Progress in Nickel MOF-Based Materials for Electrochemical Biosensor and Supercapacitor Applications
Abstract
1. Introduction
2. Ni-MOF in Electrochemical Sensing Application
2.1. Ni-MOF and Ni-MOF/Metal Oxide for Electrochemical Sensors
2.2. Ni-MOF/GO, rGO, CNT, and gCN Materials for Electrochemical Sensors
2.3. Bimetallic Ni-Based MOFs for Electrochemical Sensing Application
2.4. Other Ni-MOF-Based Materials
3. Ni-MOF in Supercapacitor Application
3.1. Ni-MOF and Ni-MOF/Metal Oxides
3.2. Ni-MOF/Carbon-Based Materials
3.3. Ni-MOF/MXene/Polymers
3.4. Ni-MOF/LDH Materials
3.5. Bimetallic and Trimetallic Ni-MOF-Based Materials
4. Conclusions, Challenges, and Future Trends/Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Shafique, M.S.; Chapa, S.O.M.; Madou, M.J. Recent Advances in MOF-Based Materials for Biosensing Applications. Sensors 2025, 25, 2473. [Google Scholar] [CrossRef]
- Dhanabalan, K.; Perumalsamy, M.; Sriram, G.; Murugan, N.; Shalu; Sadhasivam, T.; Oh, T.H. Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives. Energies 2023, 16, 4950. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal–Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.H. Implementing Metal–Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef]
- Ryoo, G.; Kim, S.K.; Lee, D.K.; Kim, Y.-J.; Han, Y.S.; Jung, K.-H. Energy Storage Performance of Electrode Materials Derived from Manganese Metal–Organic Frameworks. Nanomaterials 2024, 14, 503. [Google Scholar] [CrossRef]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal–Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef]
- Mosca, L.P.L.; Gapan, A.B.; Angeles, R.A.; Lopez, E.C.R. Stability of Metal–Organic Frameworks: Recent Advances and Future Trends. Eng. Proc. 2023, 56, 146. [Google Scholar]
- Laeim, H.; Molahalli, V.; Prajongthat, P.; Pattanaporkratana, A.; Pathak, G.; Phettong, B.; Hongkarnjanakul, N.; Chattham, N. Porosity Tunable Metal–Organic Framework (MOF)-Based Composites for Energy Storage Applications: Recent Progress. Polymers 2025, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, W.; Yin, Y.; Wang, N.; Wu, W.; Zhang, W.; Pei, S.; Liu, T.; Tao, C.; Zheng, B.; et al. Catalytic Modification of Porous Two-Dimensional Ni-MOFs on Portable Electrochemical Paper-Based Sensors for Glucose and Hydrogen Peroxide Detection. Biosensors 2023, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Yang, P.; Zhang, Y.; Fei, J.; Xie, Y. Electrochemical Behavior of β-Cyclodextrin-Ni-MOF-74/Reduced Graphene Oxide Sensors for the Ultrasensitive Detection of Rutin. Molecules 2023, 28, 4604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Yan, R.; Lei, C. Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose. Chemosensors 2024, 12, 39. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, Q.; Hu, P.; Ji, L. Tunable Non-Enzymatic Glucose Electrochemical Sensing Based on the Ni/Co Bimetallic MOFs. Molecules 2023, 28, 5649. [Google Scholar] [CrossRef]
- Dourandish, Z.; Sheikhshoaie, I.; Maghsoudi, S. Molybdenum Disulfide/Nickel-Metal Organic Framework Hybrid Nanosheets-Based Disposable Electrochemical Sensor for Determination of 4-Aminophenol in Presence of Acetaminophen. Biosensors 2023, 13, 524. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, Z.; Zong, Y.; Li, C.; Guo, W.; Guo, Y.; Zou, X. Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide. Biosensors 2024, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhong, Y.; Cai, S.; Lei, L.; Peng, J. Does Air Pollution Aggravate Health Problems in Low-Income Countries? Verification from Countries Along the Belt and Road. Sustainability 2025, 17, 1796. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Wen, J. Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability 2023, 15, 6686. [Google Scholar] [CrossRef]
- Alsalme, A.; Alsaeedi, H. Fabrication of Selective and Sensitive Hydrazine Sensor Using Sol–Gel Synthesized MoSe2 as Efficient Electrode Modifier. Crystals 2023, 13, 161. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Liu, B.; Zhang, H. Hydrogen Peroxide and Dopamine Sensors Based on Electrodeposition of Reduced Graphene Oxide/Silver Nanoparticles. Sensors 2024, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, X.; Wang, C.; Li, Z.; Li, D. A Novel Highly Sensitive Electrochemical Nitrite Sensor Based on a AuNPs/CS/Ti3C2 Nanocomposite. Nanomaterials 2022, 12, 397. [Google Scholar] [CrossRef]
- Venegas, C.J.; Bollo, S.; Sierra-Rosales, P. Carbon-Based Electrochemical (Bio)Sensors for the Detection of Carbendazim: A Review. Micromachines 2023, 14, 1752. [Google Scholar] [CrossRef]
- Ho, C.K.; Robinson, A.; Miller, D.R.; Davis, M.J. Overview of Sensors and Needs for Environmental Monitoring. Sensors 2005, 5, 4–37. [Google Scholar] [CrossRef]
- Nalakurthi, N.V.S.R.; Abimbola, I.; Ahmed, T.; Anton, I.; Riaz, K.; Ibrahim, Q.; Banerjee, A.; Tiwari, A.; Gharbia, S. Challenges and Opportunities in Calibrating Low-Cost Environmental Sensors. Sensors 2024, 24, 3650. [Google Scholar] [CrossRef]
- Islam, M.S.; Sazawa, K.; Sugawara, K.; Kuramitz, H. Electrochemical Biosensor for Evaluation of Environmental Pollutants Toxicity. Environments 2023, 10, 63. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Dinu Iacob, A.; Iticescu, C.; Georgescu, P.L. Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Contaminants in Natural Waters—A Comprehensive Review. Chemosensors 2025, 13, 65. [Google Scholar] [CrossRef]
- Hernandez-Vargas, G.; Sosa-Hernández, J.E.; Saldarriaga-Hernandez, S.; Villalba-Rodríguez, A.M.; Parra-Saldivar, R.; Iqbal, H.M.N. Electrochemical Biosensors: A Solution to Pollution Detection with Reference to Environmental Contaminants. Biosensors 2018, 8, 29. [Google Scholar] [CrossRef]
- Gupta, P.K.; Siegenthaler, J.R. Revolutionizing Electrochemical Sensing with Nanomaterial-Modified Boron-Doped Diamond Electrodes. Chemosensors 2025, 13, 183. [Google Scholar] [CrossRef]
- Ma, C.; Wen, Y.; Qiao, Y.; Shen, K.Z.; Yuan, H. A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application. Molecules 2024, 29, 2925. [Google Scholar] [CrossRef]
- Dey, K.; Santra, T.S.; Tseng, F.G. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Appl. Sci. 2025, 15, 2523. [Google Scholar] [CrossRef]
- Nagal, V.; Masrat, S.; Khan, M.; Alam, S.; Ahmad, A.; Alshammari, M.B.; Bhat, K.S.; Novikov, S.M.; Mishra, P.; Khosla, A.; et al. Highly Sensitive Electrochemical Non-Enzymatic Uric Acid Sensor Based on Cobalt Oxide Puffy Balls-like Nanostructure. Biosensors 2023, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Eskander, T.N.A.; Fathalla, M.; Zhukova, V.; Blanco, J.M.; Gonzalez, J.; Zhukov, A.; Abu-Dief, A.M. Empowering the Future: Cutting-Edge Developments in Supercapacitor Technology for Enhanced Energy Storage. Batteries 2025, 11, 232. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.K.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.U.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A Review of Supercapacitors: Materials Design, Modification, and Applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Subasinghage, K.; Gunawardane, K.; Padmawansa, N.; Kularatna, N.; Moradian, M. Modern Supercapacitors Technologies and Their Applicability in Mature Electrical Engineering Applications. Energies 2022, 15, 7752. [Google Scholar] [CrossRef]
- Mehra, P.; Saxena, S.; Bhullar, S. A Comprehensive Analysis of Supercapacitors and Their Equivalent Circuits—A Review. World Electr. Veh. J. 2024, 15, 332. [Google Scholar] [CrossRef]
- Sriram, G.; Kurkuri, M.; Oh, T.H. Recent Trends in Highly Porous Structured Carbon Electrodes for Supercapacitor Applications: A Review. Energies 2023, 16, 4641. [Google Scholar] [CrossRef]
- Argirusis, C.; Katsanou, M.-E.; Alizadeh, N.; Argirusis, N.; Sourkouni, G. Recent Advances in the Application of MOFs in Supercapacitors. Batteries 2025, 11, 181. [Google Scholar] [CrossRef]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Ding, X.; Zhu, H.; Wu, J. Mechanically Exfoliated Multilayer Graphene-Supported Ni-MOF Parallelogram Nanosheets for Enhanced Supercapacitor Performance. Nanomaterials 2025, 15, 643. [Google Scholar] [CrossRef]
- Li, Q.; Guo, H.; Xue, R.; Wang, M.; Xu, M.; Yang, W.; Zhang, J.; Yang, W. Self-Assembled Mo-Doped Ni-MOF Nanosheets-Based Electrode Material for High Performance Battery–Supercapacitor Hybrid Device. Int. J. Hydrogen Energy 2020, 45, 20820–20831. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Li, Y.; Xu, J.; Li, T.; Zhang, T. In Situ Growth of Ni-MOF Nanorods Array on Ti3C2Tx Nanosheets for Supercapacitive Electrodes. Nanomaterials 2023, 13, 610. [Google Scholar] [CrossRef]
- Wang, L.; Pan, L.; Han, X.; Ha, M.N.; Li, K.; Yu, H.; Zhang, Q.; Li, Y.; Hou, C.; Wang, H. A Portable Ascorbic Acid in Sweat Analysis System Based on Highly Crystalline Conductive Nickel-Based Metal–Organic Framework (Ni-MOF). J. Colloid Interface Sci. 2022, 616, 326–337. [Google Scholar] [CrossRef]
- Kavya, K.V.; Muthu, D.; Pattappan, D.; Vargheese, S.; Gokila, N.; Sivaramkumar, M.S.; Rajendra Kumar, R.T.; Haldorai, Y. Palladium Nanoparticles Decorated Ni-MOF Nanocomposite as an Electrochemical Platform for the Selective Detection of Dopamine. Mater. Lett. 2022, 306, 130926. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Wu, L.; Long, M.; Lin, Z.; Su, Q.; Zheng, F.; Wu, S.; Li, H.; Yu, G. 3D Co-Doped Ni-Based Conductive MOFs Modified Electrochemical Sensor for Highly Sensitive Detection of L-Tryptophan. Talanta 2022, 247, 123596. [Google Scholar] [CrossRef]
- Wan, J.; Shen, Y.; Xu, L.; Xu, R.; Zhang, J.; Sun, H.; Zhang, C.; Yin, C.; Wang, X. Ferrocene-Functionalized Ni(II)-Based Metal–Organic Framework as Electrochemical Sensing Interface for Ratiometric Analysis of Cu2+, Pb2+ and Cd2+. J. Electroanal. Chem. 2021, 895, 115374. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Wu, X.; Yuan, G.; Su, Y.; Fan, Z.; Xue, H.; Pang, H. Streamlined Synthesis of Superstructure Ni-Benzimidazole MOFs: Glucose Electrochemical Analysis. J. Colloid Interface Sci. 2024, 665, 764–771. [Google Scholar] [CrossRef]
- Cao, J.; Yun, J.; Zhang, N.; Wei, Y.; Yang, H.; Xu, Z. Bifunctional Ag@Ni-MOF for High Performance Supercapacitor and Glucose Sensor. Synth. Met. 2021, 282, 116931. [Google Scholar] [CrossRef]
- Nie, Y.; Li, Y.; Li, J.; Chen, L.; Wang, X.; Chen, T.; Cai, Z. Incorporated Ferrocene-Derivatives Endow Ni-Based MOF with High-Performance for Electrochemical Detection. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132742. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Gao, F.; Zheng, Z.; Lin, Y.; Wang, S.; Wang, Q.; Wang, Q. A Ratiometric Electrochemical Sensor for Antiepileptic Drug of Carbamazepine Based on Electroactive Ni2+-Terephthalic Acid MOF. Talanta 2025, 292, 128019. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, S.; Ghanbari, K.; Zahedi-Tabrizi, M. Development of an Electrochemical Sensor Based on Ni-Bio-MOF and a Molecular Imprinted Polymer for Determination of Diclofenac: Electrochemical and DFT Investigations. RSC Adv. 2025, 15, 16983–16998. [Google Scholar] [CrossRef]
- Atefi, A.; Moradi, S.; Salehnia, F.; Hosseini, M. A Free Enzyme Electrochemical Glucose Sensor Using an Integrated Display to CeO2 NPs/Ni-MOF-Based Sensor for Highly Sensitive Determination of Glucose in Sweat. Microchem. J. 2025, 209, 112809. [Google Scholar] [CrossRef]
- Chen, A.; Tang, N.; Wei, Y.; Shi, S.; Zhou, C.; He, Q.; Wang, W. A novel approach to simultaneous and sensitive detection of epinephrine and folic acid with nickel-metal organic framework and reduced graphene oxide based electrochemical sensor. Microchem. J. 2025, 208, 112526. [Google Scholar] [CrossRef]
- Mohseni-Sardari, F.; Mazloum-Ardakani, M.; Mohammadian-Sarcheshmeh, H.; Alizadeh, Z.; Houshmand, S. Enzyme-free glutamate sensor using peony flower-like structure of nickel metal–organic framework/MWCNTs nanocomposite. Inorg. Chem. Commun. 2025, 179, 114734. [Google Scholar] [CrossRef]
- Ji, L.; Li, F.; Jia, Q.; Yao, Y.; Zhu, X.; Li, Z.; Hu, P. Signal-amplified electrochemical monitoring of 4-chlorophenol in water environments based on Ni-BDC decorated multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2024, 12, 113532. [Google Scholar] [CrossRef]
- Chen, A.; Tang, N.; Wei, Y.; Shi, S.; Zhou, C.; He, Q.; Ding, J. Efficient and environmentally friendly uric acid voltametric sensor based on Ni-MOF and carboxylated multi-walled carbon nanotubes nanocomposites. J. Environ. Chem. Eng. 2024, 12, 113388. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, C.; Lin, C.; Zhang, J.; Liu, S.; Zhang, J. Highly sensitive detection of kaempferol using electrochemical sensors based on 3D-ordered mesh interconnect C60-GO, Ni-MOF, and β-cyclodextrin. Microchem. J. 2024, 197, 109866. [Google Scholar] [CrossRef]
- Dong, S.; Niu, H.; Sun, L.; Zhang, S.; Wu, D.; Yang, Z.; Xiang, M. Highly dense Ni-MOF nanoflake arrays supported on conductive graphene/carbon fiber substrate as flexible microelectrode for electrochemical sensing of glucose. J. Electroanal. Chem. 2022, 911, 116219. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.-T.; Gowthaman, N.S.K.; Mani, G.; Jeromiyas, N.; Shankar, S.; John, S.A. Electrocatalyst based on Ni-MOF intercalated with amino acid-functionalized graphene nanoplatelets for the determination of endocrine disruptor bisphenol A. Anal. Chim. Acta 2021, 1150, 338228. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, Y.-P.; Hu, Y.; Li, S.; Yin, Y.-M.; Wu, X.-Q.; Li, D.-S. Ti3C2@Ni-MOF heterostructure as efficient bifunctional sensor for highly sensitive detection of adenine and ascorbic acid. Mater. Lett. 2025, 398, 138933. [Google Scholar] [CrossRef]
- Dey, B.; Kushwaha, K.S.; Ullah, I.; Kamal, T.; Khan, S.; Ahmad, M.W.; Hossain, S.S.; Choudhury, A. Highly sensitive and selective electrochemical sensor for carbendazim detection in fruit juice using novel bimetallic metal–organic framework anchored graphite rod electrode. Inorg. Chem. Commun. 2025, 178, 114643. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Zeng, H.; Li, T.; Hu, D.; Li, Z.; Fu, Q.; Bateer, B. Construction of bimetallic MOF derived nickel–cobalt selenide on carbon cloth as integrated electrode for acetaminophen sensing. Microchem. J. 2025, 208, 112400. [Google Scholar] [CrossRef]
- Zaimbashi, R.; Salarizadeh, N.; Askari, M.B. Electrochemical sensor based on Ni-Co-MOF/MWCNTs nanocomposite and benzoyl ferrocene for determination of L-cysteine in the presence of tryptophan. Inorg. Chem. Commun. 2025, 171, 113403. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Li, J.; Hu, M.; Zhuang, G.; Xiong, X. Novel self-supporting CoNi-MOF with triangular cone geometry array structure for highly sensitive electrochemical sensing of fructose in beverages. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137045. [Google Scholar] [CrossRef]
- Liao, Y.; Lin, L.; Liu, J.; Zhang, X.; Li, X. Ultrasensitive simultaneous determination of hydroquinone and catechol by Ni3ZnC0.7/Ni porous composites derived from Ni/Zn-MOF. J. Electroanal. Chem. 2024, 954, 118028. [Google Scholar] [CrossRef]
- Zou, J.; Zou, J.; Li, L.; Chen, H.; Liu, S.; Gao, Y.; Huang, X.; Wang, L.; Lu, L. Enhanced electrocatalytic activity in MOFs-derived 3D hollow NiCo-LDH nanocages decorated porous biochar for simultaneously ultra-sensitive electrochemical sensing of Cu2+ and Hg2+. Talanta 2024, 279, 126624. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Liu, Y.; Zeng, X.; Jin, C.; Huo, D.; Hou, J.; Hou, C. A wearable flexible electrochemical biosensor with CuNi-MOF@rGO modification for simultaneous detection of uric acid and dopamine in sweat. Anal. Chim. Acta 2024, 1299, 342441. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yue, W.; Lei, Q.; Zhao, Z.; Sun, Y.; Xu, H.; Zhang, W.; Chen, L.; Kim, J.K.; et al. Construction of highly sensitive electrochemical immunosensor based on Au and Co3O4 nanoparticles functionalized Ni/Co bimetal conductive MOF for quantitative detection of HBsAg. Chem. Eng. J. 2024, 483, 149087. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Roushani, M.; Farokhi, S. Ni–P nanosheets derived from a metal–organic framework containing triptycene ligand: A high-performance electrochemical sensor for glucose determination. Microchem. J. 2024, 197, 109737. [Google Scholar] [CrossRef]
- Han, S.; Zhang, M.; Yang, J.; Zhang, N.; Yan, R.; Wang, L.; Gao, L.; Zhang, Z. A highly sensitive electrochemical sensor for the detection of chloramphenicol based on Ni/Co bimetallic metal–organic frameworks/reduced graphene oxide composites. J. Electroanal. Chem. 2024, 963, 118295. [Google Scholar] [CrossRef]
- Gao, H.; Chai, J.; Jin, C.; Tian, M. Molecularly imprinted electrochemical sensor based on Co–Ni-MOF/RGO nanocomposites for sensitive detection of hippuric acid. Anal. Chim. Acta 2024, 1296, 342307. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, G.; Sun, W.; Zha, X.; Wang, N.; Wang, Y. Portable electrochemical sensor for adrenaline detection using CoNi-MOF-based CS-PAM hydrogel. J. Colloid Interface Sci. 2024, 671, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-N.; Wang, M.; Liu, X.-Y.; Zhao, L.-X.; Li, S.-S. Electrochemical ratiometric biosensor based on 2D flower-like Co/Ni MOF and sea urchin-like PdCuNi for accurate quantification of alpha-fetoprotein. Chem. Eng. J. 2024, 499, 156248. [Google Scholar] [CrossRef]
- Shu, H.; Lai, T.; Yang, Z.; Xiao, X.; Chen, X.; Wang, Y. High sensitivity electrochemical detection of ultra-trace imidacloprid in fruits and vegetables using a Fe-rich FeCoNi-MOF. Food Chem. 2023, 408, 135221. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, H.; Guo, Q.; Zhang, Y.; Sun, X.; Guo, Y.; Yang, Q.; Zhang, Y. Shared hairpin structure electrochemical aptasensor based on ZrO2@Ni/Co-MOFs@AuNPs for dual-target detection of Cd2+ and S. aureus. Sens. Actuators B Chem. 2023, 396, 134648. [Google Scholar] [CrossRef]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Feng, J.; Jin, D.; Zhu, A.; Xu, Q.; Hu, X. Paper-based electrochemical immunosensor device via Ni–Co MOF nanosheet as a peroxidase mimic for the label-free detection of alpha-fetoprotein. Sens. Actuators B Chem. 2022, 373, 132736. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Z.; Lei, Q.; Sun, Y.; Zhang, W.; Zhuiykov, S.; Zhang, W.; Hu, J. Constructing and electrochemical performance of AuNPs decorated MIL-53 (Fe, Ni) MOFs–derived nanostructures for highly sensitive hydrazine detection. Appl. Surf. Sci. 2022, 596, 153573. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, L.; Wei, X.; Sun, Y.; Qi, S.; Chen, Y.; Tian, X.; Qiu, J.; Xu, D. Spongy Co/Ni-Bio-MOF-Based Electrochemical Aptasensor for Detection of Kanamycin Based on Coral-Like ZrO2@Au as an Amplification Platform. J. Electroanal. Chem. 2022, 920, 116647. [Google Scholar] [CrossRef]
- Tang, J.; Hu, T.; Li, N.; Zhu, Y.; Li, J.; Zheng, S.; Guo, J. Ag Doped Co/Ni Bimetallic Organic Framework for Determination of Luteolin. Microchem. J. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Umesh, N.M.; Jesila, J.A.; Wang, S.-F.; Vishnu, N.; Yang, Y.-J. Novel Voltammetric Detection of Norfloxacin in Urine and Blood Serum Using a Flexible Ni Foam Based Ni-Co-MOF Ultrathin Nanosheets Derived from Ni-Co-LDH. Microchem. J. 2021, 160, 105747. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, Z.; Wu, X.; Gao, J.; Liu, Y.; Yuan, Q.; Gan, W. Facile Synthesis of 2D/3D Hierarchical NiCu Bimetallic MOF for Non-Enzymatic Glucose Sensor. Microchem. J. 2021, 170, 106652. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Liu, X.; Li, Z.; Wang, C.; Jia, Q.; Zhang, Z.; Du, M. A Label-Free Enrofloxacin Electrochemical Aptasensor Constructed by a Semiconducting CoNi-Based Metal–Organic Framework (MOF). Electrochim. Acta 2021, 368, 137609. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhangsun, H.; Zhao, S.; Zhao, Y.; Wang, L. Carbon Cloth-Supported Nanorod-Like Conductive Ni/Co Bimetal MOF: A Stable and High-Performance Enzyme-Free Electrochemical Sensor for Determination of Glucose in Serum and Beverage. Food Chem. 2021, 349, 129202. [Google Scholar] [CrossRef]
- Li, S.; Xie, X.; Zhang, N.; Li, C.; Li, Y.; Jiang, M.; Huang, P.; Jin, H. Nonlinear Device Strategy of Non-Enzyme Glucose Sensor via Amorphous Ni MOF and Crystalline Ni3(PO4)2 Composite. Microchem. J. 2025, 209, 112752. [Google Scholar] [CrossRef]
- Gao, P.; Hussain, M.Z.; Gryc, D.; Mukherjee, S.; Zhou, Z.; Li, W.; Jentys, A.; Elsner, M.; Fischer, R.A. Enhanced Electrochemical Activity by MOF Superstructure Derived Ni2P@C for Ultrasensitive Sensing of Bisphenol A. Biosens. Bioelectron. 2025, 286, 117598. [Google Scholar] [CrossRef] [PubMed]
- Afruz, A.; Amiri, M.; Kaffash-Jamshid, M.; Bezaatpour, A.; Bottke, P.; Wark, M. Dual-Function Nickel Bio-MOF as a Non-Enzymatic Glucose Sensor and Efficient Supercapacitor. Electrochim. Acta 2025, 513, 145586. [Google Scholar] [CrossRef]
- Xiong, Q.; Chen, S.; Pei, L.; Liu, J.; Yang, Y.; Lu, M.; Song, Y. High-Selective Dopamine Electrochemical Sensor Based on Yolk-Shell Structural Composites Derived from Ni-MOF@COFTAPB-DVA. Microchem. J. 2025, 210, 112987. [Google Scholar] [CrossRef]
- Zhu, Z.; Lv, T.; Wang, Y.; Nan, S.; Haotian, R.; Yang, Q.; Liang, A.; Luo, A. A Novel Molecularly Imprinted Electrochemical Biosensor Based on Ni3(HITP)2-MOF and a Novel Anti-Fouling Material for the Direct Detection of Glucose in Whole Blood. Sens. Actuators B Chem. 2025, 441, 138028. [Google Scholar] [CrossRef]
- Rasheed, S.; Ikram, M.; Fatima, B.; Alomayri, T.; Hussain, D. Facile Synthesis of MOF-Derived Graphitic Carbon-Decorated NiVO4 as an Ultra-Sensitive Electrochemical Sensor for Non-Enzymatic Detection of Sarcosine. J. Alloys Compd. 2025, 1030, 180867. [Google Scholar] [CrossRef]
- Yang, C.; Li, S.; Liao, C.; Du, C.; Yao, H.; Li, Y.; Zhang, Y.; Stachewicz, U.; Liu, Y. A Wearable 3D Nanostructured Ni-MOF Electrochemical Sensor Integrated with Janus Fabric for Sweat Collecting and Nutrients Detecting. Talanta 2026, 296, 128474. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, J.; Lu, T.; Lin, W.; Quan, M.; Ye, H.; Dong, S.; Yang, Z. Ni-MOFs Grown on Carbonized Loofah Sponge for Electrochemical Glucose Detection: Effects of Different Carboxylic Acid Ligands and Reaction Temperatures on Electrochemical Performance. J. Taiwan Inst. Chem. Eng. 2024, 155, 105269. [Google Scholar] [CrossRef]
- Han, S.; Sun, R.; Zhao, L.; Yan, C.; Chu, H. Molecularly Imprinted Electrochemical Sensor Based on Synergistic Interaction of Honeycomb-Like Ni-MOF Decorated with AgNPs and N-GQDs for Ultra-Sensitive Detection of Olaquindox in Animal-Origin Food. Food Chem. 2023, 418, 136001. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Xiong, Q.; Li, Y.; Sun, M.; Wang, X.; Wang, Y.; Wu, C.; Su, G.; et al. Nanoarchitectonics of On–Off Ratiometric Signal Amplified Electrochemical Sensor for Chlorpromazine with Molecularly Imprinted Polymer Based on Ni-MOF/Fe-MOF-5 Hybrid Au Nanoparticles. Sep. Purif. Technol. 2023, 327, 124858. [Google Scholar] [CrossRef]
- Chen, C.; Ren, J.; Zhao, P.; Zhang, J.; Hu, Y.; Fei, J. A Novel Dopamine Electrochemical Sensor Based on a β-Cyclodextrin/Ni-MOF/Glassy Carbon Electrode. Microchem. J. 2023, 194, 109328. [Google Scholar] [CrossRef]
- Wei, P.; Wang, S.; Wang, W.; Niu, Z.; Rodas-Gonzalez, A.; Li, K.; Li, L.; Yang, Q. CoNi Bimetallic Metal–Organic Framework and Gold Nanoparticles-Based Aptamer Electrochemical Sensor for Enrofloxacin Detection. Appl. Surf. Sci. 2022, 604, 154369. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, K.; Duan, J.; Zheng, S.; Jiang, J. The Nickel Phosphate Rods Derived from Ni-MOF with Enhanced Electrochemical Activity for Non-Enzymatic Glucose Sensing. Talanta 2022, 247, 123587. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Jia, X.; Wang, P.; Wang, Y.; Yang, Y.; Liu, H. Bimetallic Synergy Boost TCPP(Ni)-Co MOF as the High-Performance Electrochemical Sensor for Enhanced Detection of Trace Theophylline. Microchem. J. 2022, 183, 107981. [Google Scholar] [CrossRef]
- Guo, L.; Hao, L.; Zhang, Y.; Yang, X.; Wang, Q.; Wang, Z.; Wang, C. Metal–Organic Framework Precursors Derived Ni-Doping Porous Carbon Spheres for Sensitive Electrochemical Detection of Acetaminophen. Talanta 2021, 228, 122228. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Wei, Y.; Pei, X.; Zhou, Z.; Zhang, J.; Zhang, R.; Zhang, D. MOF-Derived Porous NiO Nanorod and Microflower Structures with Enhanced Non-Enzymatic Glucose Electrochemical Sensing Performance. Int. J. Electrochem. Sci. 2021, 16, 210465. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Liu, D.; Zhang, N. MOF-Derived Porous Nanostructured Ni2P/C Material with Highly Sensitive Electrochemical Sensor for Uric Acid. Inorg. Chem. Commun. 2021, 130, 108713. [Google Scholar] [CrossRef]
- Yang, W.; Guo, H.; Fan, T.; Zhao, X.; Zhang, L.; Guan, Q.; Wu, N.; Cao, Y.; Yang, W. MoS2/Ni(OH)2 Composites Derived from In Situ Grown Ni-MOF Coating MoS2 as Electrode Materials for Supercapacitor and Electrochemical Sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126178. [Google Scholar] [CrossRef]
- Xia, K.; Yi, F.; Zheng, L.; Gao, A.; Shu, D.; Ling, J. 2D Coordination Unsaturated Ni-MOFs Hierarchical Nanosheets with Internal Electric Fields for High-Performance Hybrid Supercapacitors. J. Electroanal. Chem. 2023, 939, 117464. [Google Scholar] [CrossRef]
- Bhoite, A.A.; Patil, K.V.; Redekar, R.S.; Patil, P.S.; Sawant, V.A.; Tarwal, N.L. Solvothermal synthesis of binder-free Ni-MOF thin films for supercapacitor electrodes. J. Solid State Chem. 2023, 326, 124192. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Z.; Wang, G.; Sang, H.; Li, W.; Xu, Z. One-pot preparation of La-doped Ni-MOF nanospheres for efficient hybrid supercapacitor electrode material. Mater. Chem. Phys. 2023, 309, 128340. [Google Scholar] [CrossRef]
- Sun, X.; Chen, J.; Kong, W.; Yu, Q.; Hu, C.; Long, Y.; Dai, Y.; Gong, J.; Pu, L.; Zhang, H.; et al. Cr-doped Ni-MOF nanosheet array structure anchored on nickel foam with specific orientation for high performance supercapacitors. Electrochim. Acta 2023, 469, 143264. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Jaiswal, R.; Ilangovan, S.A.; Sujatha, S.; Ajeesh, K.S.; Tatiparti, S.S.S.V. A systematic approach for selecting suitable morphologies for supercapacitor applications through morphological stability map—A case of Ni-MOF. Ceram. Int. 2023, 49, 9382–9394. [Google Scholar] [CrossRef]
- Liu, R.; Shang, M.Y.; Liu, C.; Hao, Y.; Yang, F.; Shi, J.Y.; Chen, Y.; Wang, Y.F.; Feng, J.Q.; Yang, P.F.; et al. Effect of hydrochloric acid on the properties of Ni-MOF nanostructures as supercapacitor electrode materials. Chem. Phys. Lett. 2024, 850, 141474. [Google Scholar] [CrossRef]
- Khan, J.; Ahmed, A.; Saleem, M.I.; Al-Kahtani, A.A. Benzene-1,4-dicarboxylic acid-based Ni-MOF for efficient battery–supercapacitor hybrids: Electrochemical behavior and mechanistic insights. J. Energy Storage 2024, 100, 113455. [Google Scholar] [CrossRef]
- Khokhar, S.; Chand, P.; Anand, H. Solvent-regulated fabrication of Ni-MOF-based asymmetric supercapacitor device. Inorg. Chem. Commun. 2024, 170, 113227. [Google Scholar] [CrossRef]
- Raje, P.G.; Gurav, S.R.; Waikar, M.R.; Chodankar, G.R.; Shembade, U.V.; Moholkar, A.V.; Dongale, T.D.; Sonkawade, R.G. Exploring the role of metal concentrations on the chemically synthesized Ni-MOF nanostructures for asymmetric supercapacitor. J. Energy Storage 2024, 95, 112617. [Google Scholar] [CrossRef]
- Kashif, M.; Thangarasu, S.; Murugan, N.; Magdum, S.S.; Kim, Y.A.; Kurkuri, M.; Oh, T.-H. Interatomic interaction of 2D crumpled V2O5 nanosheets layered with Ni-MOF as a bifunctional electrocatalyst for overall water splitting and supercapacitor applications. J. Energy Storage 2024, 81, 110348. [Google Scholar] [CrossRef]
- Rajamany, R.; Prakash, S.; Ismail, Y.A. Polyvinylpyrrolidone-assisted synthesis of Ni-MOF: Enhanced supercapacitive performance through morphology control. Next Mater. 2025, 7, 100459. [Google Scholar] [CrossRef]
- Sundarraj, N.K.; Pandiyan, J.Q.S.; Al Souwaileh, A.; Wu, J.J.; Anandan, S. Sonochemical synthesis of Ni-MOF using 2-methylimidazole as an organic linker: Pushing the boundaries of energy storage. Electrochim. Acta 2025, 523, 145975. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, J.; Zhan, Q.; Liu, N.; Zhang, X.; Wang, Z.; Han, Y. Fabrication of a vanadium-doped Ni-MOF with a hydrangea-like morphology as an electrode for high-performance supercapacitors. Chem. Commun. 2025, 61, 7843–7846. [Google Scholar] [CrossRef]
- Bhanuse, G.B.; Kumar, S.; Chien, C.-W.; Fu, Y.-P. Development of heterostructured ZnCo2O4@Ni-MOF electrode for the asymmetric supercapacitor and electrocatalytic oxygen evolution reaction applications. Electrochim. Acta 2025, 511, 145371. [Google Scholar] [CrossRef]
- Wang, J.-W.; Meng, T.-L.; Ma, Y.-X.; Lei, L.; Li, J.; Ran, F. Fabrication of graphite nanosheets decorated with Ni-MOFs for high-performance supercapacitor electrode materials. Diam. Relat. Mater. 2023, 139, 110281. [Google Scholar] [CrossRef]
- Meng, T.-L.; Ma, Y.-X.; Wang, J.-W.; Li, J.; Lei, L.; Wang, F.; Ran, F. Multicomponent hierarchical Ni-MOFs/MWCNTs@Ni/Co-LDH nanohybrid as advanced electrode material for supercapacitor. J. Phys. Chem. Solids 2024, 193, 112200. [Google Scholar] [CrossRef]
- Yazdani, S.; Lashkenari, M.S.; Mehri, F. Design of a novel mixed-ligand Ni-MOF/MWCNT nanocomposite to enhance the electrochemical performance of supercapacitors. Synth. Met. 2024, 307, 117702. [Google Scholar] [CrossRef]
- Amirabad, T.N.; Ensafi, A.A.; Mousaabadi, K.Z.; Rezaei, B.; Demir, M. Binder-free engineering design of Ni-MOF ultrathin sheet-like grown on PANI@GO decorated nickel foam as an electrode for hydrogen evolution reaction and asymmetric supercapacitor. Int. J. Hydrogen Energy 2023, 48, 29471–29484. [Google Scholar] [CrossRef]
- Ji, Y.; Li, W.; You, Y.; Xu, G. In situ synthesis of M (Fe, Cu, Co and Ni)-MOF@MXene composites for enhanced specific capacitance and cyclic stability in supercapacitor electrodes. Chem. Eng. J. 2024, 496, 154009. [Google Scholar] [CrossRef]
- Shivade, D.S.; Kurade, A.N.; Bhosale, R.K.; Kundale, S.S.; Shelake, A.R.; Patil, A.D.; Waifalkar, P.P.; Kamat, R.K.; Teli, A.M.; Dongale, T.D. Folic acid-assisted in situ solvothermal synthesis of Ni-MOF/MXene composite for high-performance supercapacitors. J. Energy Storage 2024, 100, 113754. [Google Scholar] [CrossRef]
- Li, J.; Qiang, X.; Jia, B.; Wang, L.; Wu, X. Etching and surface self-assembly of Ni-MOF/MXene hybrids for excellent flexible pseudocapacitance. Appl. Surf. Sci. 2025, 695, 162867. [Google Scholar] [CrossRef]
- Shalini, S.S.; Fu, Y.-P.; Bose, A.C. Synergetic interplay of MnNi-MOF composite with 2D MXene for improved supercapacitor application. Chem. Eng. J. 2024, 500, 156751. [Google Scholar] [CrossRef]
- Gopi, R.R.; Ebenezer, T.; Prabu, H.J.; Johnson, I.; Galeb, W.; Raja, M.D.; Sundaram, S.J.; Kennedy Arockiasamy, J.S.; Sahayaraj, A.F. Synthesis and investigation of charge storage characteristics in Ni-MOF/PANI composite as an active electrode material for supercapacitor. Electrochim. Acta 2024, 507, 145130. [Google Scholar] [CrossRef]
- Wu, S.; Cai, D.; Tian, Z.; Guo, L.; Wang, Y. One-step synthesis of NiCo-MOF@LDH hybrid nanosheets for high-performance supercapacitor. J. Energy Storage 2024, 89, 111670. [Google Scholar] [CrossRef]
- Meng, T.-L.; Wang, J.-W.; Ma, Y.-X.; Chen, X.-Q.; Lei, L.; Li, J.; Ran, F. Fabrication of a novel nanohybrid via the introduction of Ni/Mn-LDH into Ni-MOFs/MWCNTs for high-performance electrochemical supercapacitor. Diam. Relat. Mater. 2024, 143, 110901. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Wu, N.; Pan, Z.; Li, C.; Chen, Y.; Cao, Y.; Yang, W. Trimesic acid-modified 2D Ni-Co-MOF for high-capacity supercapacitors. J. Alloys Compd. 2023, 934, 167779. [Google Scholar] [CrossRef]
- Jin, J.; Wang, N.; Wang, Y.; Wang, Y.; Sun, T. Synergistic effect of bimetal (Zn/Ni)–organic framework/reduced graphene oxide for high-performance supercapacitor. Appl. Surf. Sci. 2023, 615, 156435. [Google Scholar] [CrossRef]
- Khan, M.F.; Marwat, M.A.; Abdullah; Shah, S.S.; Karim, M.R.A.; Aziz, M.A.; Din, Z.U.; Ali, S.; Adam, K.M. Novel MoS2-sputtered NiCoMg MOFs for high-performance hybrid supercapacitor applications. Sep. Purif. Technol. 2023, 310, 123101. [Google Scholar] [CrossRef]
- Radhika, M.G.; Srilakshmi, R.; Tejashree, V.; Venkatesh, K.; Kamath, M.K.S.; Nagaraju, K. A new strategy for the morphology-controlled synthesis of Ni/Co MOFs for high-performance asymmetric supercapacitors. J. Energy Storage 2023, 61, 106766. [Google Scholar] [CrossRef]
- Yue, L.; Chen, L.; Wang, X.; Lu, D.; Zhou, W.; Shen, D.; Yang, Q.; Xiao, S.; Li, Y. Ni/Co-MOF@aminated MXene hierarchical electrodes for high-stability supercapacitors. Chem. Eng. J. 2023, 451, 138687. [Google Scholar] [CrossRef]
- Salehi, S.; Ehsani, M.H.; Aghazadeh, M. Direct electrosynthesis of Ni-, Co-, and Ni,Co-MOF onto porous support for high-performance supercapacitors. J. Alloys Compd. 2023, 940, 168885. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Z.; Yu, Y.; Han, Y. Electrodeposited coral-like bimetallic NiCo-MOFs on Ni foam as binder-free electrodes for high performance all solid-state asymmetric supercapacitors. Electrochim. Acta 2023, 467, 143095. [Google Scholar] [CrossRef]
- Fatima, S.; Shabbir, H.; Sharif, R.; Fahad, H.M.; Yang, J.; Shaheen, F.; Wahab, R.; Akbar, S.; Perumal, V. A novel binary composite of CuCoNi-MOF/MoO3 with exceptional capacitance as electrode material for supercapacitors. J. Energy Storage 2024, 99, 113300. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, M.; Song, F.; Xiong, Z.; He, X. Preparation of layered NiCo-MOF nanosheets for high-performance asymmetric supercapacitor electrode material. Vacuum 2024, 225, 113203. [Google Scholar] [CrossRef]
- Gurav, S.R.; Shembade, U.V.; Patil, A.V.; Waikar, M.R.; Sonkawade, A.R.; Vhatkar, R.S.; Moholkar, A.V.; Sonkawade, R.G. Time and cost efficient post-synthesized core-shell NiCo-MOFs electrode for solid-state supercapacitors. Mater. Today Sustain. 2024, 28, 101049. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ishfaq, S.; Adam, K.M.; Tahir, B.; Shaikh, M.H.; Khan, M.F.; Karim, M.R.A.; Din, Z.U.; Abdullah, S.; Ghazanfar, E. Enhancing supercapacitor performance of Ni–Co–Mn metal–organic frameworks by compositing it with polyaniline and reduced graphene oxide. RSC Adv. 2024, 14, 2102–2115. [Google Scholar] [CrossRef] [PubMed]
- Bhoite, A.A.; Sawant, V.A.; Tarwal, N.L. Solvothermal synthesis of Ni/Co-based metal-organic framework with nanosheets-like structure for high-performance supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134814. [Google Scholar] [CrossRef]
- Liang, J.; Qin, S.; Luo, S.; Wang, Y.; Feng, J.; Liu, K.; Liao, S.; Xu, Z.; Li, J. Epitaxially growing multilayer CoNi-MOFs nanosheets on activated carbon cloth for high-performance asymmetric supercapacitors. J. Power Sources 2024, 618, 235209. [Google Scholar] [CrossRef]
- Sun, L.-J.; Zhang, X.-Y.; Bai, C.; Guo, H.-L.; Han, C.-M.; Zhang, Y.-F.; Hu, H.-M. Cobalt/nickel 2D MOF nanosheets with a bithiophene-tetraterpyridyl derivative ligand for high-performance supercapacitors through boosting pseudocapacitance. J. Energy Storage 2024, 92, 112197. [Google Scholar] [CrossRef]
- Dong, W.; Liu, Z.; Sun, H.; Shi, Z.; Xu, J. Ultrathin defect-rich nanosheets of NiFe-MOF with high specific capacitance and stability for supercapacitor. Mater. Today Chem. 2024, 36, 101938. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Banerjee, A.N.; Roy, N.; Merum, D.; Nallapureddy, J.; Joo, S.W. Faradaic-dominated intercalation pseudocapacitance in bimetallic ultrathin NiMn-MOF nanosheet electrodes for high-performance asymmetric supercapacitors. Chem. Eng. J. 2024, 498, 155240. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pawar, P.S.; Pagare, P.K.; Torane, A.P. Facile solvothermal synthesis of Ni-Co MOF/rGO nanoflakes for high-performance asymmetric supercapacitor. Electrochim. Acta 2024, 477, 143745. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Liu, Y.; Mu, Q.; Shi, Y. Dual metal MOF derived Co-Ni/rGO as cathode material with synergetic effect for an asymmetric supercapacitor with enhanced performances. J. Energy Storage 2024, 84, 110864. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Wustoni, S.; Failamani, F.; Wehbe, N.; Eguchi, M.; Nara, H.; Inal, S.; Suendo, V.; Yuliarto, B. Revealing the effect of cobalt content and ligand exchange in the bimetallic Ni–Co MOF for stable supercapacitors with high energy density. J. Power Sources 2024, 603, 234423. [Google Scholar] [CrossRef]
- Feng, J.; Luo, S.; Xu, P.; Liang, J.; Qin, S.; Li, J. Understanding the phase structure evolution and charge storage mechanism of FeCoNi-MOFs as electrodes for asymmetric supercapacitors. J. Colloid Interface Sci. 2025, 684, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, R.; Yue, H.; Zhang, Y.; Lu, S.; Shang, W.; Zhang, Z.; Wen, Y. In situ electrodeposition construction of a Ni/Mn bimetallic organic framework material and its electrochemical performance in flexible supercapacitors. J. Alloys Compd. 2025, 1020, 179340. [Google Scholar] [CrossRef]
- Shabbir, H.; Fahad, H.M.; Sharif, R.; Butt, A.; Fatima, S.; Shaheen, F.; Jose, R.; Wahab, R.; Perumal, V.; Akbar, S.; et al. Synergistic effect of 3D porous tri-metallic MOF based electrode materials for highly stable asymmetric supercapacitors. Mater. Sci. Semicond. Process. 2025, 186, 109036. [Google Scholar] [CrossRef]
- Arshad, I.; Marwat, M.A.; Nawaz, H.; Hannan, M.; Abdullah, S.M.; Humayun, M.; Bououdina, M.; Hamayun, U.; Khan, M.Z.; Arif, A. Enhanced electrochemical performance of NiCoMn MOFs with Ag-citrate/SWCNT nanocomposites for high-energy supercapacitors. Diam. Relat. Mater. 2025, 155, 112246. [Google Scholar] [CrossRef]
- Li, J.; Du, R.; Wang, R.; Feng, J.; Luo, S. Rational construction of multilayer NiCo-MOF@MnO2 heterostructures with optimized charge storage behavior for asymmetric supercapacitors. Appl. Surf. Sci. 2025, 706, 163595. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mohsen, Q.; El-Shamy, O.A.A. Designing novel Bi-metallic MOFs with optimized Ni and Co ions ratios for enhanced supercapacitor performance. J. Energy Storage 2025, 105, 114777. [Google Scholar] [CrossRef]
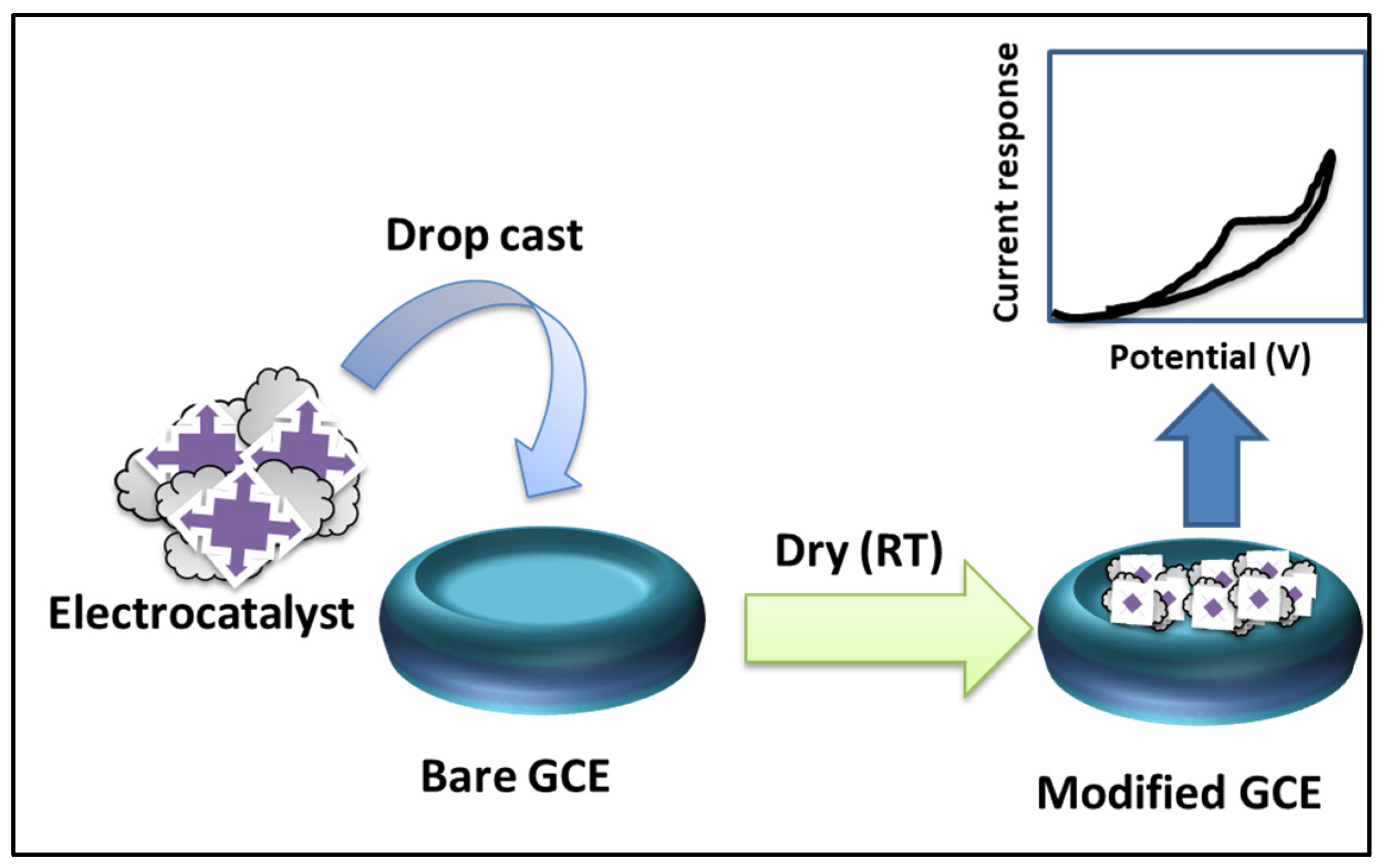

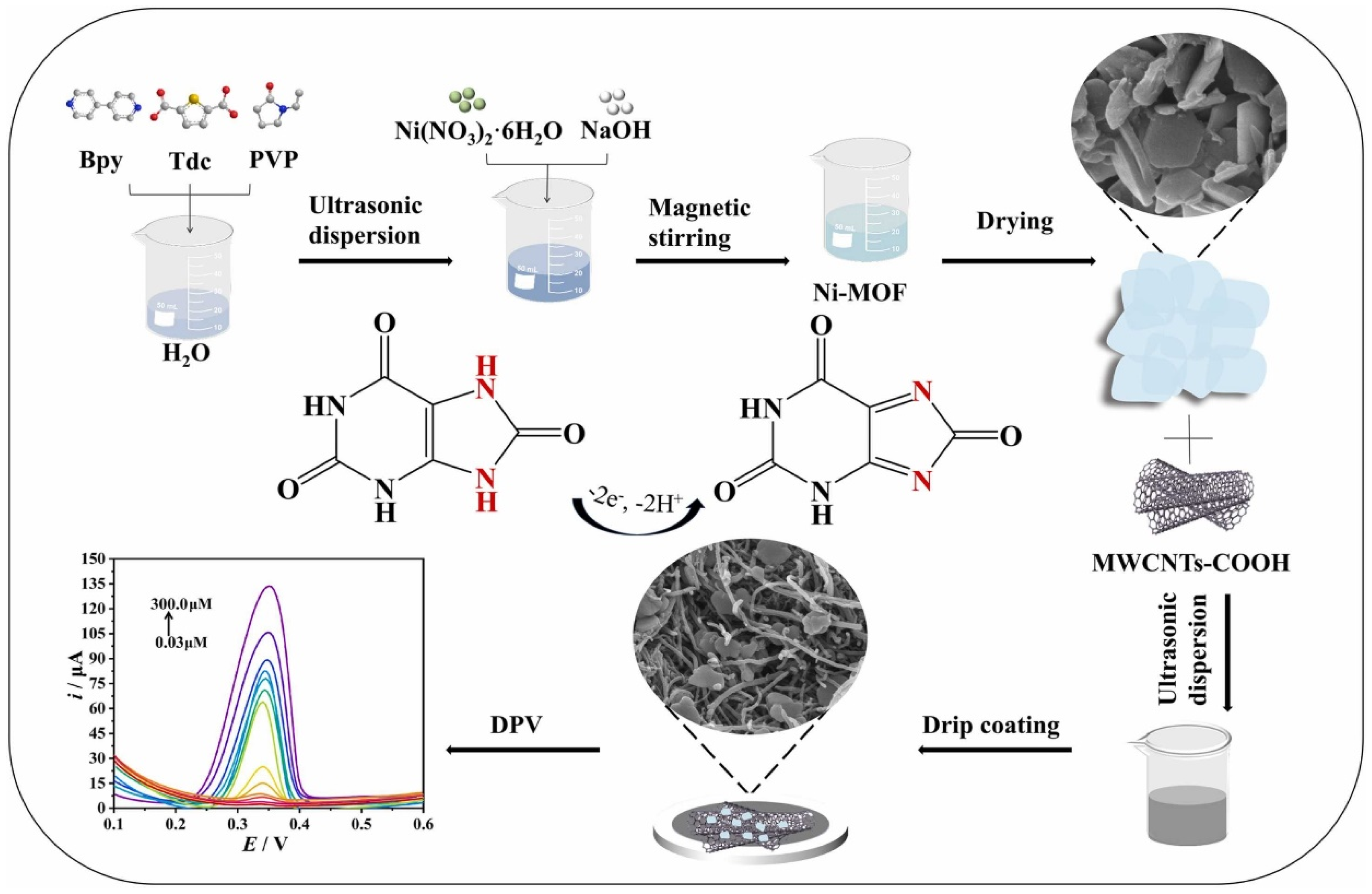
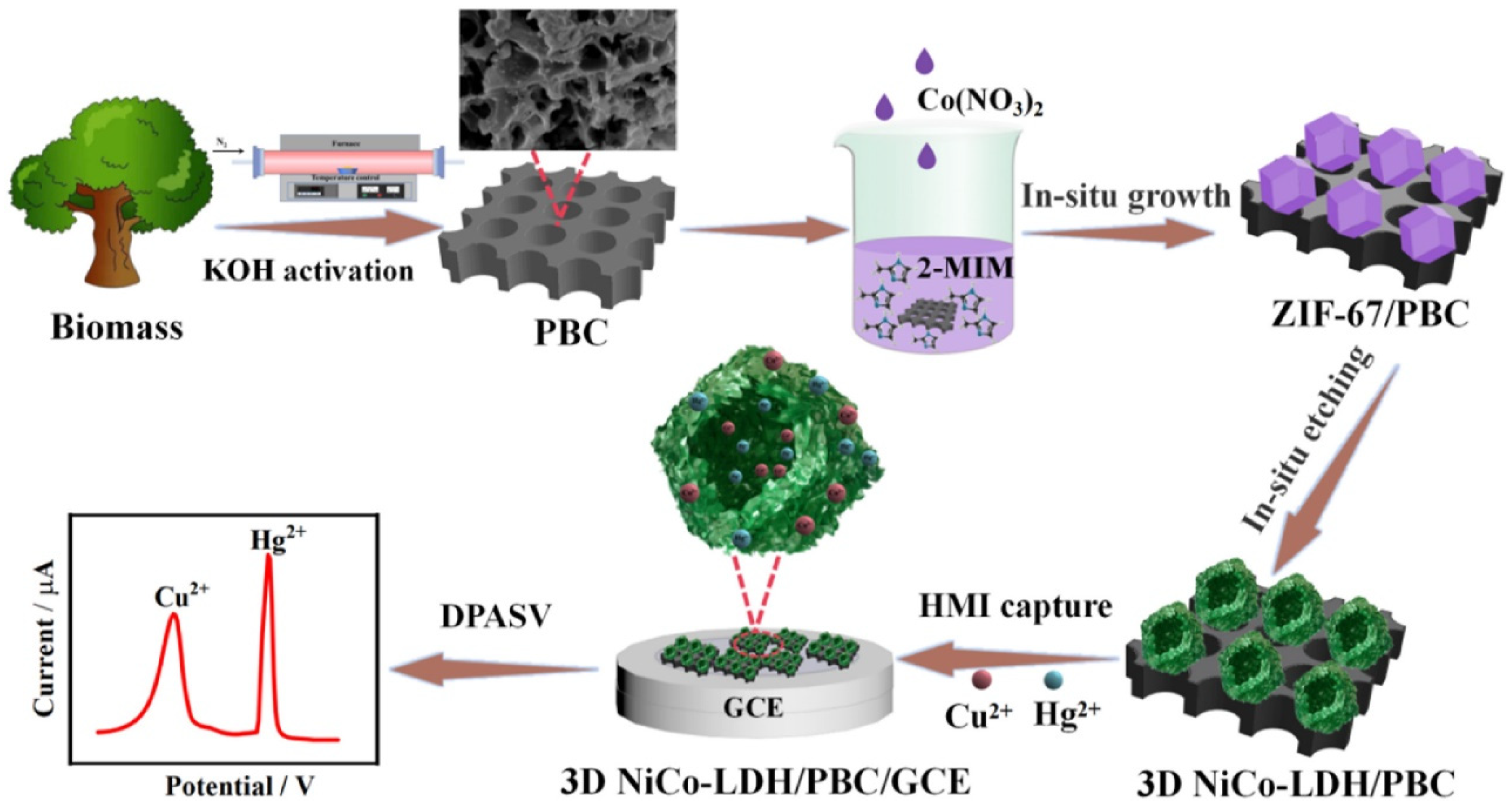
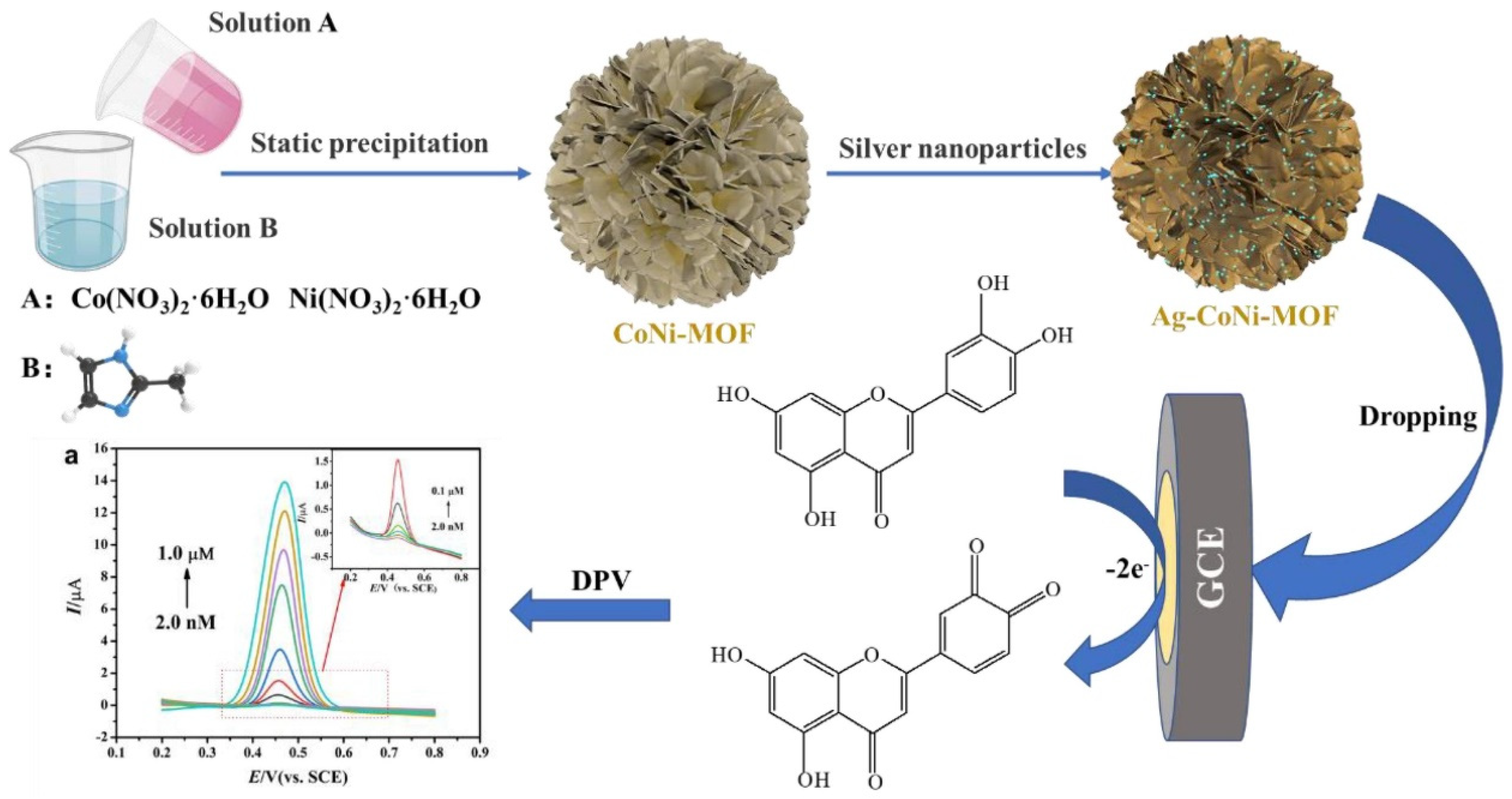
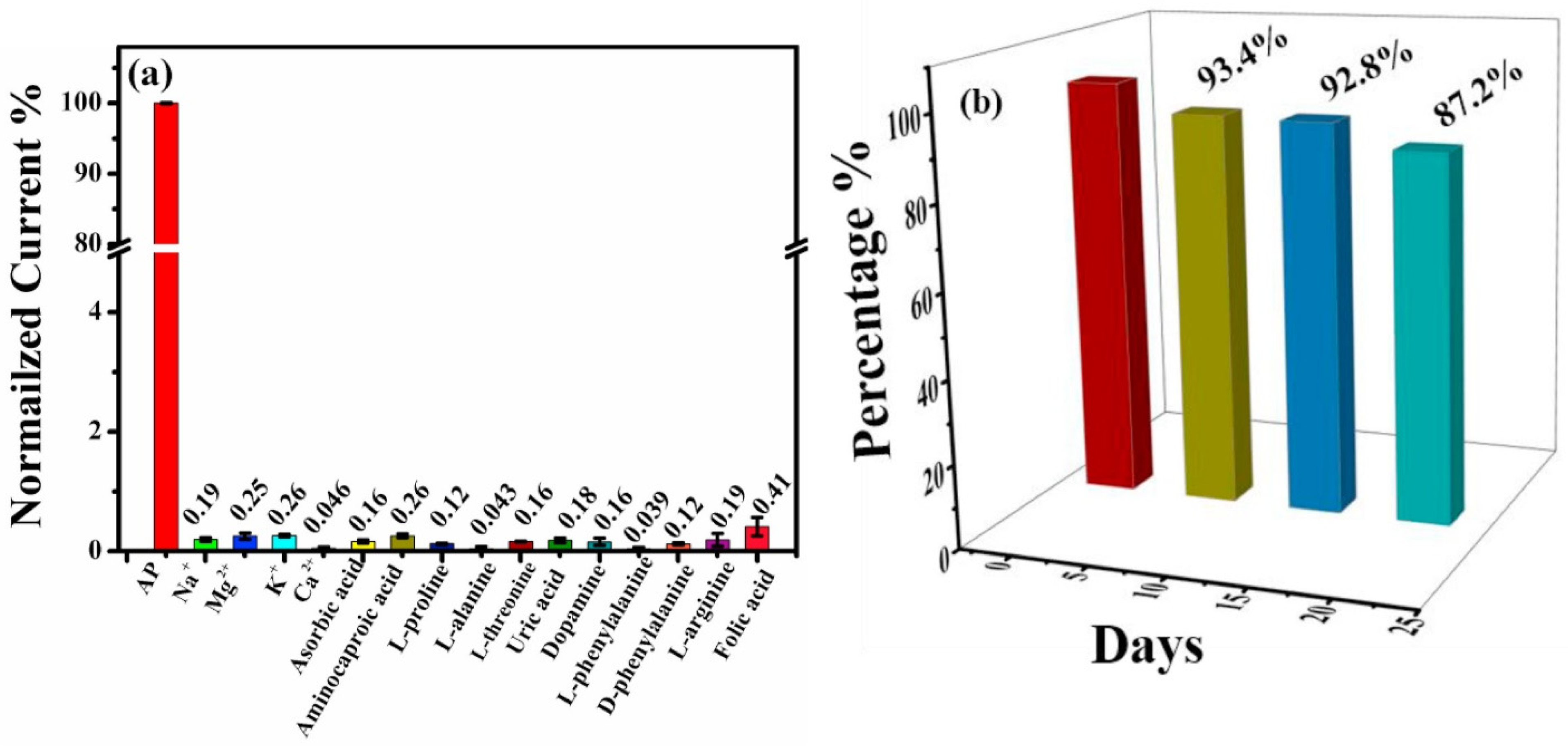
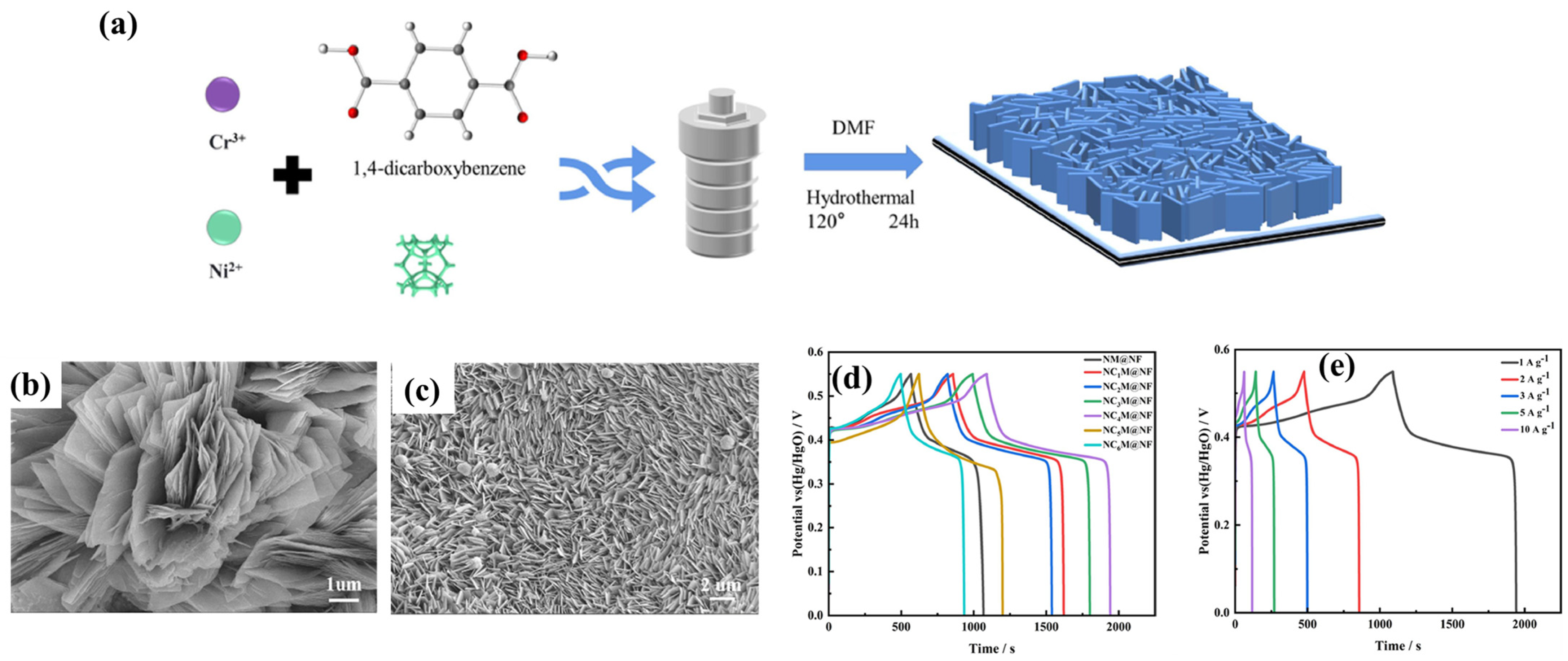
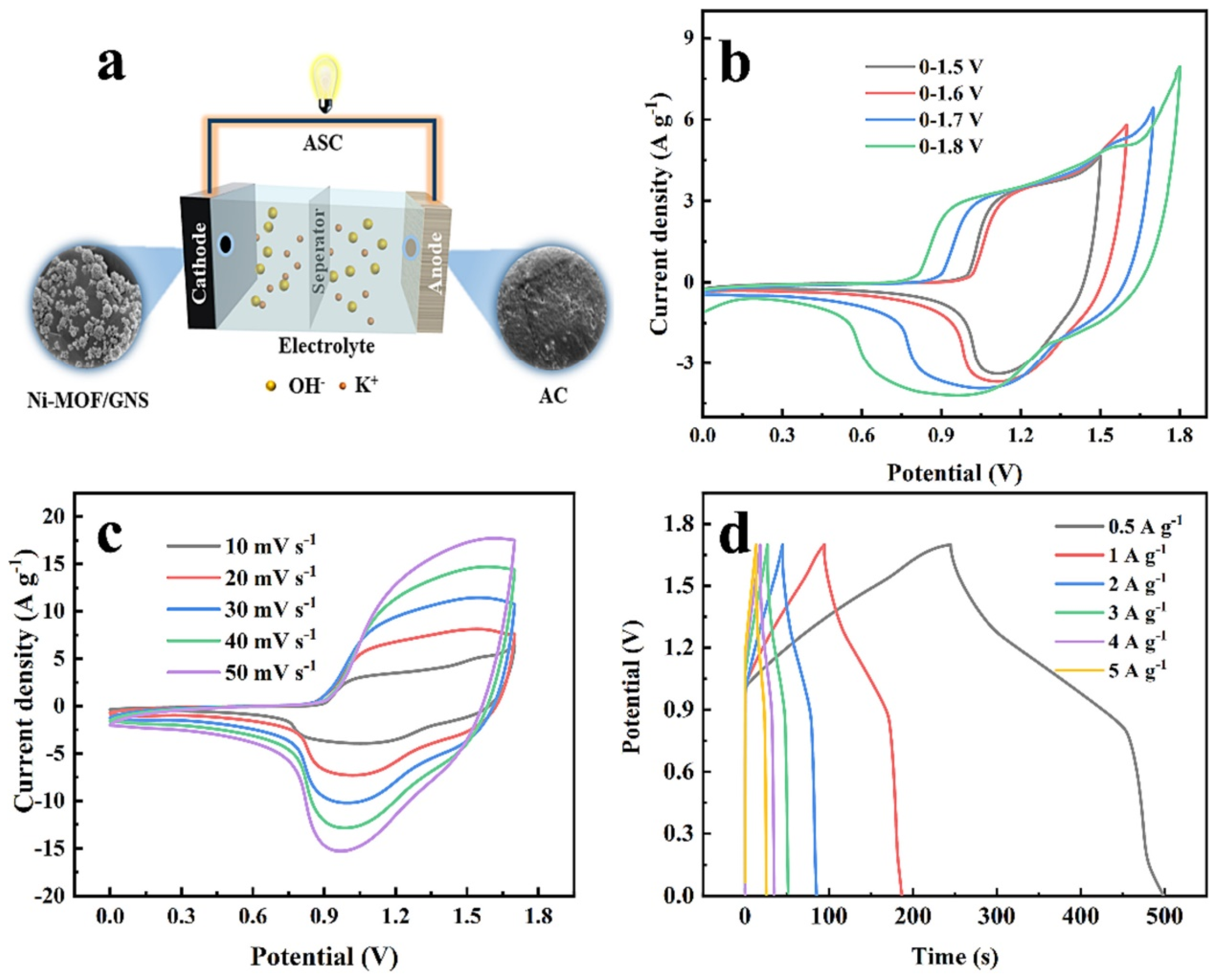

| Electrode Modifier | Sensing Analyte | LOD (µM) | Sensitivity | LR (µM) | Technique | Real Sample | Refs |
|---|---|---|---|---|---|---|---|
| Ni3(HITP)2/SPCE | AA | 1 | 0.814 μA μM−1 cm−2 | 2 to 200 | Amp | Human sweat | [41] |
| Co–Ni-MOFs-1%/GCE | L-TRP | 0.0087 | - | 0.01 to 300 | DPV | Mice plasma | [43] |
| Ni-benzimidazole MOFs | Glucose | 0.1401 | 2199.88 mA M−1 cm−2 | 0.5 to 2665.5 | Amp | - | [45] |
| Ni-MOF/CeO2 | Glucose | 30 | 2488 μA mM−1 cm−2 | 0.04 mM to 1.2 mM | Amp | Sweat | [50] |
| Ni-MOF/RGO/GCE | EP | 0.018 | - | 0.05 to 200 | LSV | Serum and urine | [51] |
| Ni-MOF/RGO/GCE | FA | 0.016 | - | 0.01 to 150 | LSV | Serum and urine | [51] |
| Ni-BDC/MWCNTs/GCE | 4-CP | 0.0165 | - | 0.1 to 50, 50 to 500 | DPV | River water | [53] |
| β-CD/Ni-MOF/C60-GO /GCE | KA | 0.058 | - | 20 to 50 | DPV | Broccoli samples | [55] |
| FxGnP-Ni-MOF | BPA | 0.000184 | - | 0.002 to 10 | Amp | River and sewage effluent water | [57] |
| Ni-Fe(PDC)/GR | CBM | 0.0032 | - | 0.05 to 320 | DPV | Strawberry and apple juice | [59] |
| Ni3ZnC0.7/Ni/GCE | HQ | 0.14 | - | 0.3 to 100 | DPV | Yellow River and tap water | [63] |
| Ni3ZnC0.7/Ni/GCE | CC | 0.21 | - | 0.5 to 100 | DPV | Yellow River and tap water | [63] |
| GCE/rGO/NiCo-BTC MOFs | CAP | 0.235 | 33.12 μA μM−1 cm−2 | 0.1 to 100 | DPV | Tap water | [68] |
| NiCu-MOF-6 | Glucose | 15 | 1832 μA mM−1 cm−2 | 0.02 to 4.93 | Amp | Human serum | [79] |
| Electrode Modifier | Specific Capacitance | Electrolyte | Current Density (A/g) | Cyclic Stability | Refs |
|---|---|---|---|---|---|
| 2D Ni-MOF | 746 C/g | 3 M KOH | 1 | 10,000 | [100] |
| La-doped Ni-MOF | 159.9 mA h/g | 2 M KOH | 1 | 5000 | [102] |
| Cr-doped Ni-MOF | 853 C/g | 3 M KOH | 1 | 5000 | [103] |
| Ni-MOF globules | 1361 F/g | 2 M KOH | 0.5 | - | [104] |
| Ni-MOF | 2567.23 F/g | 6 M KOH | 2 | 5000 | [105] |
| Ni-MOF | 565.32 C/g | 1 M KOH | 1.2 | 5000 | [106] |
| Ni-MOF nanosheets | 1124 F/g | 2 M KOH | 2 | 3000 | [107] |
| Ni-MOF | 1668.4 F/g | 1 M KOH | 10 | 10,000 | [108] |
| V2O5/Ni-MOF | 546 F/g | 1 M KOH | 1 | 10,000 | [109] |
| N-Ni MOF | 1519 F/g | 2 M KOH | 1 | 2000 | [110] |
| Ni-MOF | 221 F/g | 3 M KOH | 1 | 2000 | [111] |
| ZnCo2O4@Ni-MOF | 1800 F/g | 1 M KOH | 2 | 5000 | [113] |
| Ni-MOF/MWCNTs | 900 F/g | 6 M KOH | 0.5 | 1000 | [116] |
| Ni-MOF@MXene | 1160.5 F/g | 3 M KOH | 1 | 10,000 | [118] |
| Ni-MOF/MXene | 1406 F/g | - | 1 | 20,000 | [120] |
| NiCo-MOF@LDH | 1873.9 F/g | 2 M KOH | 0.5 | 10,000 | [123] |
| 2D NiCo-MOF | 1790 F/g | 6 M KOH | 1 | 10,000 | [125] |
| Ni/Co-MOF@TCT-NH2 | 1924 F/g | 3 M KOH | 0.5 | 10,000 | [129] |
| NiCo-MOFs | 1176 F/g | 2 M LiOH | 5 mV/s | 5000 | [134] |
| NiCo-MOF | 1070 F/g | 1 M KOH | 0.5 mA/cm2 | 5000 | [136] |
| NiFe-MOF | 15.6 F/cm2 | 1 M KOH | 2 mA/cm2 | 5000 | [139] |
| Ni-Co MOF/rGO | 1320 F/g | 1 M KOH | 4 mA/cm2 | 5000 | [141] |
| Ni/Mn-MOF | 447.5 F/g | 2 M KOH | 0.5 | 10,000 | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignesh, S.; Ahmad, K.; Oh, T.H. Progress in Nickel MOF-Based Materials for Electrochemical Biosensor and Supercapacitor Applications. Biosensors 2025, 15, 560. https://doi.org/10.3390/bios15090560
Vignesh S, Ahmad K, Oh TH. Progress in Nickel MOF-Based Materials for Electrochemical Biosensor and Supercapacitor Applications. Biosensors. 2025; 15(9):560. https://doi.org/10.3390/bios15090560
Chicago/Turabian StyleVignesh, Shanmugam, Khursheed Ahmad, and Tae Hwan Oh. 2025. "Progress in Nickel MOF-Based Materials for Electrochemical Biosensor and Supercapacitor Applications" Biosensors 15, no. 9: 560. https://doi.org/10.3390/bios15090560
APA StyleVignesh, S., Ahmad, K., & Oh, T. H. (2025). Progress in Nickel MOF-Based Materials for Electrochemical Biosensor and Supercapacitor Applications. Biosensors, 15(9), 560. https://doi.org/10.3390/bios15090560





