The Design and Application of Wearable Ultrasound Devices for Detection and Imaging
Abstract
1. Introduction
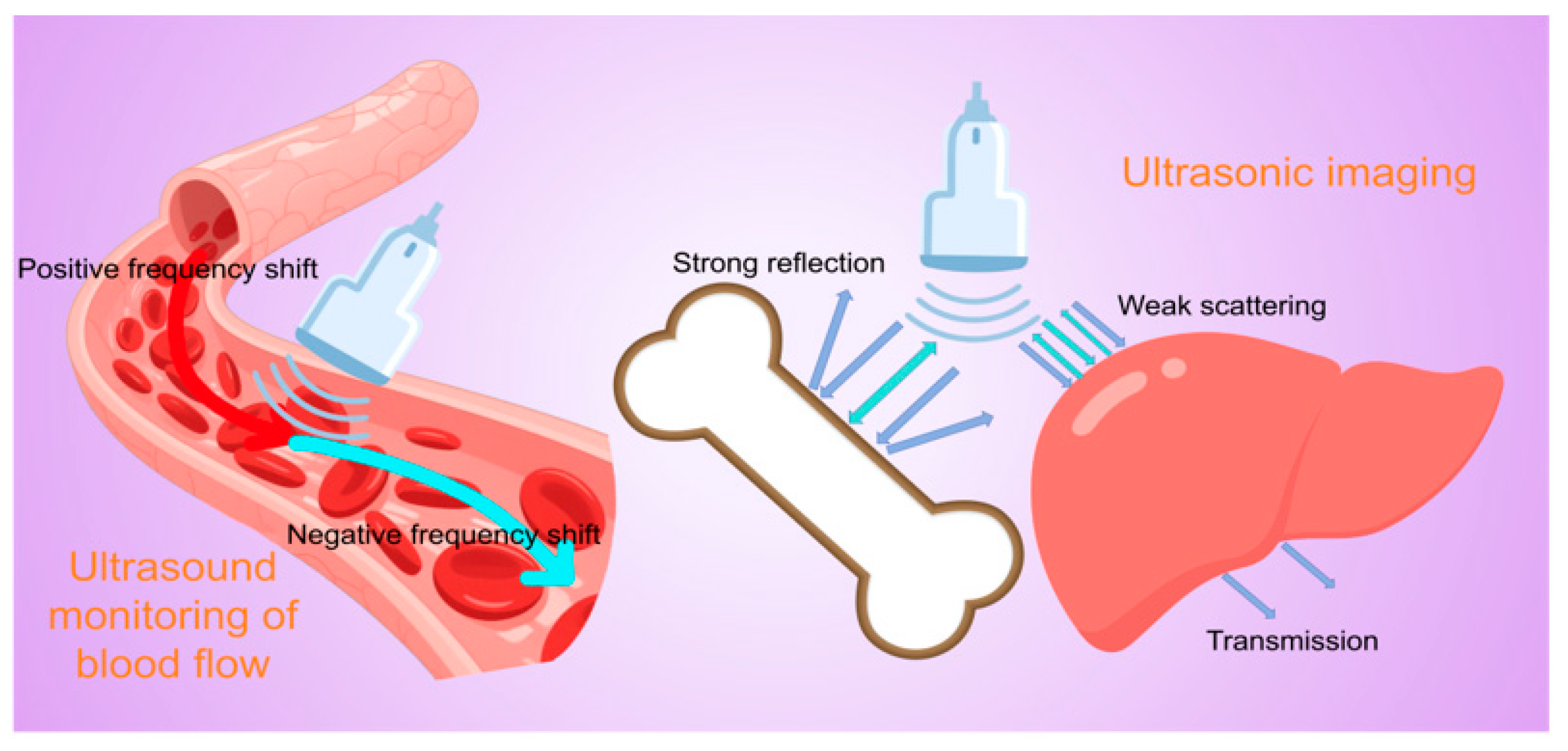
2. Principles of Ultrasonic Testing and Imaging
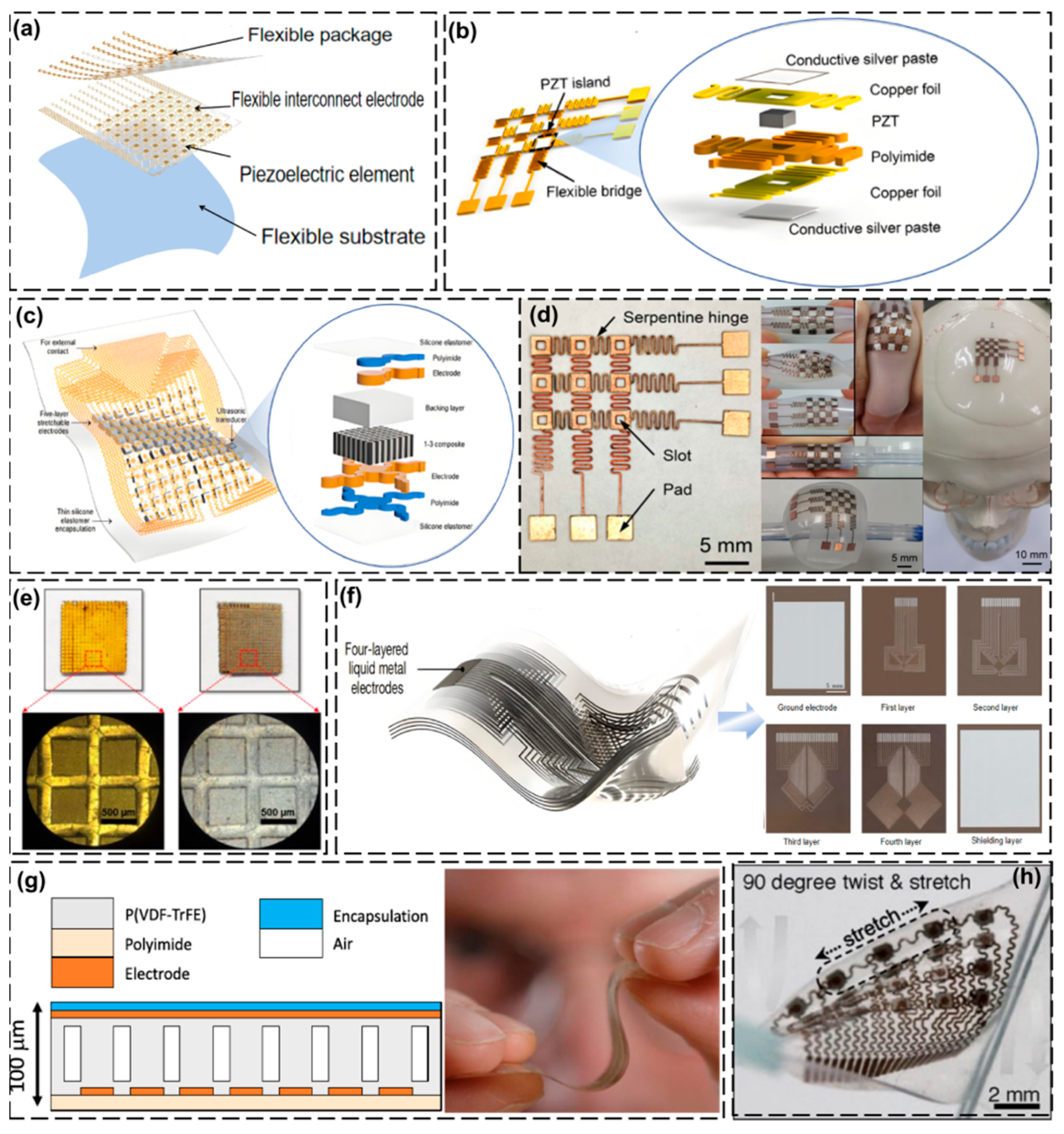
3. Design and Fabrication of Wearable Ultrasonic Transducers
3.1. Piezoelectric Elements
3.2. Interconnected Electrodes
3.3. Flexible Substrate
3.4. Flexible Packaging
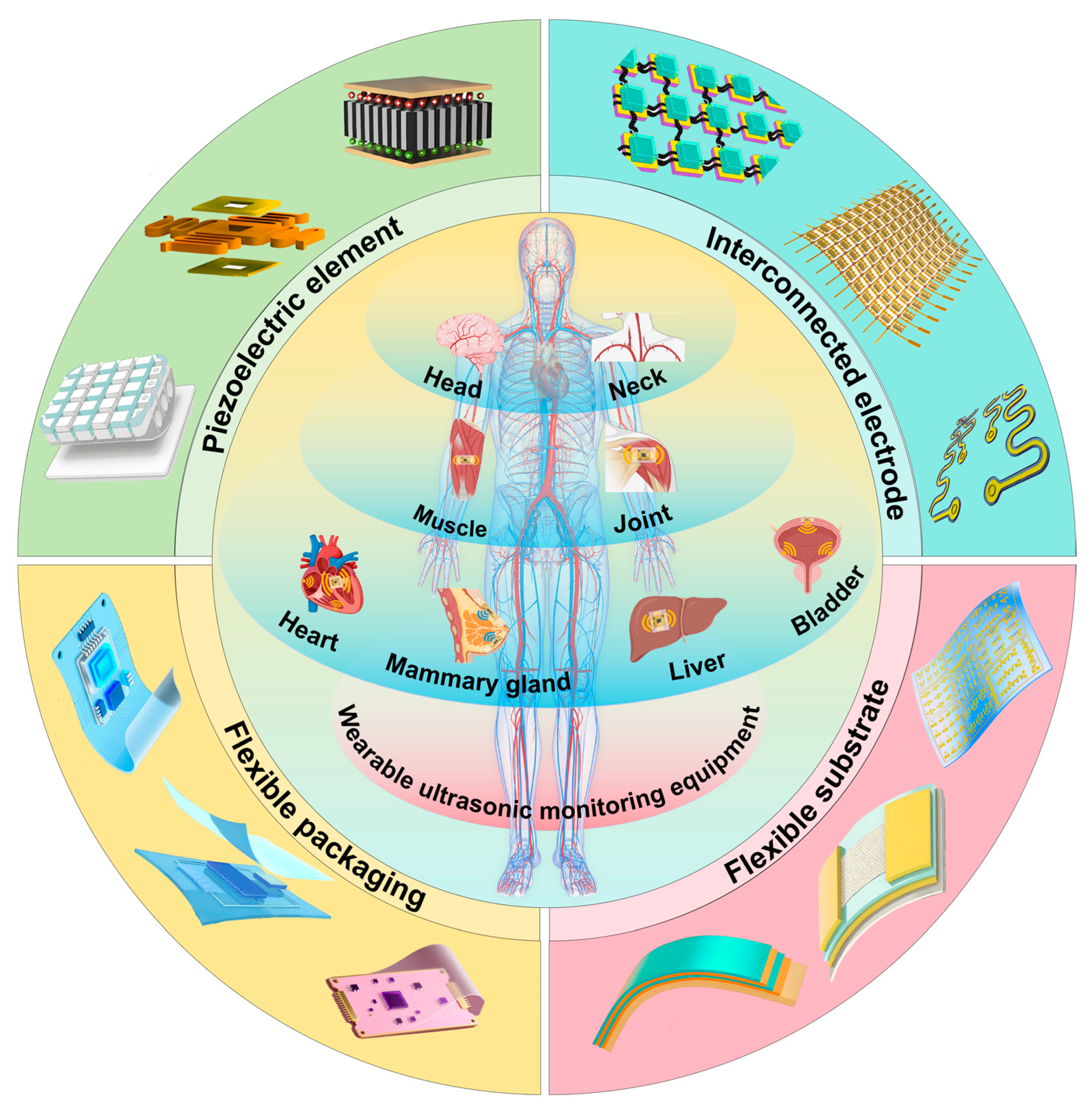
4. Application of Wearable Ultrasonic Monitoring Devices
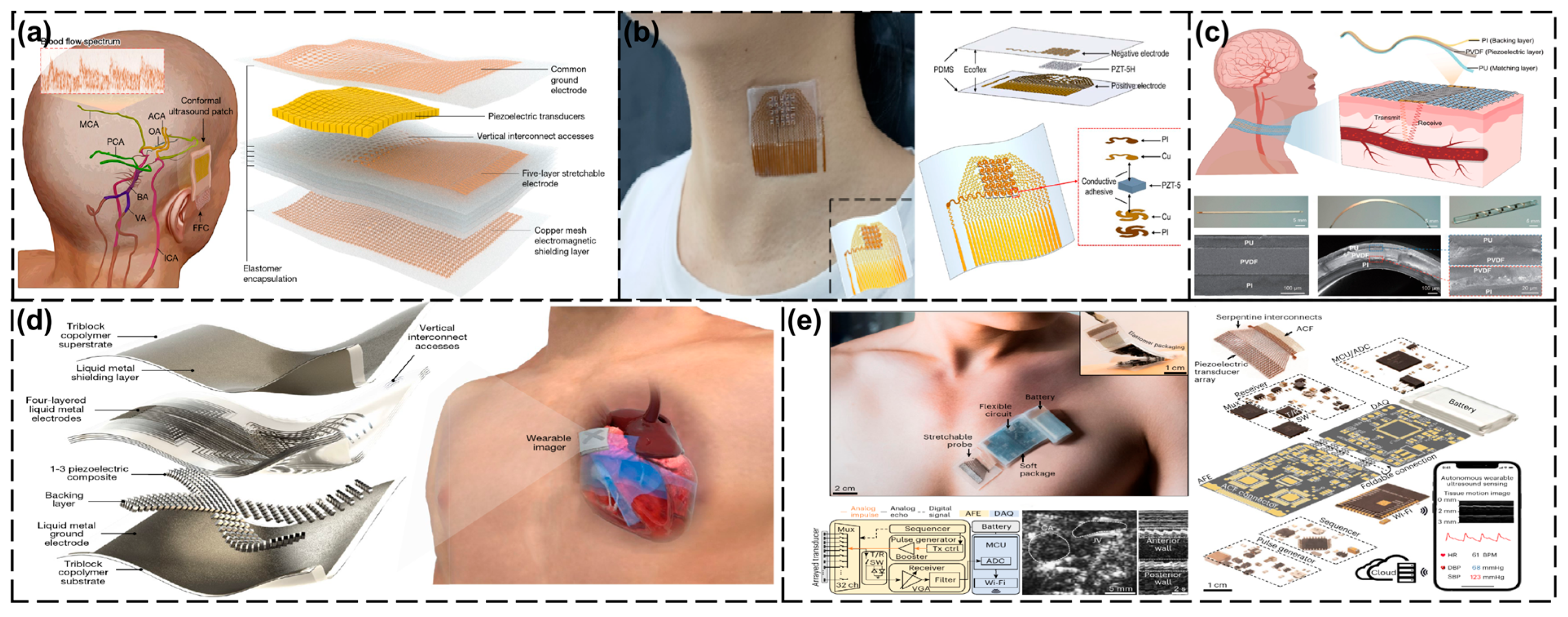
4.1. Head and Neck Area
4.2. Thoracic Cavity
4.3. Abdominal Cavity
4.4. Limbs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, N.M.; Dey, N.R.; Mia, N.M.T. Artificial Intelligence in telemedicine and remote patient monitoring: Enhancing virtual healthcare through AI-driven diagnostic and predictive technologies. Int. J. Sci. Res. Arch. 2025, 15, 1046–1055. [Google Scholar] [CrossRef]
- Kupunarapu, S.K. AI-Enabled Remote Monitoring and Telemedicine: Redefining patient engagement and care delivery. EPH-Int. J. Sci. Eng. 2016, 2, 41–48. [Google Scholar] [CrossRef]
- Rahman, A.; Kafy, A.; Kabir, J.F.; Pranto, M.T.A.; Akther, A.; Choudhury, I.A. Review of developments in sensor technology for monitoring of health-related conditions. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 240–256. [Google Scholar] [CrossRef]
- Rukundo, S.K. The impact of wearable technology on health monitoring. Res. Invent. J. Public Health Pharm. 2024, 3, 5–8. [Google Scholar] [CrossRef]
- Alegavi, S.S.; Nemade, B.; Bharadi, V.; Gupta, S.; Singh, V.; Belge, A. Revolutionizing Healthcare through Health Monitoring Applications with Wearable Biomedical Devices. Int. J. Recent Innov. Trends Comput. Commun. 2023, 11, 752–766. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Kim, J.; Yu, C.; Dagdeviren, C. An emerging era: Conformable ultrasound electronics. Adv. Mater. 2023, 36, e2307664. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, J. Emerging ultrasonic bioelectronics for personalized healthcare. Prog. Mater. Sci. 2023, 136, 101110. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Gao, X.; Damjanovic, D.; Chen, L.-Q.; Zhang, S. Ferroelectric materials toward next-generation electromechanical technologies. Science 2025, 389, eadn4926. [Google Scholar] [CrossRef]
- Addington, W.R.; Stephens, R.E.; Gilliland, K.A. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Stroke 1999, 30, 1203–1207. [Google Scholar] [CrossRef]
- Charan, G.S.; Khurana, M.S.; Kalia, R. Wearable Technology: How Healthcare Is Changing Forever. J. Chitwan Med. Coll. 2023, 13, 111–113. [Google Scholar] [CrossRef]
- Jiang, X.; Ng, W.T.; Chen, J. A miniaturized Low-Intensity ultrasound device for wearable medical therapeutic applications. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1372–1382. [Google Scholar] [CrossRef]
- Luo, X.; Tan, H.; Wen, W. Recent advances in wearable healthcare devices: From material to application. Bioengineering 2024, 11, 358. [Google Scholar] [CrossRef]
- Huang, H.; Wu, R.S.; Lin, M.; Xu, S. Emerging wearable ultrasound technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 71, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Ottakath, N.; Al-Maadeed, S.; Bouridane, A.; Chowdhury, M.E.H.; Sadasivuni, K.K. Wearable Ultrasound Devices for Continuous Health Monitoring: Current and Future Prospects. In Proceedings of the 2024 IEEE 8th Energy Conference, Doha, Qatar, 4–7 March 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Saha, T.; Ding, S.; Sandhu, S.S.; Chang, A.-Y.; Wang, J. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 2024, 7, 735–750. [Google Scholar] [CrossRef]
- Michard, F.; Wong, A.; Edul, V.K. Visualizing hemodynamics: Innovative graphical displays and imaging techniques in anesthesia and critical care. Crit. Care 2025, 29, 3. [Google Scholar] [CrossRef]
- Li, J.; Chu, H.; Chen, Z.; Yiu, C.K.; Qu, Q.; Li, Z.; Yu, X. Recent advances in materials, devices and algorithms toward wearable continuous blood pressure monitoring. ACS Nano 2024, 18, 17407–17438. [Google Scholar] [CrossRef]
- Sun, W.; Guo, Z.; Yang, Z.; Wu, Y.; Lan, W.; Liao, Y.; Wu, X.; Liu, Y. A review of recent advances in vital signals monitoring of sports and health via flexible wearable sensors. Sensors 2022, 22, 7784. [Google Scholar] [CrossRef]
- Quarato, C.M.I.; Lacedonia, D.; Salvemini, M.; Tuccari, G.; Mastrodonato, G.; Villani, R.; Fiore, L.A.; Scioscia, G.; Mirijello, A.; Saponara, A.; et al. A review on Biological Effects of Ultrasounds: Key Messages for Clinicians. Diagnostics 2023, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Bamber, J.C. Ultrasonic properties of tissues. In Ultrasound in Medicine; IOP Publishing Ltd.: Bristol, UK, 2004. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Chen, S.; Liu, Y.; Ta, D. Unveiling the potential of ultrasound in brain imaging: Innovations, challenges, and prospects. Ultrasonics 2024, 145, 107465. [Google Scholar] [CrossRef]
- Martin, D.J.; Wells, I.T.P.; Goodwin, C.R. Physics of ultrasound. Anaesth. Intensive Care Med. 2015, 16, 132–135. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, L.; Zhang, H.; Mei, Y.; Xiong, Z.; Song, E. Soft wearable electronics for evaluation of biological tissue mechanics. Soft Sci. 2024, 4, 36. [Google Scholar] [CrossRef]
- Lin, M.; Hu, H.; Zhou, S.; Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 2022, 7, 850–869. [Google Scholar] [CrossRef]
- Fenster, A.; Lacefield, J.C. Ultrasound Imaging and Therapy; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Farooq, T.; Ali, F.; Fang, H. Discrimination between different animal blood cells based on Doppler-Shifted multispectral photoacoustic signals. J. Phys. Conf. Ser. 2025, 2966, 012018. [Google Scholar] [CrossRef]
- Bulwer, B.E.; Soliman-Aboumarie, H. Fundamentals of spectral Doppler interpretation. In Clinical Applications of Echo Doppler Haemodynamics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 3–14. [Google Scholar] [CrossRef]
- Wang, F.; Jin, P.; Feng, Y.; Fu, J.; Wang, P.; Liu, X.; Zhang, Y.; Ma, Y.; Yang, Y.; Yang, A.; et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 2021, 7, eabi9283. [Google Scholar] [CrossRef]
- Meola, M.; Ibeas, J.; Lasalle, G.; Petrucci, I. Basics for performing a high-quality color Doppler sonography of the vascular access. J. Vasc. Access 2021, 22 (Suppl. S1), 18–31. [Google Scholar] [CrossRef]
- Bashein, G.; Detmer, P.R. Principles of doppler ultrasound. In Doppler in Obstetrics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–30. [Google Scholar] [CrossRef]
- Cheng, C.; Hu, C.; Zhou, S.; Zhao, H.; Yu, M. Qualitative diagnosis of solid breast mass by blood flow in solid breast mass. J. Med. Imaging Health Inform. 2021, 11, 1887–1894. [Google Scholar] [CrossRef]
- Pei, S.; Feng, Y.; Fang, S.; Jin, S.; Fan, D.; Song, F.; Wang, H. Endoleak Detection after Endovascular Aortic Aneurysm Repair Using Ultrasound Based on Nanoscale Bubble Contrast Agents and Their Effects on Vascular Smooth Muscle Cell Proliferation and Migration. J. Nanomater. 2021, 2021, 8298994. [Google Scholar] [CrossRef]
- Mandel, J.; Brun, L. Mechanical Waves in Solids; International Centre for Mechanical Sciences: Udine, Italy, 1975. [Google Scholar] [CrossRef]
- Gandomkar, M.; Soorgee, M.; Habibi, H.; Kafash, M.M. Advancing osteoporosis assessment through a numerical study utilizing ultrasonic waves in femur bone evaluation. J. Ultrasound Med. 2025, 44, 991–1006. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Point-of-Care ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, O.V. Nonlinear Acoustics in Medicine: A review. Phys. Wave Phenom. 2022, 30, 73–85. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ultrasonic estimation of soft tissue visco elastic properties. arXiv 2022, arXiv:2210.02446. [Google Scholar] [CrossRef]
- McCafferty, J. How does ultrasound work? In Point of Care Ultrasound Made Easy; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–14. [Google Scholar] [CrossRef]
- Evans, D.H. Transcranial doppler Ultrasound: Physical principles. In Neurosonology in Critical Care; Springer: Berlin/Heidelberg, Germany, 2021; pp. 99–116. [Google Scholar] [CrossRef]
- Widder, B.; Hamann, G.F. Basics of ultrasound technology. In Duplex Sonography of the Brain-Supplying Arteries; Springer: Berlin/Heidelberg, Germany, 2022; pp. 29–40. [Google Scholar] [CrossRef]
- Hausman, J.; Pollock, G. Basic science: Principles of ultrasound: Obtaining an image, resolution, depth, frequency, resonance. In Anesthesiology In-Training Exam Review; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–8. [Google Scholar] [CrossRef]
- Zatta, R.; Jain, R.; Grzyb, J.; Pfeiffer, U.R. Resolution limits in Lens-Integrated CMOS THZ cameras employing Super-Resolution imaging. In Proceedings of the 2022 47th International Conference on Infrared, Millimeter and Terahertz Waves (IRMMW-THz), Paris, France, 1–6 September 2019. [Google Scholar] [CrossRef]
- Tran, T.N.H.T.; Le, L.H.; Li, B.; Li, Y.; Nguyen, V.-H.; Ta, D. Visualization of ultrasonic-guided-wave propagation behaviors in human long bone. Biomed. Signal Process. Control 2024, 95, 106335. [Google Scholar] [CrossRef]
- Sack, I. Magnetic resonance elastography from fundamental soft-tissue mechanics to diagnostic imaging. Nat. Rev. Phys. 2022, 5, 25–42. [Google Scholar] [CrossRef]
- Oglat, A.A.; Abukhalil, T. Ultrasound elastography: Methods, clinical applications, and limitations: A review article. Appl. Sci. 2024, 14, 4308. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Dong, Y.; Cui, X.-W.; Fink, M.; Jenssen, C.; Moeller, K.; Sandrin, L.; Tsuneyoshi, S.; Tanter, M. Ultrasound elastography: A brief clinical history of an evolving technique. Ultrasound Int. Open 2024, 10, a23786926. [Google Scholar] [CrossRef]
- Kim, D.-S.; Paltiel, H.J.; White, P.J.; Sassaroli, E. Ultrasound imaging techniques and artifacts. In Pediatric Ultrasound; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–49. [Google Scholar] [CrossRef]
- Bhargava, S.K. Textbook of Color Doppler Imaging; Jaypee Brothers Medical Publisher: New Delhi, India, 2010. [Google Scholar] [CrossRef]
- Najjar, R. Redefining Radiology: A review of Artificial intelligence integration in medical imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Teertam, S.K.; Li, H.; Zhang, Z. A comprehensive review of cardiovascular disease management: Cardiac biomarkers, imaging modalities, pharmacotherapy, surgical interventions, and herbal remedies. Cells 2024, 13, 1471. [Google Scholar] [CrossRef]
- Shariati, L.; Esmaeili, Y.; Rahimmanesh, I.; Babolmorad, S.; Ziaei, G.; Hasan, A.; Boshtam, M.; Makvandi, P. Advances in nanobased platforms for cardiovascular diseases: Early diagnosis, imaging, treatment, and tissue engineering. Environ. Res. 2023, 238, 116933. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent advancements in ultrasound transducer: From material strategies to biomedical applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef]
- La, T.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2021, 7, 2100798. [Google Scholar] [CrossRef]
- Aabid, A.; Parveez, B.; Raheman, M.A.; Ibrahim, Y.E.; Anjum, A.; Hrairi, M.; Parveen, N.; Zayan, J.M. A review of Piezoelectric Material-Based Structural Control and Health Monitoring Techniques for Engineering Structures: Challenges and Opportunities. Actuators 2021, 10, 101. [Google Scholar] [CrossRef]
- He, Y.; Wan, H.; Jiang, X.; Peng, C. Piezoelectric Micromachined Ultrasound Transducer Technology: Recent advances and applications. Biosensors 2022, 13, 55. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Tyler, D.; Wood, J.; Sabir, T.; McDonnell, C.; Sayem, A.S.M.; Whittaker, N. Wearable electronic textiles. Text. Prog. 2019, 51, 299–384. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Li, Z.; Dou, H.; Zhang, W.; Yang, J.; Wu, P.; Li, D.; Mu, X. Machine learning-assisted wearable sensing systems for speech recognition and interaction. Nat. Commun. 2025, 16, 2365. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Deen, G.R.; Bates, J.S.; Maiti, C.; Lam, C.Y.K.; Pachauri, A.; AlAnsari, R.; Bělský, P.; Yoon, J.; Dodda, J.M. Smart Skin-Adhesive Patches: From design to biomedical applications. Adv. Funct. Mater. 2023, 33, 2213560. [Google Scholar] [CrossRef]
- Wang, Y.; Haick, H.; Guo, S.; Wang, C.; Lee, S.; Yokota, T.; Someya, T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022, 51, 3759–3793. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Noghanian, S.; Khan, Z.U.; Alzahrani, S.; Alharbi, S.; Alhartomi, M.; Alsulami, R. Wearable and flexible sensor devices: Recent advances in designs, fabrication methods, and applications. Sensors 2025, 25, 1377. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Qiu, Y. Advanced Flexible Skin-Like Pressure and Strain sensors for human health monitoring. Micromachines 2021, 12, 695. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhong, C.; Zhang, Y.; Qiu, Y.; Qin, L. Flexible Electronics: Advancements and applications of flexible piezoelectric composites in modern sensing technologies. Micromachines 2024, 15, 982. [Google Scholar] [CrossRef]
- Zhang, X.; Chai, J.; Zhan, Y.; Cui, D.; Wang, X.; Gao, L. Design, Fabrication, and Application of Large-Area Flexible Pressure and Strain Sensor Arrays: A Review. Micromachines 2025, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.E.; Kim, B.H. Applications of flexible and stretchable three-dimensional structures for soft electronics. Soft Sci. 2023, 3, 16. [Google Scholar] [CrossRef]
- Aliqué, M.; Simão, C.D.; Murillo, G.; Moya, A. Fully-Printed piezoelectric devices for flexible electronics applications. Adv. Mater. Technol. 2021, 6, 2001020. [Google Scholar] [CrossRef]
- Li, W.; Ke, K.; Jia, J.; Pu, J.; Zhao, X.; Bao, R.; Liu, Z.; Bai, L.; Zhang, K.; Yang, M.; et al. Recent Advances in Multiresponsive Flexible Sensors towards E-skin: A Delicate Design for Versatile Sensing. Small 2021, 18, 2103734. [Google Scholar] [CrossRef]
- Ren, D.; Yin, Y.; Li, C.; Chen, R.; Shi, J. Recent advances in flexible ultrasonic transducers: From materials optimization to imaging applications. Micromachines 2023, 14, 126. [Google Scholar] [CrossRef]
- Xue, X.; Wu, H.; Cai, Q.; Chen, M.; Moon, S.; Huang, Z.; Kim, T.; Peng, C.; Feng, W.; Sharma, N.; et al. Flexible ultrasonic transducers for wearable biomedical applications: A review on advanced materials, structural designs, and future prospects. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 71, 786–810. [Google Scholar] [CrossRef]
- Roche, E.T. Catheters gain arrays of sensors and actuators. Nat. Biomed. Eng. 2020, 4, 939–940. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, C.; Wu, D. Flexible and stretchable ultrasonic transducer array conformed to complex surfaces. IEEE Electron Device Lett. 2020, 42, 240–243. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Wang, C.; Zhang, L.; Li, X.; Lee, S.; Huang, Z.; Chen, R.; Chen, Z.; Wang, C.; et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 2018, 4, eaar3979. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Cui, Z.; Chang, W.-Y.; Kim, H.; Zhu, Y.; Jiang, X. Flexible 1–3 composite ultrasound transducers with Silver-Nanowire-Based stretchable electrodes. IEEE Trans. Ind. Electron. 2019, 67, 6955–6962. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Li, M.; Gao, X.; Yin, L.; Qi, R.; Wu, R.S.; Chen, X.; Ma, Y.; Shi, K.; et al. A wearable cardiac ultrasound imager. Nature 2023, 613, 667–675. [Google Scholar] [CrossRef]
- Van Neer, P.L.M.J.; Peters, L.C.J.M.; Verbeek, R.G.F.A.; Peeters, B.; De Haas, G.; Hörchens, L.; Fillinger, L.; Schrama, T.; Merks-Swolfs, E.J.W.; Gijsbertse, K.; et al. Flexible large-area ultrasound arrays for medical applications made using embossed polymer structures. Nat. Commun. 2024, 15, 2802. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.; Tang, M.; Wang, Y.; Xu, Z.; Yan, Y. Superior temperature stability of electromechanical properties and resonant frequency in PYN-PZT piezoelectric ceramics. J. Eur. Ceram. Soc. 2023, 44, 873–881. [Google Scholar] [CrossRef]
- Yuan, X.; Mai, Z.; Li, H.; Gao, X.; Yan, A.; Jiang, D.; Wei, X.; Jiang, H.; Dong, S. Enhanced energy density in piezoelectric PVDF-polymer nanocomposite via multiple mechanism synergetic action. Nano Energy 2024, 127, 109734. [Google Scholar] [CrossRef]
- Haider, S.T.; Shah, M.A.; Lee, D.-G.; Hur, S. A review of the recent applications of aluminum Nitride-Based Piezoelectric devices. IEEE Access 2023, 11, 58779–58795. [Google Scholar] [CrossRef]
- Fraga, M.A.; Massi, M.; Pessoa, R.S. Sputtered piezoelectric films as materials for flexible devices and sensors: A review. IEEE Sens. J. 2023, 24, 28–39. [Google Scholar] [CrossRef]
- Song, Z.; Hou, R.; Jiang, F. Recent progress in piezoelectric thin films as self-powered devices: Material and application. Front. Mater. 2024, 11, 1373040. [Google Scholar] [CrossRef]
- Nagels, S.; Deferme, W. Fabrication Approaches to interconnect based Devices for Stretchable Electronics: A review. Materials 2018, 11, 375. [Google Scholar] [CrossRef]
- Jafari, A. Stretchable Electronics: Advances in elastic conductive fibers for multifunctional applications. Org. Electron. 2024, 135, 107145. [Google Scholar] [CrossRef]
- Han, Z.; Hong, Y.; Zhu, X.; Gu, X.; Sun, Y. Electrical performance optimization of serpentine interconnect for stretchable electronics. J. Phys. Conf. Ser. 2022, 2342, 012005. [Google Scholar] [CrossRef]
- Cheng, M.; Tian, K.; Qin, T.; Li, Q.; Deng, H.; Fu, Q. Recent development on the design, preparation, and application of stretchable conductors for flexible energy harvest and storage devices. SusMat 2024, 4, e204. [Google Scholar] [CrossRef]
- Nasikhudin, N.; Fath, Y.A.; Istiqomah, I.; Rahmadani, H.; Diantoro, M.; Pujiarti, H. Silver Nanowires (AGNWs) Post-Treatment Effect in Application of flexible Transparent and Conductive Electrodes: A mini review. Mater. Sci. Forum 2024, 1118, 47–57. [Google Scholar] [CrossRef]
- Wang, P.-F.; Hu, Q.; Lv, B.; Zhu, J.-L.; Ma, W.; Dong, Z.; Wei, J.; Sun, J.-L. Facile fabrication of eutectic gallium-indium alloy nanostructure and application in photodetection. Nanotechnology 2019, 31, 145703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Soni, S.; Lee, T.; Nijhuis, C.A.; Xiang, D. Smart Eutectic Gallium–Indium: From Properties to Applications. Adv. Mater. 2022, 35, e2203391. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, L.; Hu, H.; Zhang, R. A review on polymers and their composites for flexible electronics. Mater. Adv. 2022, 4, 726–746. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Wang, R.; Zhang, J.; Lu, Y.; Hu, G.; Wang, Z.; Zhang, L. Current trends in bio-based elastomer materials. SusMat 2022, 2, 2–33. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Bu, X.; Wang, S.; Zhang, P. Flexible strain sensor based on PU film with three-dimensional porous network. Sens. Actuators A Phys. 2023, 359, 114508. [Google Scholar] [CrossRef]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2018, 31, e1807101. [Google Scholar] [CrossRef]
- Neves, L.B.; Afonso, I.S.; Nobrega, G.; Barbosa, L.G.; Lima, R.A.; Ribeiro, J.E. A review of methods to modify the PDMS surface wettability and their applications. Micromachines 2024, 15, 670. [Google Scholar] [CrossRef]
- De Menezes Atayde, C.; Doi, I. Highly stable hydrophilic surfaces of PDMS thin layer obtained by UV radiation and oxygen plasma treatments. Phys. Status Solidi. C Curr. Top. Solid State Phys. 2009, 7, 189–192. [Google Scholar] [CrossRef]
- Yu, K.; He, T. Silver-Nanowire-Based Elastic Conductors: Preparation processes and substrate adhesion. Polymers 2023, 15, 1545. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wu, Z. Flexible Substrates. In Flexible Electronic Packaging and Encapsulation Technology; Wiley Online Books: Hoboken, NJ, USA, 2024; pp. 77–122. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Wang, W.; Gong, X.; Zhao, Y.; Mu, Q.; Xue, Z.; Liu, X.; Zheng, H.; Xu, W. Recent advances in gel materials with special wettability: A review. J. Mater. Sci. 2022, 57, 13179–13201. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and challenges in conductive hydrogels: From properties to applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Lin, Z.; Duan, S.; Liu, M.; Dang, C.; Qian, S.; Zhang, L.; Wang, H.; Yan, W.; Zhu, M. Insights into Materials, Physics, and Applications in Flexible and Wearable Acoustic Sensing Technology. Adv. Mater. 2023, 36, e2306880. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Zheng, E.; Zhou, Q.; Doyley, M.; Xia, J. Dual-modal photoacoustic and ultrasound imaging: From preclinical to clinical applications. Front. Photonics 2024, 5, 1359784. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Qin, H.; Peng, C. High-Frequency 64-Element Ring-Annular Array Transducer: Development and preclinical validation for intravascular ultrasound imaging. Biosensors 2025, 15, 169. [Google Scholar] [CrossRef]
- Nong, H.; Jin, M.; Pan, C.; Zhou, H.; Zhang, C.; Pan, X.; Chen, Y.; Wei, X.; Lu, Y.; Zhao, K.; et al. Intelligent Sensing Technologies Based on flexible Wearable Sensors: A review. IEEE Sens. J. 2024, 24, 22197–22217. [Google Scholar] [CrossRef]
- Mohammadpourfazeli, S.; Arash, S.; Ansari, A.; Yang, S.; Mallick, K.; Bagherzadeh, R. Future prospects and recent developments of polyvinylidene fluoride (PVDF) piezoelectric polymer; fabrication methods, structure, and electro-mechanical properties. RSC Adv. 2023, 13, 370–387. [Google Scholar] [CrossRef]
- Ahbab, N.; Naz, S.; Xu, T.-B.; Zhang, S. A comprehensive review of Piezoelectric PVDF Polymer fabrications and characteristics. Micromachines 2025, 16, 386. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Bih, L.-I.; Chen, S.-L.; Tsai, S.-J.; Teng, C.-H. The accuracy of ultrasonic estimation of bladder volume: A comparison of portable and stationary equipment1. Arch. Phys. Med. Rehabil. 2003, 85, 138–141. [Google Scholar] [CrossRef]
- Nivedhitha, D.M.; Jeyanthi, S. Polyvinylidene fluoride, an advanced futuristic smart polymer material: A comprehensive review. Polym. Adv. Technol. 2022, 34, 474–505. [Google Scholar] [CrossRef]
- Xie, L.; Wang, G.; Jiang, C.; Yu, F.; Zhao, X. Properties and applications of flexible Poly(Vinylidene Fluoride)-Based piezoelectric materials. Crystals 2021, 11, 644. [Google Scholar] [CrossRef]
- Yuan, H.; Lei, T.; Qin, Y.; Yang, R. Flexible electronic skins based on piezoelectric nanogenerators and piezotronics. Nano Energy 2019, 59, 84–90. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Y.; Xu, F.; Duan, Y.; Yin, Z. Energy harvesters for wearable and stretchable electronics: From flexibility to stretchability. Adv. Mater. 2016, 28, 9881–9919. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible Electronics: Stretchable electrodes and their future. Adv. Funct. Mater. 2018, 29, 1805924. [Google Scholar] [CrossRef]
- Chen, S.; Cui, Z.; Wang, H.; Wang, X.; Liu, J. Liquid metal flexible electronics: Past, present, and future. Appl. Phys. Rev. 2023, 10, 021308. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Xu, Z.; Guo, J.; Tang, S.-Y.; Ma, X. Recent advancements in liquid metal enabled flexible and wearable biosensors. Soft Sci. 2023, 3, 37. [Google Scholar] [CrossRef]
- Xia, Y.; Song, D.; Zhang, M.; Wang, Z.; Shi, C.; Du, J.S.; Shin, S.H.R.; Engelhard, M.H.; Thallapally, P.K.; Orme, C.A.; et al. Achieving electrode smoothing by controlling the nucleation phase of metal deposition through polymer-substrate binding. arXiv 2025, arXiv:2502.05809. [Google Scholar] [CrossRef]
- Bury, E.; Chun, S.; Koh, A.S. Recent Advances in Deformable Circuit Components with Liquid Metal. Adv. Electron. Mater. 2021, 7, 2001006. [Google Scholar] [CrossRef]
- Guo, S.; Huang, H. Design strategies for skin-interfaced sensors. Sens. Actuators A Phys. 2024, 376, 115671. [Google Scholar] [CrossRef]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated wearable systems and Implantable biosensors: A Comprehensive review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, C.; Guo, W.; Zhu, B.; Wang, S. Wearable ultrasound devices: An emerging era for biomedicine and clinical translation. Ultrasonics 2024, 142, 107401. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, J.; Han, M. Patient-centric care: Unveiling the potential of wearable electronics in clinical practice. Wearable Electron. 2024, 1, 119–136. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, X.; Park, G.; Yang, X.; Qi, B.; Lin, M.; Huang, H.; Bian, Y.; Hu, H.; Chen, X.; et al. Transcranial volumetric imaging using a conformal ultrasound patch. Nature 2024, 629, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, H.; Ren, J.; Cheng, H.; Xie, M.; Liu, Y. Design and optimization of flexible and stretchable ultrasonic transducer array for arterial blood pressure monitoring. IEEE Sens. J. 2024, 24, 15055–15064. [Google Scholar] [CrossRef]
- Zou, J.; Guo, X.; Wu, J.; Xu, D.; Xu, K.; Chen, P.; Ta, D.; Peng, H. A weavable and wearable polymer ultrasonic transducer with a large bandwidth. Sci. China Mater. 2024, 67, 2653–2660. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, Z.; Gao, X.; Bian, Y.; Wu, R.S.; Park, G.; Lou, Z.; Zhang, Z.; Xu, X.; Chen, X.; et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat. Biotechnol. 2023, 42, 448–457. [Google Scholar] [CrossRef]
- Du, W.; Zhang, L.; Suh, E.; Lin, D.; Marcus, C.; Ozkan, L.; Ahuja, A.; Fernandez, S.; Shuvo, I.I.; Sadat, D.; et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. 2023, 9, eadh5325. [Google Scholar] [CrossRef]
- Liu, H.-C.; Zeng, Y.; Gong, C.; Chen, X.; Kijanka, P.; Zhang, J.; Genyk, Y.; Tchelepi, H.; Wang, C.; Zhou, Q.; et al. Wearable bioadhesive ultrasound shear wave elastography. Sci. Adv. 2024, 10, eadk8426. [Google Scholar] [CrossRef]
- Toymus, A.T.; Yener, U.C.; Bardakci, E.; Temel, Ö.D.; Koseoglu, E.; Akcoren, D.; Eminoglu, B.; Ali, M.; Kilic, R.; Tarcan, T.; et al. An integrated and flexible ultrasonic device for continuous bladder volume monitoring. Nat. Commun. 2024, 15, 7216. [Google Scholar] [CrossRef]
- Zhang, L.; Marcus, C.; Lin, D.; Mejorado, D.; Schoen, S.J.; Pierce, T.T.; Kumar, V.; Fernandez, S.V.; Hunt, D.; Li, Q.; et al. A conformable phased-array ultrasound patch for bladder volume monitoring. Nat. Electron. 2023, 7, 77–90. [Google Scholar] [CrossRef]
- Zhou, S.; Park, G.; Longardner, K.; Lin, M.; Qi, B.; Yang, X.; Gao, X.; Huang, H.; Chen, X.; Bian, Y.; et al. Clinical validation of a wearable ultrasound sensor of blood pressure. Nat. Biomed. Eng. 2024, 9, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, C.; Qu, M.; Lv, D.; Liu, W.; Xie, J. Continuous and Non-Invasive monitoring of blood pressure based on wearable piezoelectric micromachined ultrasonic transducers array. J. Microelectromech. Syst. 2023, 32, 437–444. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chuang, H.-C.; Xu, G.-X.; Chen, C.; Su, W.-R.; Huang, C.-C. Wearable ultrasound imaging device for dynamic Dual-Direction Shear Wave elastography of shoulder muscle. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Alvarez, J.T.; Suitor, E.L.; Swaminathan, K.; Chin, A.; Civici, U.S.; Nuckols, R.W.; Howe, R.D.; Walsh, C.J. Estimation of joint torque in dynamic activities using wearable A-mode ultrasound. Nat. Commun. 2024, 15, 5756. [Google Scholar] [CrossRef] [PubMed]
- Musick, S.; Alberico, A. Neurologic assessment of the neurocritical care patient. Front. Neurol. 2021, 12, 588989. [Google Scholar] [CrossRef]
- Parati, G.; Bilo, G.; Kollias, A.; Pengo, M.; Ochoa, J.E.; Castiglioni, P.; Stergiou, G.S.; Mancia, G.; Asayama, K.; Asmar, R.; et al. Blood pressure variability: Methodological aspects, clinical relevance and practical indications for management—A European Society of Hypertension position paper *. J. Hypertens. 2023, 41, 527–544. [Google Scholar] [CrossRef]
- Faragli, A.; Abawi, D.; Quinn, C.; Cvetkovic, M.; Schlabs, T.; Tahirovic, E.; Düngen, H.-D.; Pieske, B.; Kelle, S.; Edelmann, F.; et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail. Rev. 2020, 26, 1063–1080. [Google Scholar] [CrossRef]
- Averkiou, M.A. Imaging techniques for ultrasound contrast agents. J. Acoust. Soc. Am. 2000, 108 (Suppl. S5), 2460. [Google Scholar] [CrossRef]
- Shingina, A.; Mukhtar, N.; Wakim-Fleming, J.; Alqahtani, S.; Wong, R.J.; Limketkai, B.N.; Larson, A.M.; Grant, L. Acute liver Failure Guidelines. Am. J. Gastroenterol. 2023, 118, 1128–1153. [Google Scholar] [CrossRef]
- Huang, J.; Chan, C.-K.; Yee, S.; Deng, Y.; Bai, Y.; Chan, S.C.; Tin, M.S.; Liu, X.; Lok, V.; Zhang, L.; et al. Global burden and temporal trends of lower urinary tract symptoms: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022, 26, 421–428. [Google Scholar] [CrossRef]
- Shimizu, N.; Saito, T.; Wada, N.; Hashimoto, M.; Shimizu, T.; Kwon, J.; Cho, K.J.; Saito, M.; Karnup, S.; De Groat, W.C.; et al. Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 7885. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Sierra, T.; Duenas-Garcia, O.F.; Kim, Y.; Leung, K.; Hall, C.; Flynn, M.K. Accuracy of bladder scanner for the assessment of postvoid residual volumes in women with pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 2018, 26, 640–643. [Google Scholar] [CrossRef]
- Al-Shaikh, G.; Larochelle, A.; Campbell, C.E.; Schachter, J.; Baker, K.; Pascali, D. Accuracy of bladder scanning in the assessment of postvoid residual volume. J. Obstet. Gynaecol. Can. 2009, 31, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Pilz, N.; Picone, D.S.; Patzak, A.; Opatz, O.S.; Lindner, T.; Fesseler, L.; Heinz, V.; Bothe, T.L. Cuff-based blood pressure measurement: Challenges and solutions. Blood Press. 2024, 33, 2402368. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and response to mechanical loading on the knee. Sports Med. 2021, 52, 201–235. [Google Scholar] [CrossRef] [PubMed]
| Component | Materials/Techniques | Advantages | Challenges/Limitations | Ref(s). |
|---|---|---|---|---|
| Piezoelectric Elements | Rigid materials: PZT ceramics | High piezoelectric coefficients (PZT) | Low piezoelectric constants (ZnO/AlN) | [78,79] |
| Flexible materials: ZnO/AlN thin films, PVDF copolymers | Flexibility (films/polymers) | PVDF has poor coupling | [80,81,82] | |
| Hybrid: PZT + serpentine hinges | Hybrid balances performance and flexibility | Array spacing trade-off (crosstalk vs. stretchability) | [83,84,85] | |
| Interconnect Electrodes | Solid metals (Ag paste, Cu) | High conductivity (metals) | Solid metals fracture under strain | [85,86] |
| Liquid metals (AgNWs, EGaIn) | Stretchability (liquid metals) | Liquid metals require complex processing | [87,88,89] | |
| Design: Serpentine “island-bridge” layout | Serpentine structure enhances ductility | [90,91] | ||
| Flexible Substrate | PDMS, Ecoflex, PU, hydrogels | Biocompatibility (PDMS) | PDMS has poor adhesion | [92,93,94,95] |
| Treatments: UV/plasma activation | Hydrogels offer bioadhesion | Hydrogels dehydrate over time | [94,95] | |
| Reinforcements: CNTs, graphene | High elasticity (Ecoflex/PU) | [96,97] | ||
| Flexible Encapsulation | Hydrogel–elastomer composites | Acoustic impedance matching | Conventional hydrogels harden when dry | [98,99] |
| Silicone elastomers | Long-term adhesion (48 h) | [100,101] | ||
| Acoustic impedance matching |
| Anatomical Region | Clinical Focus | Key Technology/Device | Advantages | Ref. |
|---|---|---|---|---|
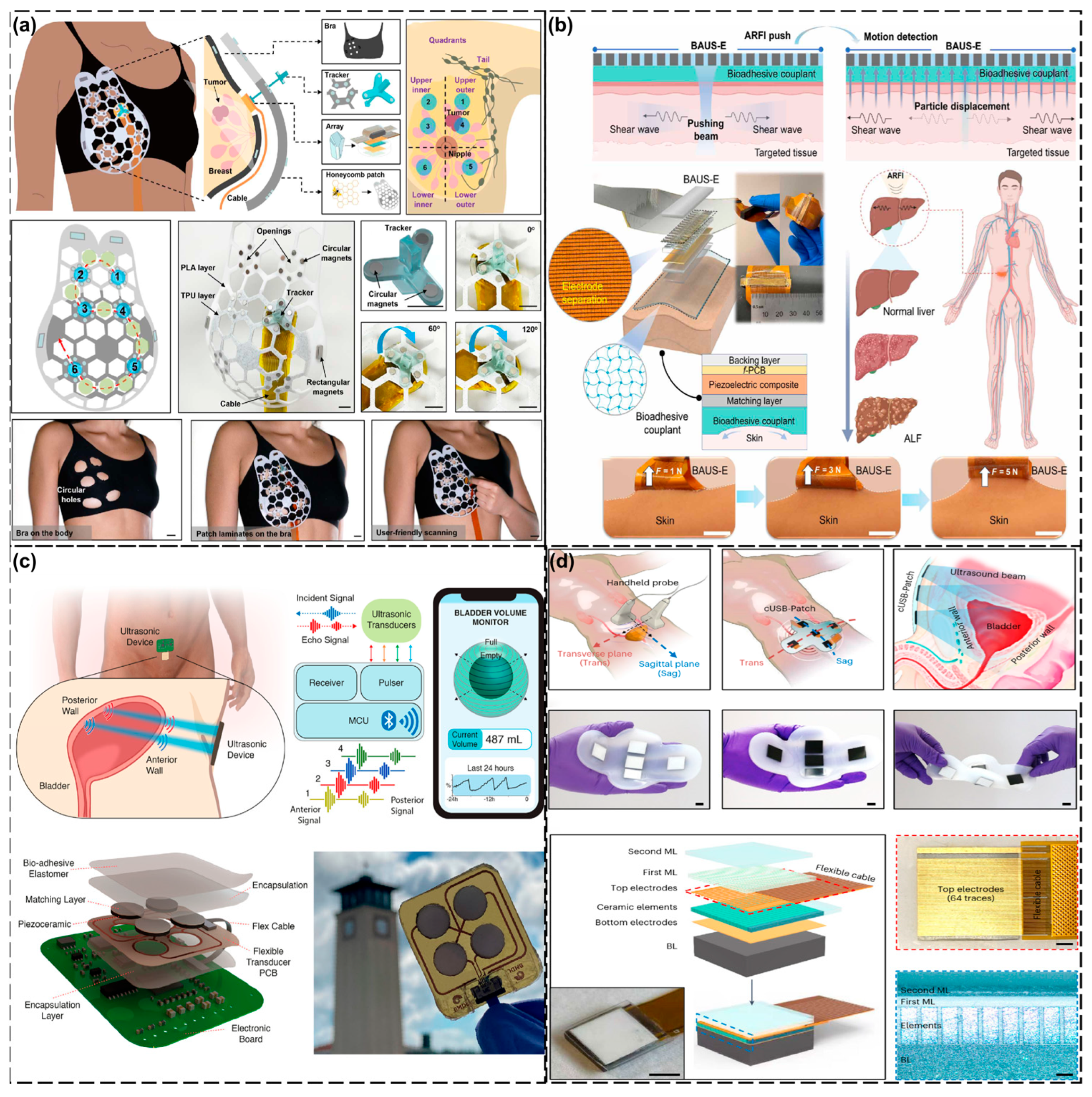
| Head and Neck | Cerebral hemodynamics | Low-frequency (2 MHz) ultrasound patch with copper mesh shielding | - Can penetrate cranial windows - SNR improvement of 5 dB - Motion-resistant long-term monitoring | [120] |
| Carotid blood pressure (ABP) | Flexible phased-array transducer (39 × 37 × 0.71 mm) | - Non-invasive, continuous monitoring - Matches invasive arterial catheter accuracy | [121] | |
| Broad tissue monitoring | Braidable polymer ultrasound transducer (PUT) with 93% bandwidth (3.68–10.08 MHz) | - Stable after 10,000 deformations - SNR comparable to commercial probes | [122] | |
| Thoracic Cavity | Cardiac function | Wearable cardiac ultrasound array + liquid metal electrodes + deep learning algorithms | - Real-time multi-angle LV imaging - Automated metrics (e.g., ejection fraction) | [75] |
| Exercise hemodynamics | Fully integrated system (USoP) with M-mode imaging | - Capable of 12-h monitoring during motion - Tracks carotid pulsations during head rotation | [123] | |
| Breast cancer screening | cUSBr-Patch with Yb/Bi-doped PIN-PMN-PT single-crystal phased array | - Wider field of view (30 mm depth) - Operator-independent imaging | [124] | |
| Abdominal Cavity | Acute Liver Failure (ALF) | Bioadhesive Ultrasound Shear Elastography (BAUS-E) | - Real-time liver stiffness tracking - Non-invasive disease progression monitoring | [125] |
| Bladder volume (LUTD) | UBVM system with multipoint reflectance + spherical fitting algorithms | - Average error of 11.17% - Wireless continuous monitoring | [126] | |
| cUSB-Patch (5-array design) | - Multi-angle imaging without probe movement - Error: 3.2–10.8% | [127] | ||
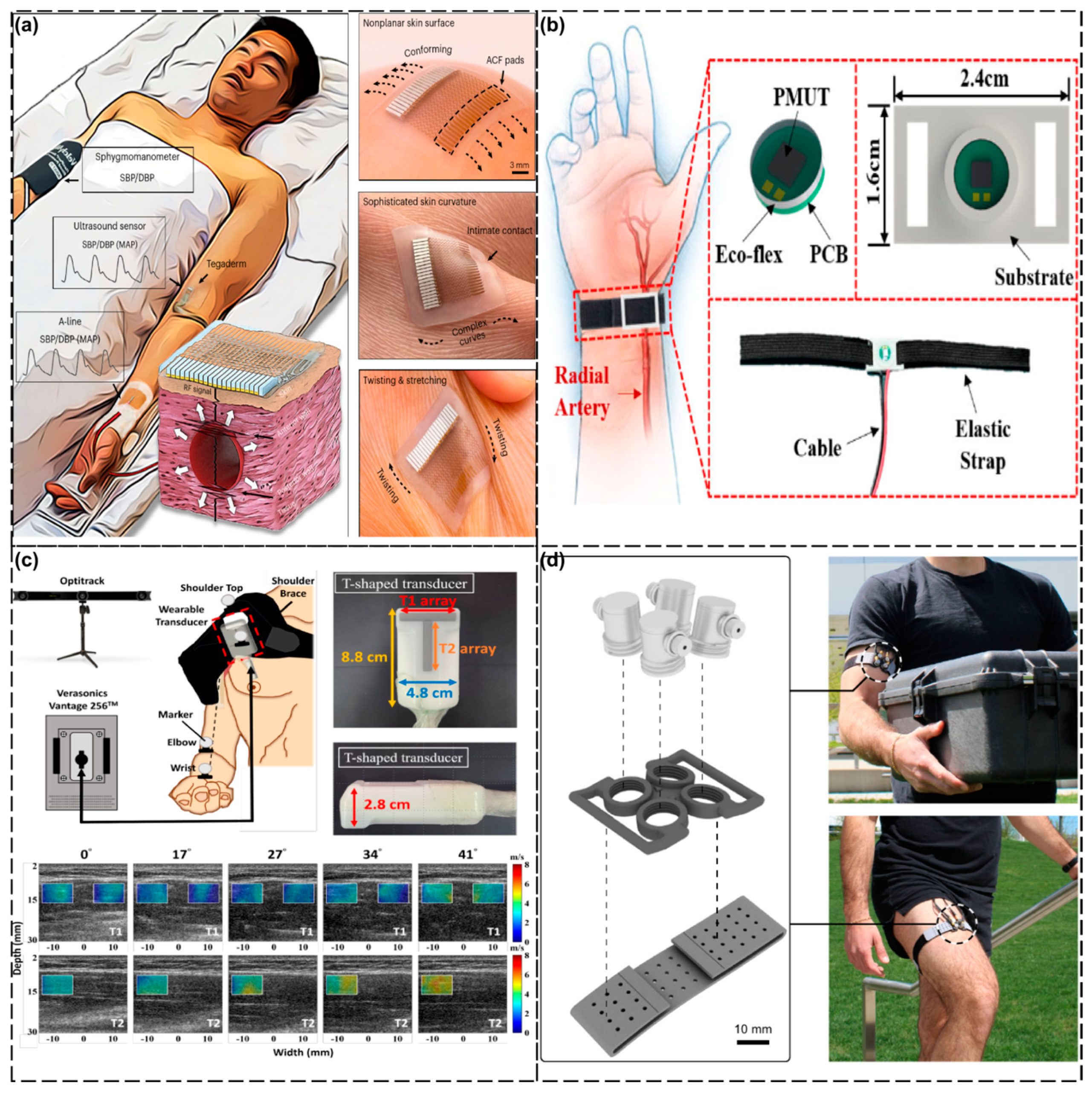
| Limbs | Continuous blood pressure | Ultra-thin flexible transducer (800 μm) | - Curved-surface conformability - Sterilization-resistant, long-term wear | [128] |
| Shoulder muscle injuries | AlN PMUT array (23 × 26) | - Accuracy: ±5 mmHg - Dynamic condition stability (e.g., hand movement) | [129] | |
| Joint torque quantification | Dual-directional shear wave elastography (DDSWE) | - Measures longitudinal/transverse SWVs during motion | [130] | |
| Wearable A-mode ultrasound system | - Torque error of <7.6% during dynamic activities (R2 > 0.92) | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Duan, J.; Qi, Q.; Fang, J.; Liu, Q.; Zhou, S.; Wu, Y. The Design and Application of Wearable Ultrasound Devices for Detection and Imaging. Biosensors 2025, 15, 561. https://doi.org/10.3390/bios15090561
Lei Y, Duan J, Qi Q, Fang J, Liu Q, Zhou S, Wu Y. The Design and Application of Wearable Ultrasound Devices for Detection and Imaging. Biosensors. 2025; 15(9):561. https://doi.org/10.3390/bios15090561
Chicago/Turabian StyleLei, Yuning, Jinjie Duan, Qi Qi, Jie Fang, Qian Liu, Shuang Zhou, and Yuxiang Wu. 2025. "The Design and Application of Wearable Ultrasound Devices for Detection and Imaging" Biosensors 15, no. 9: 561. https://doi.org/10.3390/bios15090561
APA StyleLei, Y., Duan, J., Qi, Q., Fang, J., Liu, Q., Zhou, S., & Wu, Y. (2025). The Design and Application of Wearable Ultrasound Devices for Detection and Imaging. Biosensors, 15(9), 561. https://doi.org/10.3390/bios15090561





