Development of Immunoassays for Foodborne Pathogenic Bacteria Detection Using PolyHRP for Signal Enhancement
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
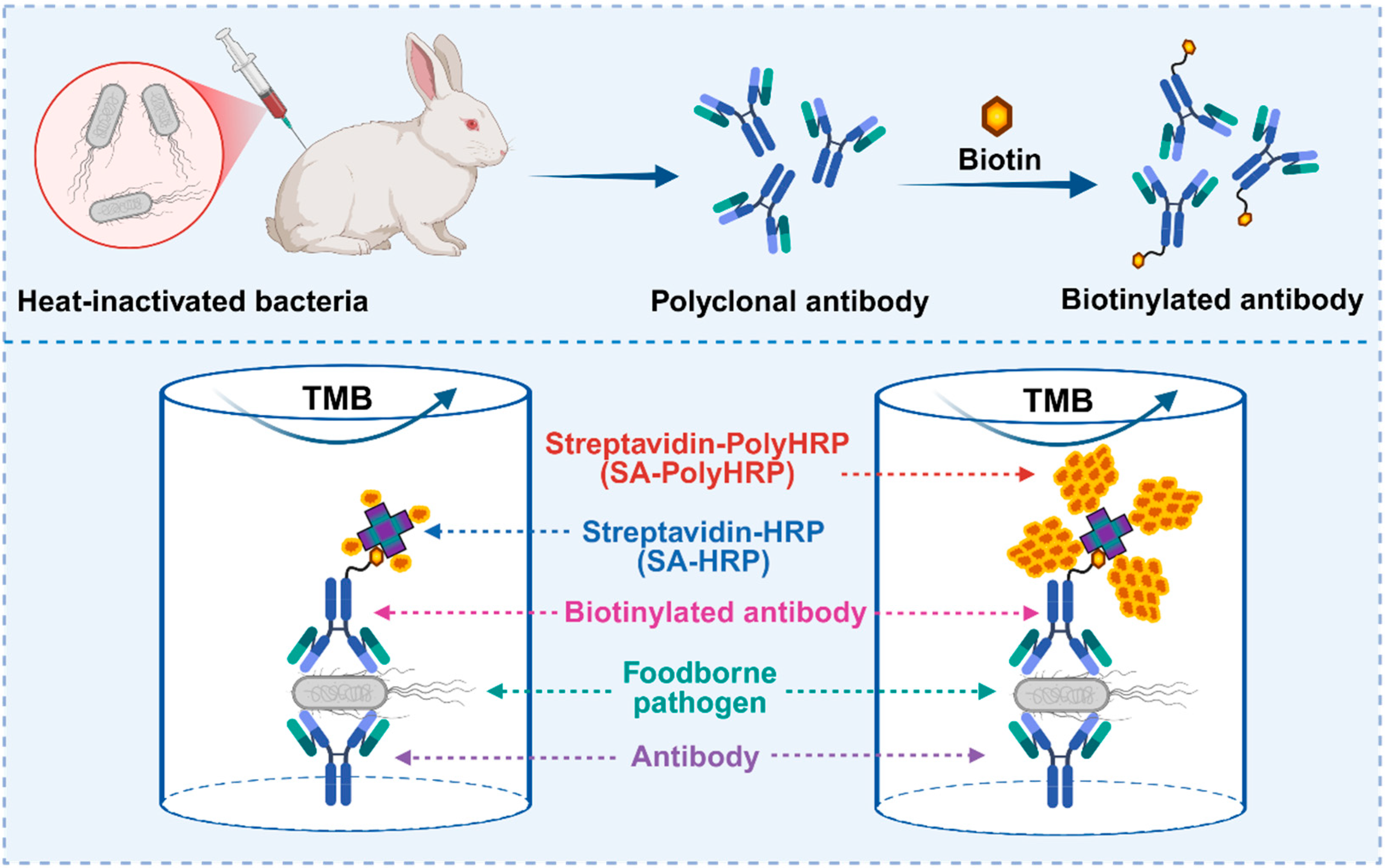
2.2. Preparation of Polyclonal Antibody Against Foodborne Pathogenic Bacteria
2.3. Biotinylation of Anti-E. coli O157:H7 pAb and Anti-S. Typhimurium pAb
2.4. Optimization of a Double-Antibody Sandwich-Based ELISA for E. coli O157:H7
2.5. Optimization of a Double-Antibody Sandwich-Based ELISA for S. Typhimurium
2.6. Development of Double-Antibody Sandwich-Based Immunoassays
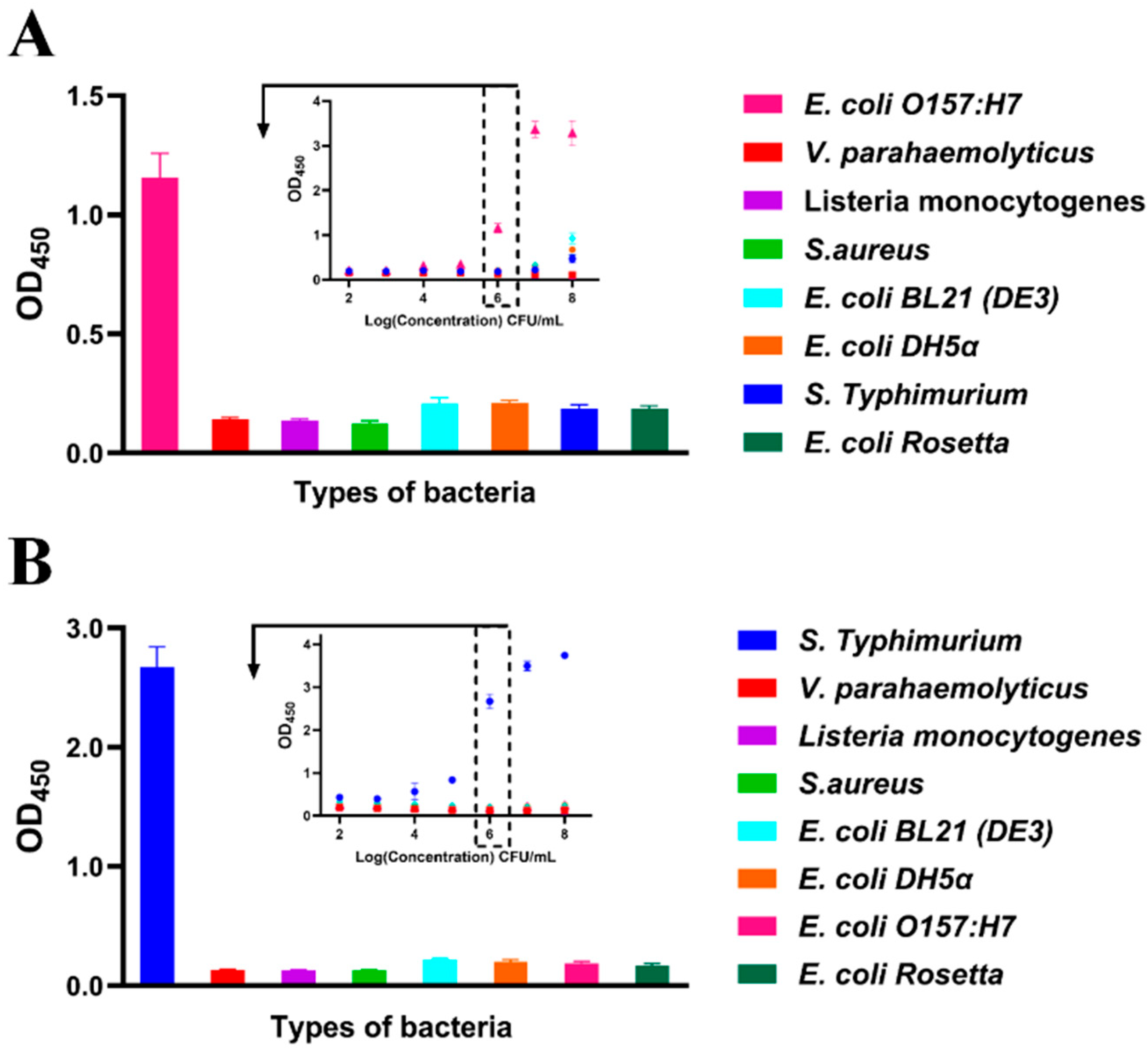
2.7. Specificity
2.8. Matrix Effect
2.9. Analysis of Food Samples
3. Result and Discussion
3.1. Characterization of the Anti-E. coli O157:H7 and Anti-S. Typhimurium pAb
3.2. Optimization for E. coli O157:H7 and S. Typhimurium Sandwich-Based ELISA
3.3. Double-Antibody Sandwich-Based ELISA for E. coli O157:H7 and S. Typhimurium
3.4. Cross-Reactivity
3.5. Spike-and-Recovery Analysis
3.6. Analysis Performance of Food Samples Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Ma, X.; Zhu, W.; Huang, Q.; Liu, Y.; Pan, J.; Ying, Y.; Xu, X.; Fu, Y. Enzymatic Catalysis in Size and Volume Dual-confined Space of Integrated Nanochannel-electrodes Chip for Enhanced Impedance Detection of Salmonella. Small 2023, 19, 2300900. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P.; Liao, X.; Dai, Y.; Yu, Q.; Yu, G.; Zhang, Y.; Wei, J.; Jing, Y.; et al. Enhancing Oriented Immobilization Efficiency: A One-for-Two Organism-Bispecific Nanobody Scaffold for Highly Sensitive Detection of Foodborne Pathogens. Anal. Chem. 2023, 95, 17135–17142. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Chen, P.; Yang, Q.; Li, S.; Liu, X.; Li, B.; Zhang, J.; Wang, J.; Yue, X.; Wang, Y. Nanobody-Induced Aggregation of Gold Nanoparticles: A Mix-and-Read Strategy for the Rapid Detection of Cronobacter Sakazakii. Anal. Chem. 2024, 96, 17602–17611. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Pan, J.; Xu, Z.; Hammock, B.D.; Li, D. Development of a Nanobody-Based Immunoassay for the Detection of Escherichia coli O157:H7 in Food Samples. Food Chem. 2025, 473, 142987. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, M.J.; Shah, H.J.; Weller, D.L.; Ray, L.C.; Smith, K.; McGuire, S.; Trevejo, R.T.; Walter, E.S.; Wymore, K.; Rissman, T.; et al. Preliminary Incidence and Trends of Infections Caused by Pathogens Transmitted Commonly through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2022. Morb. Mortal. Wkly. Rep. 2023, 72, 701–706. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Bacteriological Analytical Manual Chapter 4A: Diarrheagenic Escherichia coli (July 2020 Edition); U.S. Food and Drug Administration (FDA): College Park, MD, USA, 2020.
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 Outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Jayan, H.; Pu, H.; Sun, D.-W. Recent Development in Rapid Detection Techniques for Microorganism Activities in Food Matrices Using Bio-Recognition: A Review. Trends Food Sci. Technol. 2020, 95, 233–246. [Google Scholar] [CrossRef]
- Kothary, M.H.; Babu, U.S. Infective Dose of Foodborne Pathogens in Volunteers: A Review. J. Food Saf. 2001, 21, 49–68. [Google Scholar] [CrossRef]
- He, Y.; Jia, F.; Sun, Y.; Fang, W.; Li, Y.; Chen, J.; Fu, Y. An Electrochemical Sensing Method Based on CRISPR/Cas12a System and Hairpin DNA Probe for Rapid and Sensitive Detection of Salmonella Typhimurium. Sens. Actuators B 2022, 369, 132301. [Google Scholar] [CrossRef]
- Chen, J.C.; Patel, K.; Smith, P.A.; Vidyaprakash, E.; Snyder, C.; Tagg, K.A.; Webb, H.E.; Schroeder, M.N.; Katz, L.S.; Rowe, L.A.; et al. Reoccurring Escherichia coli O157:H7 Strain Linked to Leafy Greens–Associated Outbreaks, 2016–2019. Emerg. Infect. Dis. 2023, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.W.; Gay, J.M.; Hancock, D.D.; Gay, C.C.; Fox, L.K.; Besser, T.E. Sensitivity of Bacteriologic Culture for Detection of Escherichia coli O157:H7 in Bovine Feces. J. Clin. Microbiol. 1995, 33, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, S.; Lai, X.; Peng, J.; Lai, W. Developmental Trend of Immunoassays for Monitoring Hazards in Food Samples: A Review. Trends Food Sci. Technol. 2021, 111, 68–88. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, K.; Huang, M.; Zeng, M.; Deng, Y.; Li, S.; Chen, H.; Li, W.; Chen, Z. Research Progress on Detection Techniques for Point-of-Care Testing of Foodborne Pathogens. Front. Bioeng. Biotechnol. 2022, 10, 958134. [Google Scholar] [CrossRef]
- Yamazaki, W.; Kumeda, Y.; Uemura, R.; Misawa, N. Evaluation of a Loop-Mediated Isothermal Amplification Assay for Rapid and Simple Detection of Vibrio Parahaemolyticus in Naturally Contaminated Seafood Samples. Food Microbiol. 2011, 28, 1238–1241. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. Rapid Methods for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T. Recent Advances in Optical Biosensors for the Detection of Cancer Biomarker α-Fetoprotein (AFP). Trends Anal. Chem. 2020, 128, 115920. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Singal, A.G. Testing for AFP in Combination with Ultrasound Improves Early Liver Cancer Detection. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 947–949. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Morisseau, C.; Wagner, K.M.; Cho, Y.S.; Hammock, B.D. Development of a Highly Sensitive Enzyme-Linked Immunosorbent Assay for Mouse Soluble Epoxide Hydrolase Detection by Combining a Polyclonal Capture Antibody with a Nanobody Tracer. Anal. Chem. 2020, 92, 11654–11663. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; Wang, Y.; Wang, P.; Ji, Y.; Liu, B.; Wang, J.; González-Sapienza, G.; Wang, Y. Development of a Streptavidin-Bridged Enhanced Sandwich ELISA Based on Self-Paired Nanobodies for Monitoring Multiplex Salmonella Serogroups. Anal. Chim. Acta 2022, 1203, 339705. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of Nano-ELISA in Food Analysis: Recent Advances and Challenges. Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Sun, D.; Liu, X.; Bao, K.; Wu, L.; Kuang, H.; Pei, H.; Chen, Q. Nanobody Based Immunoassay for Alpha Fetal Protein Detection Using Streptavidin-Conjugated Polymerized Horseradish Peroxidase for Signal Amplification. Anal. Sci. 2023, 39, 2059–2065. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, S.; Gunaseelan, A.; Li, D.; Hammock, B.D.; Gomes, A.V. Improving the Sensitivity of Traditional Western Blotting via Streptavidin Containing Poly-horseradish Peroxidase (PolyHRP). Electrophoresis 2019, 40, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Li, D.; McReynolds, C.B.; Morisseau, C.; Hammock, B.D. Improved ELISA for Linoleate-Derived Diols in Human Plasma Utilizing a polyHRP-Based Secondary Tracer. Anal. Methods 2022, 14, 1810–1819. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Morisseau, C.; Gee, S.J.; Bever, C.S.; Liu, X.; Wu, J.; Hammock, B.D.; Ying, Y. Nanobody Based Immunoassay for Human Soluble Epoxide Hydrolase Detection Using Polymeric Horseradish Peroxidase (PolyHRP) for Signal Enhancement: The Rediscovery of PolyHRP? Anal. Chem. 2017, 89, 6248–6256. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, G.; Dou, W. Core-Shell Red Silica Nanoparticles Based Immunochromatographic Assay for Detection of Escherichia coli O157:H7. Anal. Chim. Acta 2018, 1038, 97–104. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Yuan, J.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Integrated Gold Superparticles into Lateral Flow Immunoassays for the Rapid and Sensitive Detection of Escherichia coli O157:H7 in Milk. J. Dairy Sci. 2020, 103, 6940–6949. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, S.; Tian, Y.; Wu, Y.; Wu, M.; Wang, Z.; Xu, D.; Hou, D.; Liu, Q. An Aggregation-Induced Emission Material Labeling Antigen-Based Lateral Flow Immunoassay Strip for Rapid Detection of Escherichia coli O157:H7. SLAS Technol. 2021, 26, 377–383. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Bai, M.; Wang, Y.; Liao, X.; Zhang, Y.; Wang, P.; Wei, J.; Zhang, H.; Wang, J.; et al. An Ultrasensitive Sandwich Chemiluminescent Enzyme Immunoassay Based on Phage-Mediated Double-Nanobody for Detection of Salmonella Typhimurium in Food. Sens. Actuators B 2022, 352, 131058. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Song, S.; Tang, L.; Kuang, H.; Xu, C. A Highly Sensitive ELISA and Immunochromatographic Strip for the Detection of Salmonella Typhimurium in Milk Samples. Sensors 2015, 15, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Rodpai, E.; Moongkarndi, P.; Tungrugsasut, W.; Phisannoradej, R.; Kanarat, S. Comparison of Multiplex Polymerase Chain Reaction and Immunoassay to Detect Salmonella spp., S. Typhimurium, and S. Enteritidis in Thai Chicken Meat. Scienceasia 2013, 39, 150–159. [Google Scholar] [CrossRef]



| Target | Spiked (CFU/mL) | Undiluted mBPWp Medium Founded (CFU/mL) | Recovery (%) | 1:10 Dilution Founded (CFU/mL) | Recovery (%) | 1:100 Dilution Founded (CFU/mL) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| E. coli O157:H7 | 1.25 × 106 | 1.4 × 106 ± 1.3 × 105 | 115% | 1.2 × 106 ± 1.0 × 105 | 93% | 1.2 × 106 ± 1.1 × 104 | 97% |
| 6.25 × 105 | 8.7 × 105 ± 3.4 × 104 | 139% | 6.2 × 105 ± 9.7 × 104 | 99% | 5.6 × 105 ± 2.1 × 104 | 90% | |
| 3.125 × 105 | 3.8 × 105 ± 2.9 × 103 | 122% | 2.6 × 105 ± 4.5 × 104 | 84% | 2.3 × 105 ± 1.3 × 104 | 74% | |
| S. Typhimurium | 1.25 × 106 | 1.5 × 106 ± 2.5 × 104 | 121% | 1.4 × 106 ± 5.6 × 104 | 109% | 1.2 × 106 ± 7.5 × 104 | 93% |
| 6.25 × 105 | 1.0 × 106 ± 8.6 × 103 | 162% | 9.3 × 105 ± 5.6 × 104 | 150% | 7.3 × 105 ± 7.7 × 104 | 116% | |
| 3.125 × 105 | 6.0 × 105 ± 3.1 × 104 | 194% | 5.2 × 105 ± 1.8 × 104 | 165% | 4.1 × 105 ± 2.6 × 104 | 130% |
| Target | Control c | Spiked with 5 CFU | |||
|---|---|---|---|---|---|
| Enrichment Period | ELISA d (CFU/mL) | Plate Counting (CFU/mL) | ELISA (CFU/mL) | Plate Counting e (CFU/mL) | |
| E. coli O157:H7 a | 5 h | N.D. | N.D. | 2.8 × 108 ± 3.0 × 106 | 2.3 × 108 |
| 18 h | N.D. | N.D. | 9.2 × 108 ± 5.5 × 107 | 7.7 × 108 | |
| S. Typhimurium b | 5 h | N.D. | N.D. | 8.2 × 108 ± 1.3 × 108 | 1.9 × 106 |
| 18 h | N.D. | N.D. | 3.5 × 1010 ± 2.0 × 109 | 2.2 × 1010 | |
| Target | Principle | Combined Technique | LOD a (CFU/mL) | Sample | Reference |
|---|---|---|---|---|---|
| E. coli O157:H7 | ELISA | PolyHRP | 1.4 × 104 | Beef | This work |
| ELISA | Core–shell red silica nanoparticles | 4.5 × 105 | Milk and pork | [28] | |
| Lateral flow immunoassay | Gold superparticles added to polymer nanobead | 5.59 × 102 | Milk | [29] | |
| Lateral flow immunoassay | Aggregation-induced emission material labeling antigen as a fluorescent probe | 105 | Beef, milk, and fruits | [30] | |
| S. Typhimurium | ELISA | PolyHRP | 6.0 × 103 | Beef | This work |
| ELISA | Nb-based ELISA | 9.15 × 103 | milk, honey, pork, and lettuce | [22] | |
| ELISA | Phage-mediated double-nanobody sandwich chemiluminescent enzyme immunoassay | 3.63 × 103 | Juice, honey, milk, and pork | [31] | |
| ELISA | Sandwich ELISA | 1.25 × 106 | Milk | [32] | |
| ELISA | IMS-ELISA | 104 | Chicken | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Pan, J.; He, Q.; Xu, Z.; Hammock, B.D.; Li, D. Development of Immunoassays for Foodborne Pathogenic Bacteria Detection Using PolyHRP for Signal Enhancement. Biosensors 2025, 15, 318. https://doi.org/10.3390/bios15050318
Zhang Y, Pan J, He Q, Xu Z, Hammock BD, Li D. Development of Immunoassays for Foodborne Pathogenic Bacteria Detection Using PolyHRP for Signal Enhancement. Biosensors. 2025; 15(5):318. https://doi.org/10.3390/bios15050318
Chicago/Turabian StyleZhang, Yijia, Junkang Pan, Qiyi He, Zhihao Xu, Bruce D. Hammock, and Dongyang Li. 2025. "Development of Immunoassays for Foodborne Pathogenic Bacteria Detection Using PolyHRP for Signal Enhancement" Biosensors 15, no. 5: 318. https://doi.org/10.3390/bios15050318
APA StyleZhang, Y., Pan, J., He, Q., Xu, Z., Hammock, B. D., & Li, D. (2025). Development of Immunoassays for Foodborne Pathogenic Bacteria Detection Using PolyHRP for Signal Enhancement. Biosensors, 15(5), 318. https://doi.org/10.3390/bios15050318








