Sophisticated Interfaces Between Biosensors and Organoids: Advancing Towards Intelligent Multimodal Monitoring Physiological Parameters
Abstract
1. Introduction
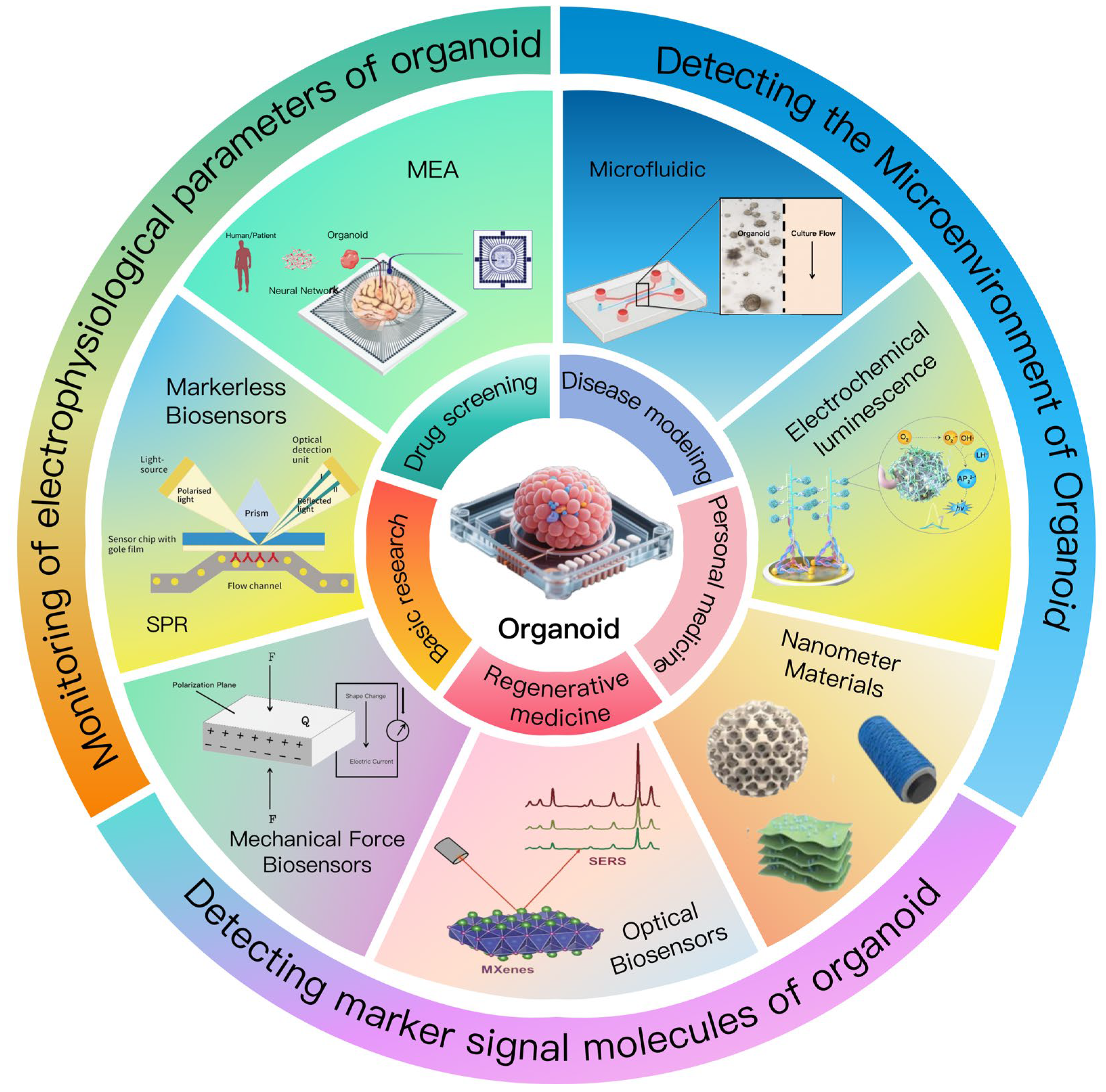
2. Organoids and Biosensors
2.1. Organoid Microenvironment Monitoring Sensors
2.1.1. Microfluidic System
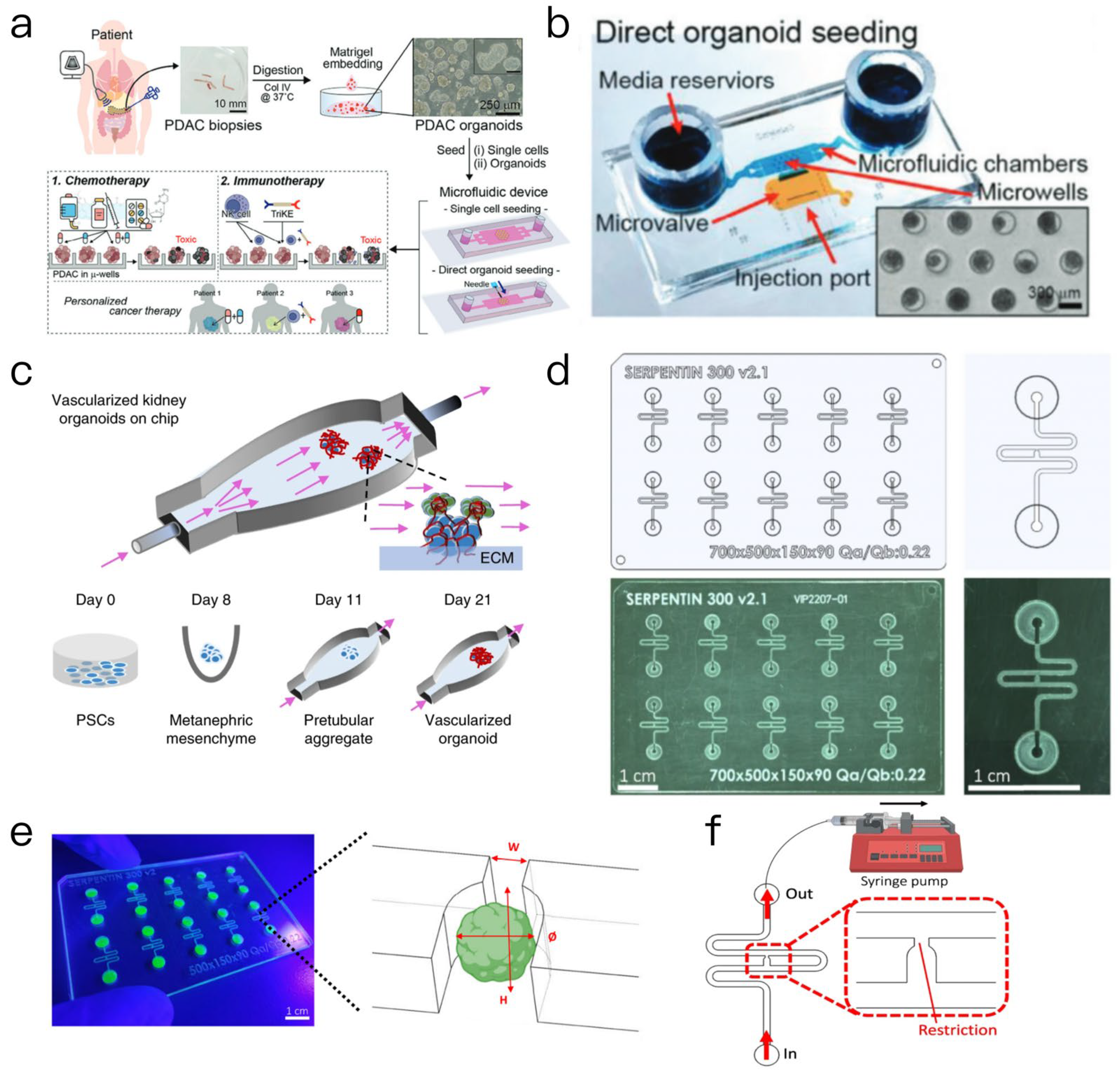
2.1.2. Electrochemical Biosensors
2.1.3. Field-Effect Transistors (FETs)
2.2. Monitoring of Organoid Electrophysiological Parameters
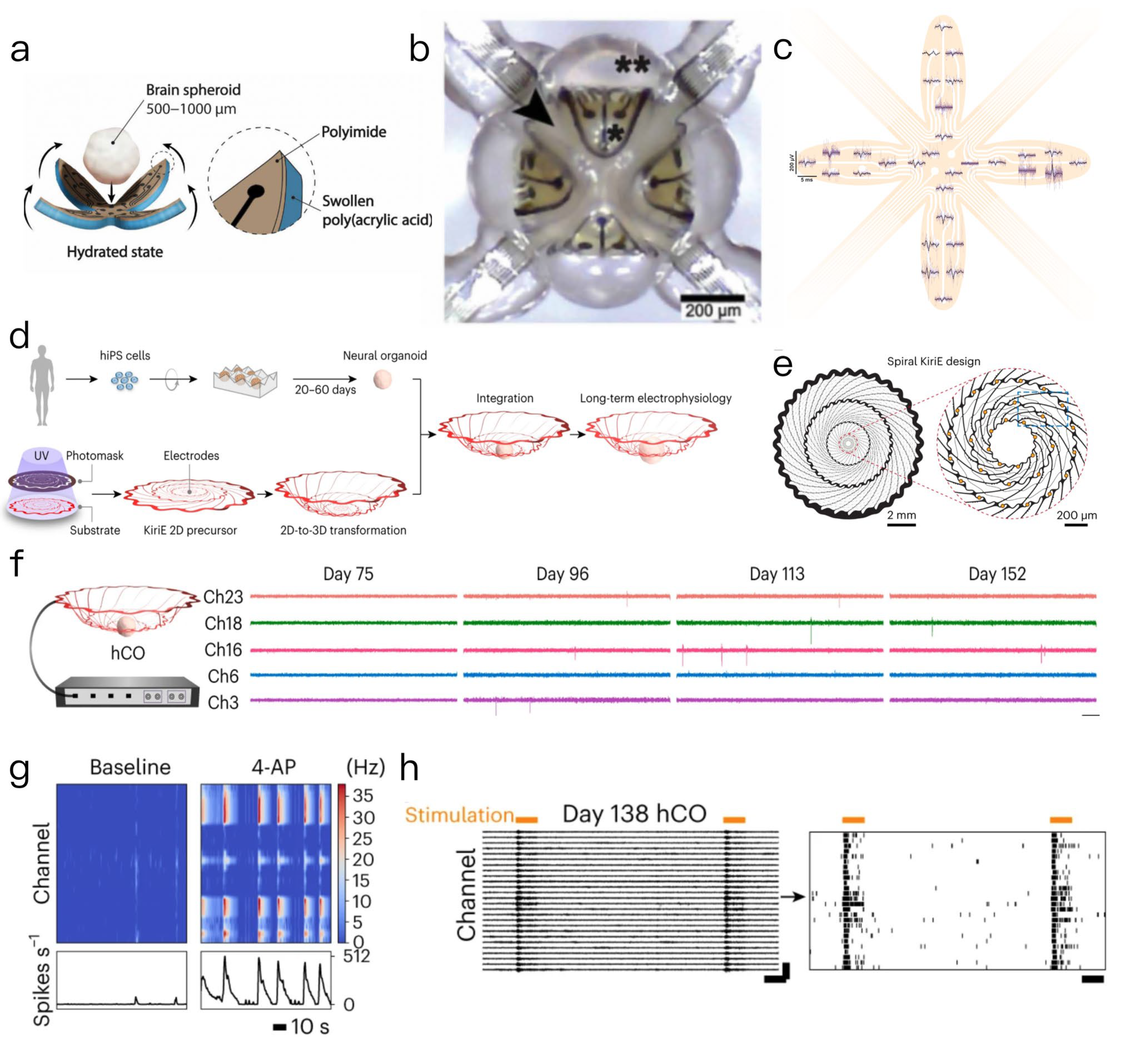
2.2.1. Microelectrode Array (MEA) Technology
2.2.2. Mechanical Force Transducers
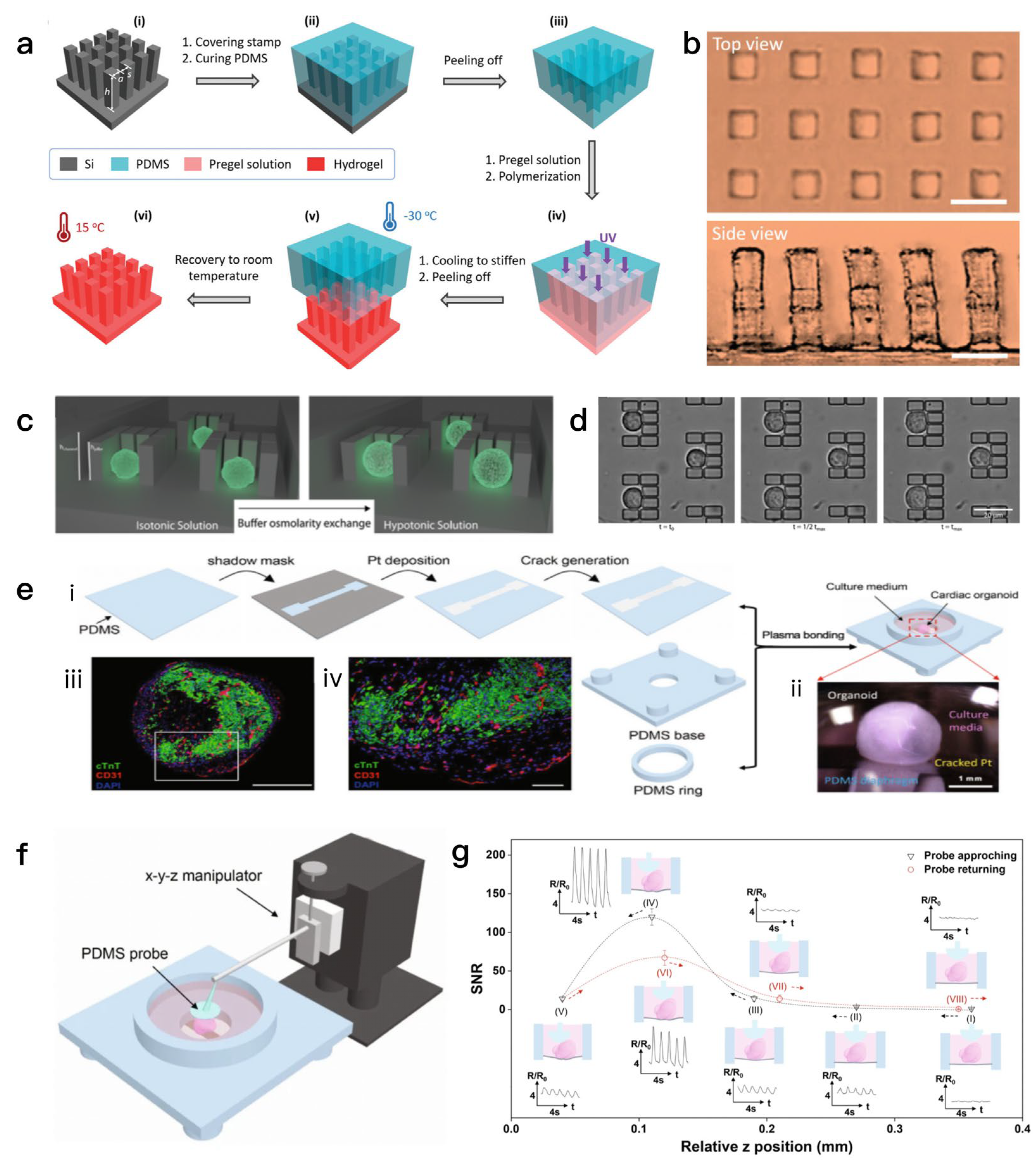
2.3. Sensors That Detect Organoid Marker Signaling Molecules
2.3.1. Optical Biosensors
2.3.2. Label-Free Sensing
| Category | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Fluorescence Imaging | Detects target molecules or biological processes by monitoring changes in fluorescence signals (e.g., intensity, wavelength shifts) [94,95]. | 1. High sensitivity, suitable for low-concentration biomolecule detection. 2. Non-destructive, enabling live-cell and organoid monitoring. 3. Widely applied in studying tumor migration, drug responses, and intracellular dynamics (e.g., calcium imaging in cardiac organoids) [97,98,99,100,101,102]. | 1. Susceptible to fluorescence background interference in complex biological samples [110]. 2. May require labeling, which could affect biological activity. |
| Raman Spectroscopy | Obtains molecular “fingerprints” by analyzing frequency shifts of scattered light after interaction with molecules, based on molecular vibrational scattering [18]. | 1. Label-free, no need for sample pretreatment. 2. Provides detailed chemical structure information (e.g., DNA, RNA, proteins) [123]. 3. Enables high-throughput analysis with optimized systems (e.g., linear illumination Raman microscope) [104]. 4. Applicable for disease classification (e.g., Alzheimer’s disease) [105]. | 1. Lower sensitivity compared to fluorescence spectroscopy for ultra-low concentration analytes. 2. Requires longer acquisition times for high-quality data. |
| Fluorescence-Raman Hybrid Technology | Integrates fluorescence and Raman spectroscopy to combine their signal outputs [96]. | 1. Complements respective shortcomings: fluorescence provides high sensitivity, while Raman offers chemical structure specificity. 2. Enables simultaneous acquisition of molecular concentration and chemical composition, improving analysis comprehensiveness and accuracy [111,112]. | 1. Increased system complexity due to integration of two techniques. 2. Potential signal crosstalk between fluorescence and Raman signals, requiring careful optimization. |
3. Integration of Multimodal Sensors in Organoids
4. Challenges and Prospects of Organoids and Biosensors
4.1. Challenge
4.2. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- HogenEsch, H.; Nikitin, A.Y. Challenges in Pre-Clinical Testing of Anti-Cancer Drugs in Cell Culture and in Animal Models. J. Control. Release 2012, 164, 183–186. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Liang, K.X. The application of brain organoid for drug discovery in mitochondrial diseases. Int. J. Biochem. Cell Biol. 2024, 170, 106556. [Google Scholar] [CrossRef]
- Singh, D.; Thakur, A.; Rakesh; Kumar, A. Advancements in Organoid-Based Drug Discovery: Revolutionizing Precision Medicine and Pharmacology. Drug Dev. Res. 2025, 86, e70121. [Google Scholar] [CrossRef]
- Mujeeb, U.R.M.; Honarvar Nazari, M.; Sencan, M. A novel semiconductor based wireless electrochemical sensing platform for chronic disease management. Biosens. Bioelectron. 2019, 124–125, 66–74. [Google Scholar] [CrossRef]
- McDonald, M.; Sebinger, D.; Brauns, L.; Gonzalez-Cano, L.; Menuchin-Lasowski, Y.; Mierzejewski, M.; Psathaki, O.E.; Stumpf, A.; Wickham, J.; Rauen, T.; et al. A mesh microelectrode array for non-invasive electrophysiology within neural organoids. Biosens. Bioelectron. 2023, 228, 115223. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.; Jung, K.B.; Kim, Y.; Jang, E.; Lee, M.O.; Son, M.Y.; Lee, H.J. Highly Stretchable 3D Microelectrode Array for Noninvasive Functional Evaluation of Cardiac Spheroids and Midbrain Organoids. Adv. Mater. 2025, 37, e2412953. [Google Scholar] [CrossRef]
- Becker, L.; Janssen, N.; Layland, S.L.; Murdter, T.E.; Nies, A.T.; Schenke-Layland, K.; Marzi, J. Raman Imaging and Fluorescence Lifetime Imaging Microscopy for Diagnosis of Cancer State and Metabolic Monitoring. Cancers 2021, 13, 5682. [Google Scholar] [CrossRef]
- Dobric, A.; Tape, C.J. High-dimensional signalling analysis of organoids. Curr. Opin. Cell Biol. 2025, 94, 102488. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, W.; Liu, R.; Li, Q.; Lee, J.; Hirschler, C.; Liu, J. Cyborg organoids integrated with stretchable nanoelectronics can be functionally mapped during development. Nat. Protoc. 2025, 1–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Chen, Y.; Yu, Y.; Diao, X.; Chiu, R.; Fang, J.; Shen, Y.; Wang, J.; Zhu, L.; et al. Human Airway Organoids and Multimodal Imaging-Based Toxicity Evaluation of 1-Nitropyrene. Environ. Sci. Technol. 2024, 58, 6083–6092. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Xu, Q.; Ikkala, O.; Peng, B. Mechanosensing of Stimuli Changes with Magnetically Gated Adaptive Sensitivity. ACS Mater. Lett. 2025, 7, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Kar, E.; Bose, N.; Dutta, B.; Mukherjee, N.; Mukherjee, S. Ultraviolet- and Microwave-Protecting, Self-Cleaning e-Skin for Efficient Energy Harvesting and Tactile Mechanosensing. ACS Appl. Mater. Interfaces 2019, 11, 17501–17512. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Wang, N.; Yang, Y.; Govindaraj, K.; Velasco, J.J.; Martinez Blanco, A.D.; Bae, N.H.; Lee, H.; Shin, S.R. Enhancing organoid technology with carbon-based nanomaterial biosensors: Advancements, challenges, and future directions. Adv. Drug Deliv. Rev. 2025, 222, 115592. [Google Scholar] [CrossRef]
- Shin, S.R.; Kilic, T.; Zhang, Y.S.; Avci, H.; Hu, N.; Kim, D.; Branco, C.; Aleman, J.; Massa, S.; Silvestri, A.; et al. Label-Free and Regenerative Electrochemical Microfluidic Biosensors for Continual Monitoring of Cell Secretomes. Adv. Sci. 2017, 4, 1600522. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- List, M.; Schmidt, S.; Christiansen, H.; Rehmsmeier, M.; Tan, Q.; Mollenhauer, J.; Baumbach, J. Comprehensive analysis of high-throughput screens with HiTSeekR. Nucleic Acids Res. 2016, 44, 6639–6648. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Castanheira, E.M.S.; Lanceros-Mendez, S.; Cardoso, V.F. Recent Advances on Cell Culture Platforms for In Vitro Drug Screening and Cell Therapies: From Conventional to Microfluidic Strategies. Adv. Healthc. Mater. 2023, 12, e2202936. [Google Scholar] [CrossRef]
- Choi, D.; Gonzalez-Suarez, A.M.; Dumbrava, M.G.; Medlyn, M.; De Hoyos-Vega, J.M.; Cichocki, F.; Miller, J.S.; Ding, L.; Zhu, M.; Stybayeva, G.; et al. Microfluidic Organoid Cultures Derived from Pancreatic Cancer Biopsies for Personalized Testing of Chemotherapy and Immunotherapy. Adv. Sci. 2024, 11, 2303088. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibanez, E.; Cifuentes, A.; Lu, W. Microfluidic biosensors for biomarker detection in body fluids: A key approach for early cancer diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-Enhanced Vascularization and Maturation of Kidney Organoids in Vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Quintard, C.; Tubbs, E.; Jonsson, G.; Jiao, J.; Wang, J.; Werschler, N.; Laporte, C.; Pitaval, A.; Bah, T.S.; Pomeranz, G.; et al. A Microfluidic Platform Integrating Functional Vascularized Organoids-on-Chip. Nat. Commun. 2024, 15, 1452. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, J.; Zhang, H.; Pang, X.; Zhai, Y.; Cheng, H.; Xu, D.; Liao, M.; Qi, Y.; Wu, D.; et al. Electroacoustic Responsive Cochlea-on-a-Chip. Adv. Mater. 2024, 36, e2309002. [Google Scholar] [CrossRef]
- Tedjo, W.; Obeidat, Y.; Catandi, G.; Carnevale, E.; Chen, T. Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array. Biosensors 2021, 11, 256. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordonez-Moran, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Debnath, K.; Heras, K.L.; Rivera, A.; Lenzini, S.; Shin, J.W. Extracellular vesicle-matrix interactions. Nat. Rev. Mater. 2023, 8, 390–402. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Zhang, H.; Shang, L.; Zhao, Y. Microfluidics for Drug Development: From Synthesis to Evaluation. Chem. Rev. 2021, 121, 7468–7529. [Google Scholar] [CrossRef]
- Gough, A.; Soto-Gutierrez, A.; Vernetti, L.; Ebrahimkhani, M.R.; Stern, A.M.; Taylor, D.L. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 252–268. [Google Scholar] [CrossRef]
- Elvira, K.S. Microfluidic technologies for drug discovery and development: Friend or foe? Trends Pharmacol. Sci. 2021, 42, 518–526. [Google Scholar] [CrossRef]
- Wei, F.; Patel, P.; Liao, W.; Chaudhry, K.; Zhang, L.; Arellano-Garcia, M.; Hu, S.; Elashoff, D.; Zhou, H.; Shukla, S.; et al. Electrochemical sensor for multiplex biomarkers detection. Clin. Cancer Res. 2009, 15, 4446–4452. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Parihar, A.; Singhal, A.; Kumar, N.; Khan, R.; Khan, M.A.; Srivastava, A.K. Next-Generation Intelligent MXene-Based Electrochemical Aptasensors for Point-of-Care Cancer Diagnostics. Nanomicro Lett. 2022, 14, 100. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Singh, D.P.; Yadav, R.M. Self-Assembled Hierarchical Formation of Conjugated 3D Cobalt Oxide Nanobead-CNT-Graphene Nanostructure Using Microwaves for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2015, 7, 15042–15051. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Brazys, E.; Ratautaite, V.; Mohsenzadeh, E.; Boguzaite, R.; Ramanaviciute, A.; Ramanavicius, A. Formation of molecularly imprinted polymers: Strategies applied for the removal of protein template (review). Adv. Colloid Interface Sci. 2025, 337, 103386. [Google Scholar] [CrossRef]
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Marks, A.; Luscombe, C.K. Molecular Design Strategies toward Improvement of Charge Injection and Ionic Conduction in Organic Mixed Ionic-Electronic Conductors for Organic Electrochemical Transistors. Chem. Rev. 2022, 122, 4325–4355. [Google Scholar] [CrossRef]
- Bakhshandeh, F.; Zheng, H.; Barra, N.G.; Sadeghzadeh, S.; Ausri, I.; Sen, P.; Keyvani, F.; Rahman, F.; Quadrilatero, J.; Liu, J.; et al. Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals. Adv. Mater. 2024, 36, e2313743. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, G.R.; Cho, E.N.; Jung, Y.S. Fabrication and Applications of 3D Nanoarchitectures for Advanced Electrocatalysts and Sensors. Adv. Mater. 2020, 32, e1907500. [Google Scholar] [CrossRef]
- Aleman, J.; Kilic, T.; Mille, L.S.; Shin, S.R.; Zhang, Y.S. Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat. Protoc. 2021, 16, 2564–2593. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent Advances in Field Effect Transistor Biosensors: Designing Strategies and Applications for Sensitive Assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef]
- Septiana, W.L.; Pawitan, J.A. Potential Use of Organoids in Regenerative Medicine. Tissue Eng. Regen. Med. 2024, 21, 1125–1139. [Google Scholar] [CrossRef]
- Bajgai, J.; Jun, M.; Oh, J.H.; Lee, J.H. A perspective on the potential use of aptamer-based field-effect transistor sensors as biosensors for ovarian cancer biomarkers CA125 and HE4. Talanta 2025, 292, 127954. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Shi, P.; You, X.; Zhao, G. Establishment and evaluation of on-chip intestinal barrier biosystems based on microfluidic techniques. Mater. Today Bio 2024, 26, 101079. [Google Scholar] [CrossRef]
- Marcos, L.F.; Wilson, S.L.; Roach, P. Tissue engineering of the retina: From organoids to microfluidic chips. J. Tissue Eng. 2021, 12, 20417314211059876. [Google Scholar] [CrossRef] [PubMed]
- Manimekala, T.; Sivasubramanian, R.; Dharmalingam, G. Nanomaterial-Based Biosensors using Field-Effect Transistors: A Review. J. Electron. Mater. 2022, 51, 1950–1973. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sheng, Z.; Hou, X.; Chen, H.; Zhang, Z.; Zhang, D.W.; Zhou, P. Ambipolar 2D Semiconductors and Emerging Device Applications. Small Methods 2021, 5, e2000837. [Google Scholar] [CrossRef]
- Yang, A.J.; Wang, S.-X.; Xu, J.; Loh, X.J.; Zhu, Q.; Wang, X.R. Two-Dimensional Layered Materials Meet Perovskite Oxides: A Combination for High-Performance Electronic Devices. ACS Nano 2023, 17, 9748–9762. [Google Scholar] [CrossRef]
- Tao, J.; Sun, W.; Lu, L. Organic small molecule semiconductor materials for OFET-based biosensors. Biosens. Bioelectron. 2022, 216, 114667. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Fan, X.; Xu, Y.; Chen, S.; Zhang, X.; Man, B.; Yang, C.; Du, J. Review on two-dimensional material-based field-effect transistor biosensors: Accomplishments, mechanisms, and perspectives. J. Nanobiotechnol. 2023, 21, 144. [Google Scholar] [CrossRef]
- Xiao, W.-H.; Hu, Y.; Yan, K.; Tang, L.-M.; Chen, X.; D’aGosta, R.; Yang, K. Phonon-limited carrier mobility modeling of two-dimensional semiconductors based on first principles. J. Phys. Condens. Matter 2025, 37, 263001. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Q.; Zeng, T.; Ye, K.; Zhou, H.; Han, Z.; Zeng, Y.; Fang, B.; Lv, W.; Geng, L.; et al. Van der Waals Antiferroelectric CuCrP2S6-Based Artificial Synapse for High-Precision Neuromorphic Computation. Small 2025, 21, e2502676. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Ren, Y.; Liu, Y.; Wu, X.; Li, Z.; Ren, J. Biomaterials and biosensors in intestinal organoid culture, a progress review. J. Biomed. Mater. Res. Part A 2020, 108, 1501–1508. [Google Scholar] [CrossRef]
- Otero, J.; Ulldemolins, A.; Farré, R.; Almendros, I. Oxygen Biosensors and Control in 3D Physiomimetic Experimental Models. Antioxidants 2021, 10, 1165. [Google Scholar] [CrossRef]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. Semin. Cell Dev. Biol. 2022, 144, 41–54. [Google Scholar] [CrossRef]
- Caipa Garcia, A.L.; Kucab, J.E.; Al-Serori, H.; Beck, R.S.S.; Fischer, F.; Hufnagel, M.; Hartwig, A.; Floeder, A.; Balbo, S.; Francies, H.; et al. Metabolic Activation of Benzo (a) pyrene by Human Tissue Organoid Cultures. Int. J. Mol. Sci. 2022, 24, 606. [Google Scholar] [CrossRef]
- Skottvoll, F.S.; Hansen, F.A.; Harrison, S.; Boger, I.S.; Mrsa, A.; Restan, M.S.; Stein, M.; Lundanes, E.; Pedersen-Bjergaard, S.; Aizenshtadt, A.; et al. Electromembrane Extraction and Mass Spectrometry for Liver Organoid Drug Metabolism Studies. Anal. Chem. 2021, 93, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, Y.; Wei, D. Two-Dimensional Field-Effect Transistor Sensors: The Road toward Commercialization. Chem. Rev. 2022, 122, 10319–10392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, C.M.; Huynh, M.A.; Vu, H.H.; Nguyen, T.K.; Nguyen, N.T. Field effect transistor based wearable biosensors for healthcare monitoring. J. Nanobiotechnol. 2023, 21, 411. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Shu, J.; Zhang, G.J. Flexible Field-Effect Transistor Sensors for Next-Generation Health Monitoring: Materials to Advanced Applications. Small 2025, e2504059. [Google Scholar] [CrossRef]
- Lv, S.; He, E.; Luo, J.; Liu, Y.; Liang, W.; Xu, S.; Zhang, K.; Yang, Y.; Wang, M.; Song, Y.; et al. Using Human-Induced Pluripotent Stem Cell Derived Neurons on Microelectrode Arrays to Model Neurological Disease: A Review. Adv. Sci. 2023, 10, e2301828. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 2021, 24, 1488–1500. [Google Scholar] [CrossRef]
- Yang, W.; Ouyang, Q.; Zhu, Z.; Wu, Y.; Fan, M.; Liao, Y.; Guo, X.; Xu, Z.; Zhang, X.; Zhang, Y.; et al. A biosensing system employing nonlinear dynamic analysis-assisted neural network for drug-induced cardiotoxicity assessment. Biosens. Bioelectron. 2023, 222, 114923. [Google Scholar] [CrossRef]
- Sharf, T.; van der Molen, T.; Glasauer, S.M.K.; Guzman, E.; Buccino, A.P.; Luna, G.; Cheng, Z.; Audouard, M.; Ranasinghe, K.G.; Kudo, K.; et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat. Commun. 2022, 13, 4403. [Google Scholar] [CrossRef]
- Huang, Z.; Jia, K.; Tan, Y.; Yu, Y.; Xiao, W.; Zhou, X.; Yi, J.; Zhang, C. Advances in cardiac organoid research: Implications for cardiovascular disease treatment. Cardiovasc. Diabetol. 2025, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y.; Wang, J.; Xu, T.; Zhang, X. Integrated Mini-Pillar Platform for Wireless Real-Time Cell Monitoring. Research 2024, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Volmert, B.; Kiselev, A.; Juhong, A.; Wang, F.; Riggs, A.; Kostina, A.; O’Hern, C.; Muniyandi, P.; Wasserman, A.; Huang, A.; et al. A patterned human primitive heart organoid model generated by pluripotent stem cell self-organization. Nat. Commun. 2023, 14, 8245. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Akouissi, O.; Liebi, L.; Furfaro, I.; Maulà, D.; Savoia, N.; Remy, A.; Nikles, L.; Roux, A.; Stoppini, L.; et al. The E-Flower: A Hydrogel-Actuated 3D MEA for Brain Spheroid Electrophysiology. Sci. Adv. 2024, 10, eadp8054. [Google Scholar] [CrossRef]
- Yang, X.; Forró, C.; Li, T.L.; Miura, Y.; Zaluska, T.J.; Tsai, C.-T.; Kanton, S.; McQueen, J.P.; Chen, X.; Mollo, V.; et al. Kirigami Electronics for Long-Term Electrophysiological Recording of Human Neural Organoids and Assembloids. Nat. Biotechnol. 2024, 42, 1836–1843. [Google Scholar] [CrossRef]
- Didier, C.M.; Fox, D.; Pollard, K.J.; Baksh, A.; Iyer, N.R.; Bosak, A.; Li Sip, Y.Y.; Orrico, J.F.; Kundu, A.; Ashton, R.S.; et al. Fully Integrated 3D Microelectrode Arrays with Polydopamine-Mediated Silicon Dioxide Insulation for Electrophysiological Interrogation of a Novel 3D Human, Neural Microphysiological Construct. ACS Appl. Mater. Interfaces 2023, 15, 37157–37173. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.; Xi, Y.; Ruan, T.; Cao, J.; Xu, M.; Zheng, K.; Du, Z.; Wei, N.; Wang, X.; et al. An Efficient MEMS Microelectrode Array with Reliable Interelectrode Insulation Processes for In Vivo Neural Recording. Small 2025, 21, e2407950. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Yang, X.; Kang, J. Unveiling the potential of amorphous nanocatalysts in membrane-based hydrogen production. Mater. Horiz. 2024, 11, 4885–4910. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, R.; Chen, X.; Chen, Z.; Zhu, J.J. In Vivo Voltammetric Imaging of Metal Nanoparticle-Catalyzed Single-Cell Electron Transfer by Fermi Level-Responsive Graphene. Research 2023, 6, 0145. [Google Scholar] [CrossRef]
- Hang, X.; He, S.; Dong, Z.; Minnick, G.; Rosenbohm, J.; Chen, Z.; Yang, R.; Chang, L. Nanosensors for single cell mechanical interrogation. Biosens. Bioelectron. 2021, 179, 113086. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Li, R.; Wang, S.; Geng, Z.; Shi, Z.; Jing, Y.; Xu, K.; Wei, Y.; Wang, G.; et al. Standardization and consensus in the development and application of bone organoids. Theranostics 2025, 15, 682–706. [Google Scholar] [CrossRef] [PubMed]
- Sato, T. Role of Patient-Derived Tumor Organoids in Advanced Cancer Research. J. Nippon. Med. Sch. 2025, 92, 234–241. [Google Scholar] [CrossRef]
- Abdel Fattah, A.R.; Daza, B.; Rustandi, G.; Berrocal-Rubio, M.A.; Gorissen, B.; Poovathingal, S.; Davie, K.; Barrasa-Fano, J.; Condor, M.; Cao, X.; et al. Actuation enhances patterning in human neural tube organoids. Nat. Commun. 2021, 12, 3192. [Google Scholar] [CrossRef]
- Tian, Y.; Hou, L.X.; Zhang, X.N.; Du, M.; Zheng, Q.; Wu, Z.L. Engineering Tough Supramolecular Hydrogels with Structured Micropillars for Tunable Wetting and Adhesion Properties. Small 2024, 20, e2308570. [Google Scholar] [CrossRef]
- Cortelli, G.; Grob, L.; Patruno, L.; Cramer, T.; Mayer, D.; Fraboni, B.; Wolfrum, B.; de Miranda, S. Determination of Stiffness and the Elastic Modulus of 3D-Printed Micropillars with Atomic Force Microscopy-Force Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 7602–7609. [Google Scholar] [CrossRef]
- Fajrial, A.K.; Liu, K.; Gao, Y.; Gu, J.; Lakerveld, R.; Ding, X. Characterization of Single-Cell Osmotic Swelling Dynamics for New Physical Biomarkers. Anal. Chem. 2021, 93, 1317–1325. [Google Scholar] [CrossRef]
- Tang, Z.X.; Wang, B.; Li, Z.R.; Huang, Z.; Zhao, H.X.; Long, L.S.; Zheng, L.S. Enhancing the performance of molecule-based piezoelectric sensors by optimizing their microstructures. Chem. Sci. 2024, 15, 18060–18066. [Google Scholar] [CrossRef]
- Lyu, Q.; Gong, S.; Lees, J.G.; Yin, J.; Yap, L.W.; Kong, A.M.; Shi, Q.; Fu, R.; Zhu, Q.; Dyer, A.; et al. A soft and ultrasensitive force sensing diaphragm for probing cardiac organoids instantaneously and wirelessly. Nat. Commun. 2022, 13, 7259. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, Z.; Le, X.; Xie, J.; Lee, C. Soft Robotic Perception System with Ultrasonic Auto-Positioning and Multimodal Sensory Intelligence. ACS Nano 2023, 17, 4985–4998. [Google Scholar] [CrossRef]
- Xiao, Z.; Ren, Z.; Zhuge, Y.; Zhang, Z.; Zhou, J.; Xu, S.; Xu, C.; Dong, B.; Lee, C. Multimodal In-Sensor Computing System Using Integrated Silicon Photonic Convolutional Processor. Adv. Sci. 2024, 11, e2408597. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Y.; Ma, G.; Zhang, M.; Fu, J.; Luo, C.; Yuan, L.; Long, Y. Overview of 3D Printing Multimodal Flexible Sensors. ACS Appl. Mater. Interfaces 2024, 16, 65779–65795. [Google Scholar] [CrossRef] [PubMed]
- Maly, P.; Brixner, T. Fluorescence-Detected Pump-Probe Spectroscopy. Angew. Chem. Int. Ed. Engl. 2021, 60, 18867–18875. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yao, Z.; Zhang, H.; Li, Z.; Chen, Z.; Xiong, H. Transient Stimulated Raman Excited Fluorescence Spectroscopy. J. Am. Chem. Soc. 2023, 145, 7758–7762. [Google Scholar] [CrossRef]
- Qian, N.; Xiong, H.; Wei, L.; Shi, L.; Min, W. Merging Vibrational Spectroscopy with Fluorescence Microscopy: Combining the Best of Two Worlds. Annu. Rev. Phys. Chem. 2025, 76, 279–301. [Google Scholar] [CrossRef]
- Ansaryan, S.; Chiang, Y.C.; Liu, Y.C.; Tan, J.; Lorenzo-Martin, L.F.; Lutolf, M.P.; Tolstonog, G.; Altug, H. Spatiotemporal Interrogation of Single Spheroids Using Multiplexed Nanoplasmonic-Fluorescence Imaging. Small Methods 2025, e2500106. [Google Scholar] [CrossRef]
- Huang, K.; Li, M.; Li, Q.; Chen, Z.; Zhang, Y.; Gu, Z. Image-based profiling and deep learning reveal morphological heterogeneity of colorectal cancer organoids. Comput. Biol. Med. 2024, 173, 108322. [Google Scholar] [CrossRef]
- Luo, X.; Chen, M.; Shan, H.; Yu, X.; Lin, Q.; Tao, Q.; Wei, X.; Lv, C.; Chen, Z.; Zhuo, F.; et al. Label-Free 3D Photoacoustic Imaging of Tumor Organoids for Volumetric Drug Screening. Adv. Sci. 2025, e17226. [Google Scholar] [CrossRef]
- Fatima, M.; Abbas, N. Expanding Horizons in Advancements of FRET Biosensing Technologies. Biosensors 2025, 15, 452. [Google Scholar] [CrossRef]
- Tirgar, P.; Vikstrom, A.; Sepulveda, J.M.R.; Srivastava, L.K.; Amini, A.; Tabata, T.; Higo, S.; Bub, G.; Ehrlicher, A. Heart-on-a-Miniscope: A Miniaturized Solution for Electrophysiological Drug Screening in Cardiac Organoids. Small 2025, 21, e2409571. [Google Scholar] [CrossRef]
- Barth, B.M.; Sharma, R.; Altinoglu, E.I.; Morgan, T.T.; Shanmugavelandy, S.S.; Kaiser, J.M.; McGovern, C.; Matters, G.L.; Smith, J.P.; Kester, M.; et al. Bioconjugation of calcium phosphosilicate composite nanoparticles for selective targeting of human breast and pancreatic cancers in vivo. ACS Nano 2010, 4, 1279–1287. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, V.; Garnaik, U.C.; Dada, R.; Qamar, I.; Goel, V.K.; Agarwal, S. Unveiling the Molecular Secrets: A Comprehensive Review of Raman Spectroscopy in Biological Research. ACS Omega 2024, 9, 50049–50063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, M.; Zhao, X.; Wang, Y.; Li, D. Fourier Raman light field microscopy based on surface-enhanced Raman scattering. Opt. Lett. 2024, 49, 4693–4696. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Kral, M.; Vrtelka, O.; Setnicka, V. Comparative study of Raman spectroscopy techniques in blood plasma-based clinical diagnostics: A demonstration on Alzheimer’s disease. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123392. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; Charron, G.; Cortes, E.; Kneipp, J.; de la Chapelle, M.L.; Langer, J.; Prochazka, M.; Tran, V.; Schlucker, S. Towards Reliable and Quantitative Surface-Enhanced Raman Scattering (SERS): From Key Parameters to Good Analytical Practice. Angew. Chem. Int. Ed. Engl. 2020, 59, 5454–5462. [Google Scholar] [CrossRef]
- Lian, S.; Li, X.; Lv, X. Recent Developments in SERS Microfluidic Chips: From Fundamentals to Biosensing Applications. ACS Appl. Mater. Interfaces 2025, 17, 10193–10230. [Google Scholar] [CrossRef]
- Skinner, W.H.; Robinson, N.; Hardisty, G.R.; Fleming, H.; Geddis, A.; Bradley, M.; Gray, R.D.; Campbell, C.J. SERS microsensors for pH measurements in the lumen and ECM of stem cell derived human airway organoids. Chem. Commun. 2023, 59, 3249–3252. [Google Scholar] [CrossRef]
- Mou, L.; Mandal, K.; Mecwan, M.M.; Hernandez, A.L.; Maity, S.; Sharma, S.; Herculano, R.D.; Kawakita, S.; Jucaud, V.; Dokmeci, M.R.; et al. Integrated biosensors for monitoring microphysiological systems. Lab Chip 2022, 22, 3801–3816. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.F. Multifunctional graphene magnetic nanosheet decorated with chitosan for highly sensitive detection of pathogenic bacteria. J. Mater. Chem. B 2013, 1, 3950–3961. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Xue, S.; Zhou, R.; Wang, C.; Guo, Z.; Wang, Y.; Cai, J. “Raman plus X” dual-modal spectroscopy technology for food analysis: A review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70102. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; Abbasi, H.; Bakker Schut, T.C.; Van Driel, P.; Hardillo, J.A.U.; Santos, I.P.; Barroso, E.M.; Koljenovic, S.; Vahrmeijer, A.L.; Baatenburg de Jong, R.J.; et al. The complementary value of intraoperative fluorescence imaging and Raman spectroscopy for cancer surgery: Combining the incompatibles. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2364–2376. [Google Scholar] [CrossRef]
- Reuter, C.; Hauswald, W.; Burgold-Voigt, S.; Hubner, U.; Ehricht, R.; Weber, K.; Popp, J. Imaging Diffractometric Biosensors for Label-Free, Multi-Molecular Interaction Analysis. Biosensors 2024, 14, 398. [Google Scholar] [CrossRef]
- Hanifa Lestari, T.F.; Irkham, I.; Pratomo, U.; Gaffar, S.; Zakiyyah, S.N.; Rahmawati, I.; Topkaya, S.N.; Hartati, Y.W. Label-free and label-based electrochemical detection of disease biomarker proteins. ADMET DMPK 2024, 12, 463–486. [Google Scholar]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of organoid culture in cancer research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Zhang, Y.; Zhong, H.; Cai, X.; Guan, R. Recent progress on the organoids: Techniques, advantages and applications. Biomed. Pharmacother. 2025, 185, 117942. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, S.; Lee, Y.J.; He, Y.; Kong, E.M.; Li, H.; Anastasio, M.A.; Popescu, G. Live-dead assay on unlabeled cells using phase imaging with computational specificity. Nat. Commun. 2022, 13, 713. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Tao, H.; Liu, F.; Hu, B.; Wu, M.; Yu, H. Research on adaptive impedance control technology of upper limb rehabilitation robot based on impedance parameter prediction. Front. Bioeng. Biotechnol. 2023, 11, 1332689. [Google Scholar] [CrossRef]
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J.H. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262. [Google Scholar] [CrossRef]
- He, Z.; Li, F.; Zuo, P.; Tian, H. Principles and Applications of Resonance Energy Transfer Involving Noble Metallic Nanoparticles. Materials 2023, 16, 3083. [Google Scholar] [CrossRef]
- Bankoglu Yola, B.; Ozdemir, N.; Yola, M.L. A Review Study on Molecularly Imprinting Surface Plasmon Resonance Sensors for Food Analysis. Biosensors 2024, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.; Bockova, M.; Slaby, J.; Sohrabi, F.; Capkova, M.; Homola, J. Surface plasmon resonance biosensors and their medical applications. Biosens. Bioelectron. 2025, 278, 117308. [Google Scholar] [CrossRef] [PubMed]
- Zemaitis, K.J.; Lin, V.S.; Ahkami, A.H.; Winkler, T.E.; Anderton, C.R.; Velickovic, D. Expanded Coverage of Phytocompounds by Mass Spectrometry Imaging Using On-Tissue Chemical Derivatization by 4-APEBA. Anal. Chem. 2023, 95, 12701–12709. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lu, Q.; Liu, S.; Yan, X. Postionization Mass Spectrometry Imaging: Past, Present, and Future. Mass Spectrom. Rev. 2024. [Google Scholar] [CrossRef]
- Dayanidhi, D.L.; Watlington, W.K.; Mantyh, J.B.; Rupprecht, G.; Hsu, D.S. Effects and Eradication of Mycoplasma Contamination on Patient-derived Colorectal Cancer Organoid Cultures. Cancer Res. Commun. 2023, 3, 1952–1958. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Li, J.; Zhou, J.; Yang, H.; Yu, F.; Yu, F.; Khutsishvili, D.; Wang, Z.; Jiang, S.; et al. Droplet-engineered organoids recapitulate parental tissue transcriptome with inter-organoid homogeneity and inter-tumor cell heterogeneity. Fundam. Res. 2024, 4, 1506–1514. [Google Scholar] [CrossRef]
- Matsumoto, M.; Morimoto, Y.; Sato, T.; Takeuchi, S. Microfluidic Device to Manipulate 3D Human Epithelial Cell-Derived Intestinal Organoids. Micromachines 2022, 13, 2082. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Zhou, S.; Wang, M.; Jiang, J.; Wu, W.; Liu, T.; Xu, W.; Zhang, J.; Liu, D.; et al. Mechanical force drives the initial mesenchymal-epithelial interaction during skin organoid development. Theranostics 2023, 13, 2930–2945. [Google Scholar] [CrossRef]
- Ronzetti, M.; Simeonov, A. A comprehensive update on the application of high-throughput fluorescence imaging for novel drug discovery. Expert Opin. Drug Discov. 2025, 20, 785–797. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Y.; Chen, R.; Fan, H.; Kong, L.; Cao, X. A novel perspective on bone tumors: Advances in organoid research. Front. Pharmacol. 2025, 16, 1550163. [Google Scholar] [CrossRef]
- El-Sewify, I.M.; Shenashen, M.A.; El-Agamy, R.F.; Emran, M.Y.; Selim, M.S.; Khairy, M.; Shahat, A.; Selim, M.M.; Elmarakbi, A.; Ebara, M.; et al. Fluorescent sensor/tracker for biocompatible and real-time monitoring of ultra-trace arsenic toxicants in living cells. J. Hazard. Mater. 2024, 478, 135429. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Mori, T.; Uchide, T.; Azakami, D.; Fukushima, R.; Yoshida, T.; et al. Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture. Sci. Rep. 2020, 10, 9393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Oyang, L.; Peng, Q.; Liu, Q.; Xu, X.; Wu, N.; Tan, S.; Yang, W.; Han, Y.; Lin, J.; et al. Organoids: Opportunities and challenges of cancer therapy. Front. Cell Dev. Biol. 2023, 11, 1232528. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, Y.; Hao, Z.; Qu, M.; Huang, C.; Wang, Z.; Yang, J.; Liang, Q.; Jia, Y.; Song, Q.; et al. Graphene-based wearable biosensors for point-of-care diagnostics: From surface functionalization to biomarker detection. Mater. Today Bio 2025, 32, 101667. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Zhang, W.; Tang, J.; Liu, J. Rational design matrix materials for organoid development and application in biomedicine. Regen. Biomater. 2025, 12, rbaf038. [Google Scholar] [CrossRef]
- Narazaki, G.; Miura, Y.; Pavlov, S.D.; Thete, M.V.; Roth, J.G.; Avar, M.; Shin, S.; Kim, J.I.; Hudacova, Z.; Heilshorn, S.C.; et al. Scalable production of human cortical organoids using a biocompatible polymer. Nat. Biomed. Eng. 2025, 1–9. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, Q.; Wang, Q.; Qiu, H.; Li, H.; Xu, T. Fully integrated microneedle biosensor array for wearable multiplexed fitness biomarkers monitoring. Biosens. Bioelectron. 2024, 265, 116697. [Google Scholar] [CrossRef]
- Chimene, D.; Saleem, W.; Longbottom, N.; Ko, B.; Jeevarathinam, A.S.; Horn, S.; McShane, M.J. Long-Term Evaluation of Inserted Nanocomposite Hydrogel-Based Phosphorescent Oxygen Biosensors: Evolution of Local Tissue Oxygen Levels and Foreign Body Response. ACS Appl. Bio Mater. 2024, 7, 3964–3980. [Google Scholar] [CrossRef]
- Xue, J.; Chu, Y.; Huang, Y.; Chen, M.; Sun, M.; Fan, Z.; Wu, Y.; Chen, L. A tumorigenicity evaluation platform for cell therapies based on brain organoids. Transl. Neurodegener. 2024, 13, 53. [Google Scholar] [CrossRef]
- Cooper, R.M.; Wright, J.A.; Ng, J.Q.; Goyne, J.M.; Suzuki, N.; Lee, Y.K.; Ichinose, M.; Radford, G.; Ryan, F.J.; Kumar, S.; et al. Engineered bacteria detect tumor DNA. Science 2023, 381, 682–686. [Google Scholar] [CrossRef]
- Pang, X.; Hu, Y.; Dai, Z.; Lou, Q.; Xu, W.; Chen, L. Precision medicine research progress based on colorectal cancer organoids. Discov. Oncol. 2025, 16, 1181. [Google Scholar] [CrossRef]
- Motoike, S.; Inada, Y.; Toguchida, J.; Kajiya, M.; Ikeya, M. Jawbone-like organoids generated from human pluripotent stem cells. Nat. Biomed. Eng. 2025, 1–19. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, S.; Huang, Y.; Li, J.; Chen, Y.; Gao, L.; Dai, H. Portable multimodal platform with carbon nano-onions as colorimetric and fluorescent signal output for trypsin detection. Talanta 2025, 281, 126819. [Google Scholar] [CrossRef]

| Category | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Electrochemical sensors (basic) | Generate electrical signals (current, potential changes) through the reaction between biological recognition elements on the electrode surface and target substances to reflect the concentration of the substances. | High sensitivity, fast response speed, can be miniaturized and integrated, suitable for real-time monitoring. | Face challenges in sensitivity and selectivity in complex biological environments (such as organoid culture media) and are susceptible to interfering substances. |
| Material-modified electrochemical sensors | Modify electrodes with high specific surface area nanomaterials (such as carbon nanotubes, graphene, MXene and other two-dimensional materials) to enhance electrochemical reaction rates; optimize chemical properties through surface functionalization/modification of electrodes to improve specificity for target molecules. | 1. Nanomaterials significantly enhance the electrochemical reaction rate of electrodes and improve the response capability of sensors. 2. Surface modification can reduce interference and enhance specificity for target molecules. 3. New two-dimensional materials (e.g., MXene) exhibit excellent electrochemical performance and sensitivity, suitable for detection in complex biological environments. | Rely on sophisticated material modification techniques; the dispersibility and stability of nanomaterials may affect the performance consistency of sensors. |
| Molecularly Imprinted Polymer (MIP) technology | Construct specific recognition sites on the electrode surface to accurately identify target analytes. | 1. Significantly improve the selectivity of sensors for target molecules and reduce interference from structurally similar compounds (e.g., glucose sensors eliminate interference from other sugars). 2. Relatively low preparation cost and good stability. | The uniformity of recognition sites may be insufficient, and non-specific binding may occur in complex biological samples. |
| Organic Electrochemical Transistors (OECTs) | Act as signal amplifiers, amplifying detection signals through the sensitivity of organic semiconductor channels to changes in ion concentration. | 1. Greatly improve sensor sensitivity, enabling the detection of extremely low concentrations of biomarkers. 2. Suitable for integrated design, enhancing real-time monitoring capabilities. | Sensitive to environmental conditions (such as humidity and temperature); long-term stability needs further optimization. |
| Integration and miniaturization of electrochemical sensors | Rely on precision manufacturing technologies such as 3D printing and microfabrication to miniaturize sensors and integrate them into the same system (e.g., organoid culture systems) for simultaneous multi-parameter monitoring. | 1. Reduce costs, improve portability and real-time monitoring capabilities. 2. Can be directly embedded into culture systems to continuously monitor metabolic indicators (e.g., glucose, lactate), enhancing monitoring accuracy. 3. Streamline experimental procedures, reduce sample handling, and minimize potential interference. | Miniaturized manufacturing requires high process precision; the compatibility and long-term stability of integrated systems need continuous optimization. |
| Category | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Impedance Sensors | Identify target molecules or cell states by monitoring changes in electrical impedance (current/voltage fluctuations) caused by cell growth, adhesion, or barrier function variations [1,113]. | 1. Enables real-time monitoring of electrophysiological properties, reflecting cell growth, function, and viability [1,112]. 2. Evaluates multiple biological characteristics (barrier function, cell adhesion, proliferation) [118]. 3. Frequency-dependent responses allow cell classification and state monitoring [119]. 4. Label-free, avoiding interference from exogenous markers. | 1. Limited to monitoring changes related to cell quantity or structure (e.g., junctions), providing less specific molecular information. 2. May be affected by environmental factors (e.g., medium conductivity) leading to signal variations. |
| Surface Plasmon Resonance (SPR) | Uses interactions between light and surface plasmons (on a thin metal film, e.g., gold/silver) to monitor refractive index changes caused by biomolecular binding on the surface, detected via shifts in SPR angle [114,120]. | 1. Label-free detection based on refractive index changes, suitable for real-time monitoring of biomolecular interactions [114]. 2. High sensitivity and rapid response, enabling kinetic analysis of molecular binding [119]. 3. Applicable for drug efficacy evaluation and new drug development [115,121,122]. 4. Supports high-throughput analysis. | 1. Relies on a metal film surface, limiting detection to molecules that can bind to the surface (requires specific immobilization). 2. Sensitive to non-specific binding, which may interfere with results in complex biological samples. |
| Mass Spectrometry Imaging (MSI) | Combines mass spectrometry with imaging: ionizes molecules on organoid surfaces using specific matrices, analyzes their mass-to-charge ratios via mass spectrometry, and generates spatial distribution images of molecular species [123,124]. | 1. Provides label-free metabolomics analysis with spatial distribution of multiple molecules in organoids [116,117]. 2. Enables cellular-level probing of metabolic changes, useful for drug metabolism and toxicity assessment [116]. 3. Combines mass spectrometry with imaging to offer both molecular identity and spatial location [123]. | 1. Requires appropriate matrix selection for organoid sections to enhance ionization efficiency [124], adding complexity to sample preparation. 2. May have lower spatial resolution compared to optical imaging techniques for small-scale organoid structures. |
| Technical Type | Application in Organoids | Advantages | Disadvantages |
|---|---|---|---|
| Microfluidic Technology | 1. Centered on microfluidic chips, realizing three-dimensional (3D) culture of organoids to simulate human organ functions. 2. Capable of mimicking tumor microenvironments and metastasis processes for drug testing and mechanism research. 3. Enabling efficient screening of drug compounds, such as anti-cancer drugs. 4. Iintegrating sensors to real-time monitor key parameters in culture media, including pH value, oxygen partial pressure, and nutrient concentration. | 1. Can precisely control microenvironments, such as fluid perfusion and chemical gradients, promoting more biomimetic organ structures and functional maturation. 2. Can integrate sensors for real-time monitoring of cellular responses. 3. In drug screening, it can simulate in vivo drug metabolism processes to more accurately predict drug biological effects and toxicity. 4. Can address the problem of insufficient cell quantity in traditional culture. | 1. Traditional microfluidic chips hardly replicate in vivo flow. 2. There are challenges in achieving in vivo functional vascularization of induced pluripotent stem cell (iPSC)-derived organoids in vitro. 3. Constructing vascularized organ chips remains difficult. |
| Electrochemical Sensors | 1. Detecting biomarkers in organoids, such as metabolic molecules like glucose and lactate, to real-timely reflect the functional status of organoids. 2. Improving the detection capability for specific biomarkers through optimized design and adoption of new materials. | 1. Based on the principle of electrochemical reactions, they can directly detect the concentration of target molecules. 2. The application of new two-dimensional materials, etc., enhances electrochemical performance and sensitivity. 3. The trend of miniaturization and integration reduces costs and improves portability and real-time monitoring capabilities. | 1. Face challenges in sensitivity and selectivity in complex biological environments. 2. Are susceptible to environmental factors and culture materials, and sensor performance may fluctuate, affecting data reliability. |
| Microelectrode array (MEA) technology | 1. Record or stimulate neural electrical activity, such as recording the spontaneous electrical activity of neural organoids to reflect the connections and network activities among neurons. 2. Record the electrophysiological characteristics of cardiac organoids for drug screening and regenerative medicine research. 3. Combine multimodal data fusion strategies with mechanical stress loading, etc., to quantify changes in electrical activity. | 1. It can detect the electrical signals of neurons or cardiomyocytes in a high-throughput manner. 2. The three-dimensional electrode structure can increase the contact area with the organoids, improving the signal quality and stability. 3. And it can achieve long-term stable recording and precise regulation of the electrophysiological activities of the organoids. | 1. Traditional planar electrodes are limited by space when contacting three-dimensional tissues, resulting in poor signal acquisition. 2. Some existing electrophysiological recording techniques cannot perform long-term suspension recording while maintaining the morphology of organoids. |
| Mechanical Force Sensors | 1. Monitoring the response of organoids to external mechanical stimuli, such as the contractile force of cardiac organoids. 2. Combining with other technologies to synchronously monitor mechanical and biochemical signals, etc. 3. Valuating biomechanical properties of cells, such as adhesiveness and migration ability. | 1. Can provide real-time and continuous biophysical data, revealing the interaction between cells and the microenvironment. 2. Emerging ultrasensitive sensors can achieve monitoring of tiny forces. 3. Integrating with multimodal sensing systems provides more comprehensive biological information. | 1. Traditional force sensors face challenges in monitoring complex 3D soft tissues, and their contact and adaptability with organoids need improvement. 2. Some sensors may have stability issues during long-term use. |
| Optical Sensors | 1. Optical sensors using fluorescence imaging to detect tumor cell migration and response to drugs, achieving live-cell calcium imaging of cardiac organoids. 2. Analyzing the chemical structure and metabolic changes of molecules in organoids through Raman spectroscopy. 3. Detecting low-concentration molecules by Surface-Enhanced Raman Spectroscopy (SERS) technology. | 1. Feature high sensitivity and non-destructive detection advantages. 2. Can provide information on the chemical composition and concentration of molecules. 3. SERS technology has extremely high sensitivity in detecting low-concentration molecules. 4. Enables rapid imaging and dynamic monitoring of organoids. | 1. Fluorescence sensors may be interfered by fluorescent background signals in biological samples. 2. Raman spectroscopy signals may be weak when detecting complex samples. 3. System integration and miniaturization still need further improvement. |
| Label-Free Sensing | 1. Monitoring the electrophysiological properties, growth status, and barrier function, etc., of organoids through impedance sensors. 2. Analyzing the effect of drugs on tumor organoids using Surface Plasmon Resonance (SPR) technology. 3. Detecting metabolic changes in organoids by adopting Mass Spectrometry Imaging (MSI) technology. | 1. No exogenous markers are required, avoiding interference from exogenous markers and improving the reliability of experimental results. 2. Impedance sensors can real-timely monitor multiple biological characteristics. 3. SPR technology has the characteristics of high sensitivity and real-time detection. 4. MSI can simultaneously monitor the spatial distribution of multiple molecules. | 1. In cell classification and status monitoring by impedance technology, the impedance response differences of different cells or states need precise identification. 2. SPR technology has strong dependence on the sensing surface. 3. MSI technology has high requirements for sample processing and matrix selection, etc. |
| Nanomaterial Technology | 1. Modifying electrodes to reduce interface impedance and enhance the electrochemical performance of electrodes, such as graphene-modified electrodes; serving as SERS substrates, such as gold nanoparticles, to enhance Raman scattering signals. 2. Being used to develop new biosensors, such as sensors made of carbon-based nanomaterials. | 1. Nanomaterials have unique physical and chemical properties, which can enhance the sensitivity, selectivity, and conductivity of sensors. 2. They have good biocompatibility with biological tissues, which is conducive to the work of sensors in biological environments. 3. They can achieve the detection of trace substances. | 1. The biocompatibility of some nanomaterials may have problems during long-term culture. 2. The preparation and modification processes of nanomaterials may be complex, increasing costs. 3. They may have stability issues in complex biological environments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, S.; Chen, Y.; Wang, M.; Liu, Y.; Qu, Z.; Du, L.; Wu, C. Sophisticated Interfaces Between Biosensors and Organoids: Advancing Towards Intelligent Multimodal Monitoring Physiological Parameters. Biosensors 2025, 15, 557. https://doi.org/10.3390/bios15090557
Chen Y, Liu S, Chen Y, Wang M, Liu Y, Qu Z, Du L, Wu C. Sophisticated Interfaces Between Biosensors and Organoids: Advancing Towards Intelligent Multimodal Monitoring Physiological Parameters. Biosensors. 2025; 15(9):557. https://doi.org/10.3390/bios15090557
Chicago/Turabian StyleChen, Yuqi, Shuge Liu, Yating Chen, Miaomiao Wang, Yage Liu, Zhan Qu, Liping Du, and Chunsheng Wu. 2025. "Sophisticated Interfaces Between Biosensors and Organoids: Advancing Towards Intelligent Multimodal Monitoring Physiological Parameters" Biosensors 15, no. 9: 557. https://doi.org/10.3390/bios15090557
APA StyleChen, Y., Liu, S., Chen, Y., Wang, M., Liu, Y., Qu, Z., Du, L., & Wu, C. (2025). Sophisticated Interfaces Between Biosensors and Organoids: Advancing Towards Intelligent Multimodal Monitoring Physiological Parameters. Biosensors, 15(9), 557. https://doi.org/10.3390/bios15090557







