superior Oxidase-like Mimic for Colorimetric Detection of Glucose and Choline. Talanta 2024, 271, 125708. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Zhang, J.; Lei, L.; Su, X. Construction of a Laccase Mimic Enzyme with Fluorescence Properties for Kanamycin Multi-Mode Analysis. Chem. Eng. J. 2023, 471, 144184. [Google Scholar] [CrossRef]
- Yu, Z.; Liao, Y.; Liu, J.; Wu, Q.; Cheng, Y.; Huang, K. A Smartphone-Based Gold Nanoparticle Colorimetric Sensing Platform for Kanamycin Detection in Food Samples. Anal. Methods 2023, 15, 4282–4288. [Google Scholar] [CrossRef]
- Olson, L.P.; Whitcomb, D.R.; Rajeswaran, M.; Blanton, T.N.; Stwertka, B.J. The Simple Yet Elusive Crystal Structure of Silver Acetate and the Role of the Ag-Ag Bond in the Formation of Silver Nanoparticles during the Thermally Induced Reduction of Silver Carboxylates. Chem. Mater. 2006, 18, 1667–1674. [Google Scholar] [CrossRef]
- Jiang, W.; Zheng, J.; Su, J.; Tang, Y.; Wu, Y.; Cao, Y.; Cao, W. Bimetallic Ag2CrO4 Nanoparticles with Dual-Enzyme-Mimic Activities in Colorimetric Sensor for Sensitive and Highly Selective Detection of Dimethoate in Vegetables. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 332, 125841. [Google Scholar] [CrossRef]
- Nakano, M.; Fujiwara, T.; Koga, N. Thermal Decomposition of Silver Acetate: Physico-Geometrical Kinetic Features and Formation of Silver Nanoparticles. J. Phys. Chem. C 2016, 120, 8841–8854. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Qu, L.; Yu, L.; Li, Z. Highly Active Silver-Based Laccase-like Nanozyme for Colorimetric Distinction and Detection of Phenylenediamine Isomers. New J. Chem. 2025, 49, 10429–10439. [Google Scholar] [CrossRef]
- Nian, Q.; Yang, H.; Meng, E.; Wang, C.; Xu, Q.; Zhang, Q. Efficient Adsorptive Removal of Aminoglycoside Antibiotics from Environmental Water. Chemosphere 2023, 337, 139379. [Google Scholar] [CrossRef]
- Zheng, T.; Li Sip, Y.Y.; Leong, M.B.; Huo, Q. Linear Self-Assembly Formation between Gold Nanoparticles and Aminoglycoside Antibiotics. Colloids Surf. B Biointerfaces 2018, 164, 185–191. [Google Scholar] [CrossRef]
- Tian, Y.; Mou, Y.; Zhang, W.; Sun, Z.; Yin, Y.; Han, L.; Chen, D.; Guo, Y.; Sun, X.; Li, F.; et al. A Fluorescence and Colorimetric Dual-Mode Aptasensor for Kanamycin Detection. Biosens. Bioelectron. 2025, 268, 116911. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Zhou, P.; Wang, C.; Tao, H.; Wu, Y. Colorimetric Detection of Kanamycin Residue in Foods Based on the Aptamer-Enhanced Peroxidase-Mimicking Activity of Layered WS2 Nanosheets. J. Agric. Food Chem. 2021, 69, 2884–2893. [Google Scholar] [CrossRef]
- Xu, C.; Ying, Y.; Ping, J. Colorimetric Aggregation Assay for Kanamycin Using Gold Nanoparticles Modified with Hairpin DNA Probes and Hybridization Chain Reaction-Assisted Amplification. Microchim. Acta 2019, 186, 448. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, T.; Kumar, J.V.; Rhim, J.-W.; Shin, G.H.; Kim, J.T. Yellow-Emitting Carbon Dots as ‘off-on’ Fluorescence Probes in Paper-Based Micro Kit for Selective Detection of Silver Ions and Kanamycin in Animal-Derived Food. J. Environ. Chem. Eng. 2025, 13, 117706. [Google Scholar] [CrossRef]
- Bai, L.; Ye, T.; Zhu, D.; Sun, D.; Zhang, S.; Lu, Y.; Yuan, M.; Cao, H.; Hao, L.; Wu, X.; et al. Spherical Nucleic Acids with Tailored DNA Conformation via Bromide Backfilling for the Detection of Kanamycin. Luminescence 2022, 37, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Moore, J.H.; Harbaugh, S.; Chavez, J.; Chou, C.-F.; Swami, N.S. Multiplexed Assessment of Engineered Bacterial Constructs for Intracellular β-Galactosidase Expression by Redox Amplification on Catechol-Chitosan Modified Nanoporous Gold. Microchim. Acta 2021, 189, 4. [Google Scholar] [CrossRef]
- Wang, J.; Lu, T.; Hu, Y.; Wang, X.; Wu, Y. A Label-Free and Carbon Dots Based Fluorescent Aptasensor for the Detection of Kanamycin in Milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117651. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, L.; Wei, X.; Sun, Y.; Qi, S.; Chen, Y.; Tian, X.; Qiu, J.; Xu, D. Spongy Co/Ni-Bio-MOF-Based Electrochemical Aptasensor for Detection of Kanamycin Based on Coral-like ZrO2@Au as an Amplification Platform. J. Electroanal. Chem. 2022, 920, 116647. [Google Scholar] [CrossRef]
- Yao, X.; Shen, J.; Liu, Q.; Fa, H.; Yang, M.; Hou, C. A Novel Electrochemical Aptasensor for the Sensitive Detection of Kanamycin Based on UiO-66-NH2/MCA/MWCNT@rGONR Nanocomposites. Anal. Methods 2020, 12, 4967–4976. [Google Scholar] [CrossRef]
- Écija-Arenas, Á.; Kirchner, E.-M.; Hirsch, T.; Fernández-Romero, J.M. Development of an Aptamer-Based SPR-Biosensor for the Determination of Kanamycin Residues in Foods. Anal. Chim. Acta 2021, 1169, 338631. [Google Scholar] [CrossRef]
- Li, A.; Li, H.; Ma, Y.; Wang, T.; Liu, X.; Wang, C.; Liu, F.; Sun, P.; Yan, X.; Lu, G. Bioinspired Laccase-Mimicking Catalyst for on-Site Monitoring of Thiram in Paper-Based Colorimetric Platform. Biosens. Bioelectron. 2022, 207, 114199. [Google Scholar] [CrossRef]
- Huang, H.; Bai, J.; Li, J.; Lei, L.; Zhang, W.; Yan, S.; Li, Y. Fluorometric and Colorimetric Analysis of Alkaline Phosphatase Activity Based on a Nucleotide Coordinated Copper Ion Mimicking Polyphenol Oxidase. J. Mater. Chem. B 2019, 7, 6508–6514. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Preparation of Laccase Mimicking Nanozymes and Their Catalytic Oxidation of Phenolic Pollutants. Catal. Sci. Technol. 2021, 11, 3402–3410. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Lei, L.; Huang, H.; Li, Y. Colorimetric Logic Gate for Protamine and Trypsin Based on the Bpy-Cu Nanozyme with Laccase-like Activity. Sens. Actuators B Chem. 2022, 357, 131429. [Google Scholar] [CrossRef]
- Gu, H.; Li, P.; Wang, J.; Niu, N.; Chen, L. Bioinspired Bimetallic Metal–Organic Framework Nanozyme with Laccase-Mimicking Activity for Detection and Removal of Phenolic Contaminants. Microchem. J. 2024, 201, 110568. [Google Scholar] [CrossRef]
- Guan, M.; Wang, M.; Qi, W.; Su, R.; He, Z. Biomineralization-Inspired Copper-Cystine Nanoleaves Capable of Laccase-like Catalysis for the Colorimetric Detection of Epinephrine. Front. Chem. Sci. Eng. 2021, 15, 310–318. [Google Scholar] [CrossRef]
- Maity, T.; Jain, S.; Solra, M.; Barman, S.; Rana, S. Robust and Reusable Laccase Mimetic Copper Oxide Nanozyme for Phenolic Oxidation and Biosensing. ACS Sustain. Chem. Eng. 2022, 10, 1398–1407. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tan, W.; Jin, P.; Zhang, P.; Li, K. Bioinspired Coassembly of Copper Ions and Nicotinamide Adenine Dinucleotides for Single-Site Nanozyme with Dual Catalytic Functions. Anal. Chem. 2023, 95, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Qu, L.; Yu, L.; Li, Z. 2-Methylbenzimidazole-Copper Nanozyme with High Laccase Activity for Colorimetric Differentiation and Detection of Aminophenol Isomers. Talanta 2024, 279, 126630. [Google Scholar] [CrossRef] [PubMed]


| Method | Material System | Linear Range (μM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| Colorimetry | CH3COOAg * | 0.01–0.1 | 3.99 | This work |
| Colorimetry | CuNCs@SiO2-DNA-AgNCs * | 0.1–0.8 | 14.5 | [64] |
| Colorimetry | WS2 Nanosheets * | 0.1–0.5 | 60 | [65] |
| Colorimetry | AuNPs * | 1–40 | 680 | [66] |
| Fluorescence | Y-CDs * | 6.66–66.6 | 51.9 | [67] |
| Fluorescence | AuNPs * | 0.2–10 | 71.53 | [68] |
| Fluorescence | NPG/MCH/Cat-Chit * | 0.1–0.8 | 23.6 | [69] |
| Fluorescence | CDs * | 0.04–0.24 | 18 | [70] |
| Electrochemistry | Co/Ni-Bio-MOF-ZrO2@Au * | 10–1000 | 37 | [71] |
| Electrochemistry | UiO-66-NH2/MCA/MWCNT@rGONR * | 0.025–0.9 | 13 | [72] |
| Surface Plasmon Resonance | rGO * | 1–100 | 285 | [73] |
| Added (nM) | Found (nM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|
| 0 | - | - | - |
| 20 | 18.8 | 94.0 | 6.8 |
| 40 | 36.6 | 91.5 | 2.4 |
| 60 | 56.6 | 94.4 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Chai, T.-Q.; Li, J.-X.; Dai, J.-J.; Xu, L.; Qin, W.-L.; Yang, F.-Q. CH3COOAg with Laccase-like Activity for Differentiation and Detection of Aminoglycoside Antibiotics. Biosensors 2025, 15, 570. https://doi.org/10.3390/bios15090570
Zhu H, Chai T-Q, Li J-X, Dai J-J, Xu L, Qin W-L, Yang F-Q. CH3COOAg with Laccase-like Activity for Differentiation and Detection of Aminoglycoside Antibiotics. Biosensors. 2025; 15(9):570. https://doi.org/10.3390/bios15090570
Chicago/Turabian StyleZhu, Huan, Tong-Qing Chai, Jia-Xin Li, Jing-Jing Dai, Lei Xu, Wen-Ling Qin, and Feng-Qing Yang. 2025. "CH3COOAg with Laccase-like Activity for Differentiation and Detection of Aminoglycoside Antibiotics" Biosensors 15, no. 9: 570. https://doi.org/10.3390/bios15090570
APA StyleZhu, H., Chai, T.-Q., Li, J.-X., Dai, J.-J., Xu, L., Qin, W.-L., & Yang, F.-Q. (2025). CH3COOAg with Laccase-like Activity for Differentiation and Detection of Aminoglycoside Antibiotics. Biosensors, 15(9), 570. https://doi.org/10.3390/bios15090570






